Abstract
Serum 25-hydroxyvitamin D [25(OH)D] concentrations are the best indicator of vitamin D nutritional status. We measured serum 25(OH)D concentrations in 94 healthy controls and in 41 subjects (aged 4–22 years) with steroid-sensitive nephrotic syndrome (SSNS) in remission. Children with remitted SSNS had significantly lower 25(OH)D concentrations than healthy controls (median 16.4 ng/ml versus 23.9 ng/ml, P <0.001). In a multivariable logistic regression model, the odds ratios (OR) of vitamin D insufficiency [25(OH)D <20 ng/ml] were independently increased in SSNS subjects [OR 11.2 (95% confidence interval 3.5–36.2)], non-whites [OR 12.9 (4.6–36.2)], older children [OR 1.20 per year (1.06–1.36)], and winter months [OR 6.7 (2.5–18.4)]. Within the SSNS subjects, multiple linear regression determined that serum 25(OH)D concentrations were not associated with SSNS disease characteristics measured in this study, such as duration of disease, number of relapses, cumulative glucocorticoids, and interval since last relapse. In conclusion, children with remitted SSNS have lower serum 25(OH)D concentrations than healthy controls. This difference persisted after adjusting for the potential confounding effects of age, race, season, and milk intake. Children with remitted SSNS may benefit from routine measurement of 25(OH)D, but the clinical significance of low 25(OH)D in this population remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with idiopathic nephrotic syndrome (NS) and preserved renal function often exhibit abnormal vitamin D metabolism [1]. Serum concentrations of 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2 D], the active metabolite of vitamin D, are decreased [2, 3] or in the normal range [4, 5, 6]. More importantly, serum concentrations of 25-hydroxyvitamin D [calcidiol or 25(OH)D], the best marker of body vitamin D stores and overall vitamin D nutritional status [7], are decreased in children with NS [2, 3, 4, 5, 6]. These low 25(OH)D concentrations stem from urinary excretion of vitamin D-binding protein, the carrier protein for 25(OH)D [8]. In NS, suboptimal 25(OH)D concentrations may lead to secondary hyperparathyroidism [9] and metabolic bone disease, notably osteomalacia [10, 11, 12]. A recent case series reported altered bone histology in eight children with NS and normal glomerular filtration rate (GFR), five of whom had serum 25(OH)D less than 30 ng/ml [13]. Although the authors attributed the histological changes to NS disease activity and glucocorticoid therapy [13], suboptimal 25(OH)D concentrations may have contributed to some of the changes.
Most studies of NS have examined vitamin D metabolism during periods of heavy proteinuria [2, 3, 4], but children with NS typically respond quickly and completely to high-dose glucocorticoids [14]. As a result, children with steroid-sensitive nephrotic syndrome (SSNS) are only intermittently proteinuric, during either their first attack or their relapses. During remissions, serum 25(OH)D concentrations are higher than the 25(OH)D concentrations during urinary relapses [5] and are reportedly comparable to the levels in healthy children [6]. Consequently, concern regarding vitamin D nutritional status in childhood NS has focused upon treatment-resistant NS, with its long-standing proteinuria, rather than upon SSNS [15].
Current guidelines from the National Kidney Foundation (NKF) concerning bone metabolism are lacking regarding the measurement of 25(OH)D in persons with NS and normal GFR [16]. In persons with elevated parathyroid hormone (PTH) levels and GFR below 60 ml/min per 1.73 m2, however, the NKF recommends routine measurement of serum 25(OH)D and supplementation with ergocalciferol (vitamin D2) when serum 25(OH)D is less than 30 ng/ml [16]. Before children with NS can be considered candidates for routine 25(OH)D screening, the prevalence of suboptimal 25(OH)D concentrations in this population should be ascertained.
The objective of this study was to assess the vitamin D nutritional status, as measured by serum 25(OH)D, in children and adolescents with remitted SSNS, compared with healthy controls. Our hypothesis was that patients with remitted SSNS are at increased risk for vitamin D insufficiency, which was defined as a serum 25(OH)D concentration <20 ng/ml.
Materials and methods
Study design and subjects
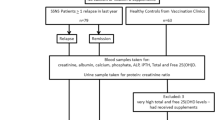
A cross-sectional study was performed to compare children with remitted SSNS and healthy controls. The protocol was approved by the Human Subjects Institutional Review Board at The Children’s Hospital of Philadelphia, and all subjects and parents provided written informed consent.
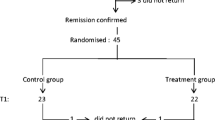
Children with SSNS were identified through a systematic review of all clinic charts for patients seen over the previous 3 years in the nephrology clinics at The Children’s Hospital of Philadelphia and St. Christopher’s Hospital for Children, both in Philadelphia (Pa., USA). Subjects fulfilling the International Study of Kidney Disease in Children criteria for the diagnosis of SSNS [17] (negative or trace protein by urine dipstick within 8 weeks of initial glucocorticoid treatment) were eligible, provided they had had a documented relapse within the 12 months prior to the study visit. Subjects were excluded if they had (1) GFR <80 ml/min per 1.73 m2 as estimated by the Schwartz formula [18] or (2) other chronic medical conditions or medications, unrelated to the nephrotic syndrome, that could potentially affect bone health or vitamin D status. Study visits were scheduled at least 14 days after urinary remission of the most recent relapse. Negative or trace protein by urine dipstick was documented at the time of the study visit.
Healthy controls were recruited from the general pediatric clinics and from the surrounding community, using newspaper advertisements and flyers. Subjects with chronic medical conditions or medications potentially affecting growth, pubertal development, nutritional status, or dietary intake were excluded. Controls were required to be between the 3rd and 97th percentiles for height and body mass index (BMI) for age and gender.
Measurements and assays
SSNS and control subjects were evaluated in the Nutrition and Growth Laboratory at the Children’s Hospital of Philadelphia, where blood was drawn and anthropometric measurements were performed. Weight (kg) was measured using a digital electronic stand-on scale and height (cm) was measured using a wall-mounted stadiometer. Age- and gender-specific standard deviation scores (z-scores) for height, weight, and BMI were calculated using the Centers for Disease Control and Prevention growth charts [19].
Circulating 25(OH)D and 1,25(OH)2 D concentrations were measured by radioimmunoassay with 125I-labeled tracers [20, 21]. Intact PTH was measured in the SSNS subjects only, using a second-generation immunoradiometric assay [22]. The normal range for intact PTH was 10–65 pg/ml. These assays were performed in the laboratory of Dr. Bruce W. Hollis (Medical University of South Carolina, Charleston, S.C., USA).
Subjects completed a food frequency questionnaire at the time of the study visit. Participants were asked to estimate average milk intake per day over the previous 28 days, including milk with cereal, chocolate milk, hot chocolate made from milk, and Lactaid milk.
NS disease characteristics
The inpatient and outpatient medical charts of the SSNS children were reviewed for date of diagnosis of SSNS, total number of relapses, date of last relapse, renal biopsy results, and prior steroid-sparing therapy with alkylating agents (chlorambucil, cyclophosphamide) or cyclosporine. All doses of prednisone and methylprednisolone in the interval between the date of diagnosis and the study visit were documented. Methylprednisolone doses were converted to prednisone equivalents, and total glucocorticoid exposure was summarized as the cumulative milligrams and milligrams per kilogram of prednisone equivalents between the first dose and the last dose.
Statistical analyses
Initial analyses were descriptive. Continuous variables were expressed as means ±standard deviation (SD) or as medians with total and interquartile ranges and compared using unpaired t -tests or Wilcoxon rank sum tests, as appropriate. Categorical variables were expressed as proportions and compared using chi-squared tests.
Given the known decrease in 25(OH)D concentrations during the fall and winter, the season of study visit was divided into winter and non-winter, with winter broadly defined as a study date falling from November through March [23, 24]. Patient race/ethnicity was characterized as either white or non-white. Children of Hispanic ethnicity were considered non-white.
Serum 25(OH)D concentrations were used to categorize the vitamin D nutritional status of the study subjects. Previously described cutoffs for serum 25(OH)D were used. A patient was considered to have hypovitaminosis D if 25(OH)D was <30 ng/ml [16], vitamin D insufficiency if 25(OH)D was <20 ng/ml, and vitamin D deficiency if 25(OH)D was <10 ng/ml [25, 26].
Multivariable logistic regression
To determine the characteristics associated with vitamin D insufficiency, we built an explanatory multivariable logistic regression model. Variables whose unadjusted, bivariable odds ratios (OR) had P <0.25 were eligible for inclusion in the multivariable model. Using a backwards deletion strategy, these variables were used to construct the adjusted multivariable model [27]. Multiplicative interaction terms were incorporated to evaluate the effects of race and season on the relationship between SSNS and vitamin D status. Overall model fit and calibration were assessed by the Hosmer-Lemeshow goodness-of-fit test [27]. Model discrimination was assessed using the area under the receiver operator characteristics (ROC) curve. Multivariable logistic regression for hypovitaminosis D and vitamin D deficiency was not performed since few study subjects had vitamin D deficiency and nearly all subjects had hypovitaminosis D [27].
Multiple linear regression
To determine if SSNS subject characteristics affected serum 25(OH)D concentrations, a multiple linear regression model was fit, using 25(OH)D as the dependent variable. Candidate independent variables included demographic characteristics and SSNS disease and treatment characteristics, such as duration of NS, number of relapses, time since last relapse, cumulative dose of glucocorticoids, and duration of glucocorticoid therapy.
Variables were screened using bivariable analysis. A backwards deletion strategy was used to determine the final, parsimonious regression model. Model fit was confirmed by (1) using a forward selection strategy after the bivariable analysis and (2) initially including all candidate variables and then employing a backwards deletion strategy [28]. Variables were assessed for collinearity and multiplicative interaction. Model specification, model fit, and the assumptions of the linear regression model were also assessed.
All analyses were conducted using Stata 7.0 statistical software (Stata Corporation, College Station, Tex., USA). Two-sided tests of hypotheses were used and a P value <0.05 was considered to be statistically significant.
Results
Subject and disease characteristics
The characteristics of the control and SSNS patients are presented in Table 1. The healthy controls were slightly older than the SSNS subjects. The significant male predominance in the SSNS subjects was consistent with the gender pattern of the disease [29]. The SSNS subjects had lower height and higher BMI z-scores compared with control subjects, findings consistent with long-term glucocorticoid use.
The disease and glucocorticoid treatment of the SSNS subjects are summarized in Table 2. Of the SSNS subjects, 26 (63.4%) were on glucocorticoid therapy at the time of the study visit; 14 (34.1%) of the SSNS subjects had been in remission for less than 8 weeks. There were 7 subjects that had undergone renal biopsies: 3 had mesangial proliferation, 2 had minimal change disease, and 2 had focal segmental glomerulosclerosis. Of the 41 patients, 6 patients (14.6%) had been treated with cyclosporine, while 11 patients (26.8%) had been treated with at least one course of an alkylating agent (cyclophosphamide or chlorambucil).
Serum 25(OH)D, 1,25(OH)2 D, and PTH concentrations
Circulating 25(OH)D concentrations were significantly lower in SSNS subjects than in control subjects. As shown in Fig. 1, SSNS subjects had a median 25(OH)D concentration of 16.4 ng/ml (mean 17.4±7.6 ng/ml), while control subjects had a median 25(OH)D concentration of 23.9 ng/ml (mean 23.9±10.3 ng/ml) (P =0.0002). The distributions of 25(OH)D concentrations differed significantly between the two groups, as shown in Fig. 2 (P =0.001). Nearly all (90.2%) of the SSNS children had hypovitaminosis D, 68.3% were vitamin D insufficient, and 19.5% were vitamin D deficient. Although most control subjects had hypovitaminosis D, only 30.8% were vitamin D insufficient and 10.6% vitamin D deficient.
Box and whisker plots of 25-hydroxyvitamin D [25(OH)D] concentrations in controls and patients with steroid-sensitive nephrotic syndrome (SSNS). The horizontal lines within each box designate the median values, the lower and upper horizontal lines at the top and bottom of the boxes delineate the 25th and 75th percentiles, and the whiskers mark the upper and lower adjacent values. Outliers are designated by asterisks. The difference between the median 25(OH)D concentrations is statistically significant (P=0.0002)
Circulating 1,25(OH)2 D concentrations were higher in SSNS children than in controls (Fig. 3). SSNS subjects had a median 1,25(OH)2 D concentration of 47.1 pg/ml (mean 50.1±16.5 pg/ml), while control subjects had significantly lower 1,25(OH)2 D concentrations, with a median of 37.8 pg/ml (mean of 41.2±14.5 pg/ml) (P =0.0007).
Box and whisker plots of 1,25(OH)2 D concentrations in control and SSNS patients. The horizontal lines within each box designate the median values, the lower and upper horizontal lines at the top and bottom of the boxes delineate the 25th and 75th percentiles, and the whiskers mark the upper and lower adjacent values. Outliers are designated by asterisks. The difference between the median 1,25(OH)2 D concentrations is statistically significant (P =0.0007)
Serum PTH concentrations were available in the SSNS patients only. The mean serum intact PTH was 22.9±10.6 pg/ml (median 21.4 pg/ml, interquartile range 14.6–27.0 pg/ml). PTH concentrations were negatively correlated with 25(OH)D concentration (r=−0.30, P =0.07) and positively correlated with 1,25(OH)2 D (r=0.3491, P =0.03).
Factors associated with vitamin D insufficiency
To determine factors independently associated with vitamin D insufficiency, a parsimonious multivariable logistic regression model was fit using subjects with SSNS and healthy controls (Table 3). The candidate variables were the factors listed in Table 1. The characteristics independently associated with vitamin D insufficiency were SSNS, older age, non-white race, and a study date during wintertime. Sex and anthropometric z-scores were not associated with vitamin D insufficiency in the final model. When modeled with the other variables in Table 3, increased milk intake was associated with a decreased OR of vitamin D insufficiency (OR 0.89 per cup/day milk intake, 95% confidence interval 0.76–1.05), but this association did not achieve statistical significance (P =0.16) and was excluded from the final model. For the final model, the Hosmer-Lemeshow goodness-of-fit test statistic was 0.78. The area under the ROC curve was 0.88.
The interaction between race and season could not be tested via logistic regression, since the combination of wintertime study visit and non-white race perfectly predicted vitamin D insufficiency in both the SSNS and control subjects. All of the 20 study subjects (12 controls, 8 SSNS) who were non-white and examined during the wintertime were vitamin D insufficient. The percentage of white subjects who were vitamin D insufficient in the winter was 41.4%. This difference in proportions suggests that the impact of winter upon serum 25(OH)D may be more detrimental for non-whites than whites.
Factors associated with 25(OH)D concentration in subjects with SSNS
To determine whether NS disease characteristics affected 25(OH)D concentrations, a multiple linear regression model was fit, using only the SSNS patients (Table 4). Candidate variables for this model included general characteristics of all study participants (Table 1) as well as disease and treatment characteristics specific to SSNS (Table 2). After the bivariable analysis, the SSNS disease characteristics that were initially eligible for the multiple regression model were duration of NS, duration of glucocorticoid therapy, and cumulative glucocorticoid dose (unadjusted for body mass). Of note, there was no evidence of an association between serum 25(OH)D and the time interval since last relapse (P =0.33). Age, race, height z-score, season of study visit, and milk intake were also eligible for the multiple regression model.
In the final adjusted regression model, older age, non-white race, lower milk intake, and a study visit during wintertime were each independently associated with lower 25(OH)D concentrations (Table 4). For example, 25(OH)D concentrations were 9.25 ng/ml lower, on average, in non-whites, compared with whites. After adjusting for other potential confounders, no SSNS disease characteristics were significantly associated with circulating 25(OH)D. Duration of NS and duration of glucocorticoid therapy were strongly collinear; neither factor was significant when included individually in the model. Although its P value was >0.05, season of study visit remained in the final model, since season is a known determinant of vitamin D status, and its inclusion improved overall model fit as measured by the adjusted R 2 statistic. Multiplicative interaction between race and season of study visit was evaluated but was not significant. The adjusted R 2 statistic for the final model was 0.59.
Discussion
In this cross-sectional study, children with remitted SSNS had lower serum 25(OH)D concentrations, and hence worse vitamin D nutritional status, than local healthy controls. Serum concentrations of 25(OH)D, not 1,25(OH)2 D or even the 1,25(OH)2 D index, are the best indicator of total body stores of vitamin D [7]. This difference in 25(OH)D concentrations was substantial and persisted after adjusting for potential confounders such as age, race, sex, anthropometric measurements, milk intake, and the season of the study visit. The odds of having vitamin D insufficiency, defined as a 25(OH)D concentration <20 ng/ml, were greatly increased in children with SSNS. Older age, non-white race, and a study visit during winter were also independently associated with greater odds of vitamin D insufficiency.
To our knowledge, this study is the first to report that children with remitted NS have worse vitamin D nutritional status than healthy controls. Prior pediatric studies that documented low 25(OH)D concentrations in NS examined children with active nephrosis, either during the presenting episode or during a relapse [2, 3, 4]. Freundlich et al. [5] studied children during relapse and remission of their NS but did not include comparisons with healthy controls. Huang et al. [6] found that children with remitted NS had 25(OH)D concentrations similar to those of healthy controls but did not adjust for season, which is known to affect serum 25(OH)D [24]. In adult NS, studies of 25(OH)D have also focused upon patients with heavy or persistent proteinuria [9, 10, 11, 12] and excluded patients in remission.
In children with remitted SSNS, repeated episodes of proteinuria are the most likely cause of the low 25(OH)D concentrations. Children with SSNS usually suffer multiple relapses, with 60% experiencing five or more [14]. In this study, the median number of relapses was ten. During relapses, the response to glucocorticoids is reliable but not always instantaneous, and proteinuria may persist for weeks before resolving [17, 30]. Recurring periods of prolonged proteinuria may eventually produce deficits of circulating 25(OH)D. In this study, however, number of relapses, duration of NS, time since last relapse, and other markers of SSNS disease severity were not independently associated with the serum 25(OH)D concentration (Table 4). SSNS disease characteristics that were not measured in this study, such as cumulative duration of relapses or severity of proteinuria in each relapse, may be more closely associated with decrements in circulating 25(OH)D.
Decreases in sunlight exposure may contribute to the lower 25(OH)D concentrations in children with SSNS. Exposure to ultraviolet rays is required for dermal synthesis of vitamin D3, the precursor to 25(OH)D and 1,25(OH)2 D. In SSNS children, decreased sunlight exposure may stem from decreased outdoor activity. Skin changes related to glucocorticoid use may cause SSNS children to wear additional clothing or apply topical sunscreens, two measures that prevent dermal synthesis of vitamin D3 [31, 32]. Sunlight exposure is difficult to quantify, however, and was not measured in this study.
Decreased dietary intake of vitamin D is another possible cause of the lower 25(OH)D concentrations in SSNS. Children with SSNS and their families are advised to restrict sodium intake, due to edema and glucocorticoid-induced salt and water retention. In addition to being fortified with vitamin D, milk contains approximately 125 mg sodium/8 ounces. At the Children’s Hospital of Philadelphia, families are counseled to limit milk intake to 1.5 cups per day due to the sodium content. In this study, the milk intake was similar between the SSNS and control groups in the month prior to the study visit. However, SSNS subjects may limit milk intake during active relapses. Finally, SSNS subjects were not grossly malnourished; in fact, 41% were obese, compared with 3% of control subjects.
Besides NS, other factors independently associated with vitamin D insufficiency were non-white race, a study visit during winter, and older age. An association of non-white race with lower circulating 25(OH)D was expected. Prior studies have found that blacks [33, 34], Asians [35], and Hispanics [36] have lower 25(OH)D concentrations than whites. The seasonal variation of serum 25(OH)D is also well known [23] and arises from the atmospheric attenuation of ultraviolet radiation during cold-weather months, due to the increased zenith angle of the sun [24]. In contrast, the association of older age with worse vitamin D nutritional status, in both control and SSNS children, was unexpected. Older age has been associated with decreased circulating 25(OH)D, but these studies have examined the elderly [37] rather than children. Most pediatric studies of 25(OH)D have examined children within a narrow age range [23, 34, 38, 39], limiting their ability to show a relationship between 25(OH)D and age. The age-related decrease in 25(OH)D was not attributable to decreased milk intake in older children. The authors are not aware of other pediatric studies showing an age-related decrease in circulating 25(OH)D, and this finding will need to be confirmed in future studies.
The high prevalence of 25(OH)D insufficiency in the children with SSNS suggests that serum 25(OH)D may need to be routinely checked in all children with NS. In the past, altered bone and vitamin D status was thought to occur only in children with long-standing, massive proteinuria, like those with Finnish-type congenital NS or treatment-resistant NS [12, 15]. In this study, however, children with SSNS, which constitutes the vast majority of childhood idiopathic NS, had lower 25(OH)D concentrations than healthy children. Furthermore, the children with SSNS were in remission for at least 14 days prior to the date of the study visit. Because the duration of NS and the number of relapses were not significant in the multiple regression model, even children with infrequent relapses may be at risk for vitamin D insufficiency and deficiency.
The high prevalence of hypovitaminosis D and vitamin D insufficiency in children with SSNS suggests that many children with SSNS may benefit from vitamin D repletion. When serum 25(OH)D is less than 30 ng/ml, NKF guidelines recommend high-dose supplementation with ergocalciferol (vitamin D2) for at least 6 months, although these guidelines are intended for persons with GFR ≤60 ml/min and elevated PTH [16]. In this study, nearly all (90.2%) of the children with SSNS meet this cutoff [25(OH)D <30 ng/ml] for high-dose vitamin D therapy. It is unclear, however, whether the NKF guidelines are applicable to children with SSNS with decreased 25(OH)D but normal GFR and PTH.
Although they had higher 25(OH)D concentrations than the SSNS children, the control children also had a high prevalence of hypovitaminosis D. Admittedly, there are no uniform definitions of what 25(OH)D concentrations are low, suboptimal, or normal [40]. Since population-based reference ranges vary with diet, geography, and sunlight exposure, health-based reference limits may be more appropriate. One approach defines a “low” 25(OH)D concentration (hypovitaminosis D) as the concentration of 25(OH)D above which vitamin D supplementation no longer decreases PTH [26]. Vitamin D insufficiency refers to 25(OH)D concentrations low enough to eventually cause anatomical, physiological, or biochemical abnormalities, such as rickets. Vitamin D deficiency describes even lower 25(OH)D concentrations and the definite existence of such abnormalities, which may reverse with vitamin D repletion [41]. The cutoff 25(OH)D concentrations for these categories are somewhat arbitrary, although the cutoffs of 30 ng/ml, 20 ng/ml, and 10 ng/ml used in this study have been previously used [16, 26]. Several recent studies have documented that ostensibly healthy children often have hypovitaminosis D or more severe states of low 25(OH)D [23, 34, 38, 39]. Most notably, a cross-sectional study of 307 healthy adolescents from the Boston area reported a 42.0% prevalence of serum 25(OH)D concentrations ≤20 ng/ml [39], which is even greater than the 30.9% prevalence in our healthy controls. The nearly universal occurrence of hypovitaminosis D in our controls suggests that measurement of 25(OH)D may be useful in otherwise healthy children, as well as in children with SSNS.
This study has several important limitations. Firstly, this study’s cross-sectional design precludes definitive conclusions regarding causal inference. A prospective, longitudinal cohort that measures vitamin D metabolites before and after onset of SSNS would be the preferred study design. Secondly, this study examined SSNS patients from two medical centers in one city, so the results may not be applicable to other pediatric nephrology practices, elsewhere in the United States or abroad. However, these two centers draw upon a diverse patient population and serve as the main pediatric nephrology referral centers for a large metropolitan area. Thirdly, this study examined children whose SSNS had relapsed within the previous 12 months. These results may not apply to children with long-dormant SSNS, a population whose 25(OH)D status has not been addressed. Fourthly, diet and sunlight exposure may confound the relationship between SSNS and 25(OH)D, and this study collected only limited information regarding diet and no information regarding sunlight exposure. Although they are difficult to quantify, diet and sunlight exposure should be measured in future studies of SSNS to help determine whether low 25(OH)D concentrations in SSNS are due to proteinuria or due to decreased sunlight exposure and dietary intake.
Finally, the clinical significance of low 25(OH)D levels, in both the SSNS and control children, is uncertain. Surprisingly, SSNS patients did not demonstrate secondary hyperparathyroidism. Measurements of PTH were unavailable in the healthy controls, so we could not determine whether SSNS patients had higher PTH concentrations than control patients. The SSNS children in this study are unlikely to have abnormal bone densities, since they are a subset of a group of NS children whose dual-energy X-ray absorptiometry results were normal [42]. This present study did not measure serum markers of bone formation and resorption, such as bone-specific alkaline phosphatase and serum pyridinoline, or utilize bone biopsies. Further studies are necessary to determine whether low 25(OH)D status leads to clinically significant metabolic bone disease in SSNS. Low 25(OH)D concentrations have also been linked to non-skeletal consequences, such as certain cancers, cardiovascular disease, and type 1 diabetes [43]. Studies larger than the present one, with long-term follow-up, are required to determine whether hypovitaminosis D is associated with these diseases in persons with SSNS.
In summary, children with remitted SSNS have a worse vitamin D nutritional status, as measured by serum 25(OH)D concentrations, than healthy controls. This difference persists after adjusting for the confounding effects of age, race, season, and milk intake. Nearly all (90.2%) of the SSNS children had serum 25(OH)D concentrations less than 30 ng/ml, the cutoff below which the NKF recommends high-dose supplementation with vitamin D in persons with decreased GFR and increased PTH. Children with SSNS may benefit from routine measurement of 25(OH)D, which could lead to earlier detection of vitamin D insufficiency and earlier treatment of its consequences. Additional studies are needed to determine the clinical significance of suboptimal 25(OH)D concentrations in both healthy children and in children with remitted SSNS.
References
Alon U, Chan JC (1984) Calcium and vitamin D homeostasis in the nephrotic syndrome: current status. Nephron 36:1–4
Auwerx J, De Keyser L, Bouillon R, De Moor P (1986) Decreased free 1,25-dihydroxycholecalciferol index in patients with the nephrotic syndrome. Nephron 42:231–235
Grymonprez A, Proesmans W, Van Dyck M, Jans I, Goos G, Bouillon R (1995) Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol 9:278–281
Freundlich M, Bourgoignie JJ, Zilleruelo G, Jacob AI, Canterbury JM, Strauss J (1985) Bone modulating factors in nephrotic children with normal glomerular filtration rate. Pediatrics 76:280–285
Freundlich M, Bourgoignie JJ, Zilleruelo G, Abitbol C, Canterbury JM, Strauss J (1986) Calcium and vitamin D metabolism in children with nephrotic syndrome. J Pediatr 108:383–387
Huang JP, Bai KM, Wang BL (1992) Vitamin D and calcium metabolism in children with nephrotic syndrome of normal renal function. Chin Med J (Engl) 105:828–832
Hollis BW (1996) Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int 58:4–5
Barragry JM, France MW, Carter ND, Auton JA, Beer M, Boucher BJ, Cohen RD (1977) Vitamin-D metabolism in nephrotic syndrome. Lancet II:629–632
Goldstein DA, Haldimann B, Sherman D, Norman AW, Massry SG (1981) Vitamin D metabolites and calcium metabolism in patients with nephrotic syndrome and normal renal function. J Clin Endocrinol Metab 52:116–121
Malluche HH, Goldstein DA, Massry SG (1979) Osteomalacia and hyperparathyroid bone disease in patients with nephrotic syndrome. J Clin Invest 63:494–500
Mittal SK, Dash SC, Tiwari SC, Agarwal SK, Saxena S, Fishbane S (1999) Bone histology in patients with nephrotic syndrome and normal renal function. Kidney Int 55:1912–1919
Tessitore N, Bonucci E, D’Angelo A, Lund B, Corgnati A, Valvo E, Lupo A, Loschiavo C, Fabris A, et al. (1984) Bone histology and calcium metabolism in patients with nephrotic syndrome and normal or reduced renal function. Nephron 37:153–159
Freundlich M, Jofe M, Goodman WG, Salusky IB (2004) Bone histology in steroid-treated children with non-azotemic nephrotic syndrome. Pediatr Nephrol 19:400–407
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639
Alon US (1995) Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol 9:791–792
National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–S202
International Study of Kidney Disease in Children (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 98:561–564
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60
Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL (1993) Determination of vitamin D status by radioimmunoassay with an125I-labeled tracer. Clin Chem 39:529–533
Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL (1996) Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with125I-labeled tracer. Clin Chem 42:586–592
Bouillon R, Coopmans W, Degroote DE, Radoux D, Eliard PH (1990) Immunoradiometric assay of parathyrin with polyclonal and monoclonal region-specific antibodies. Clin Chem 36:271–276
Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S (1995) Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone 17:513–516
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378
Cannata-Andia JB, Gomez Alonso C (2002) Vitamin D deficiency: a neglected aspect of disturbed calcium metabolism in renal failure. Nephrol Dial Transplant 17:1875–1878
McKenna MJ, Freaney R (1998) Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int 8 [Suppl 2]:S3–S6
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd ed. Wiley, New York, pp 91–202
Sun GW, Shook TL, Kay GL (1996) Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol 49:907–916
Sherbotle JR, Hoyer JR (1991) Idiopathic nephrotic syndrome: minimal-change disease and focal segmental glomerulosclerosis. In: Jacobson HB, Striker GE, Klahr S (eds) The principles and practice of nephrology. Decker, Philadelphia, pp 288–292
Arbeitsgemeinschaft fur Padiatrische Nephrologie (1988) Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet I:380–383
Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF (1987) Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 64:1165–1168
Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF (1992) Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab 75:1099–1103
Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA (2002) Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192
Gessner BD, Plotnik J, Muth PT (2003) 25-Hydroxyvitamin D levels among healthy children in Alaska. J Pediatr 143:434–437
Shaw NJ, Pal BR (2002) Vitamin D deficiency in UK Asian families: activating a new concern. Arch Dis Child 86:147–149
Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR (2002) Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777
Holick MF, Matsuoka LY, Wortsman J (1989) Age, vitamin D, and solar ultraviolet. Lancet II:1104–1105
Cheng S, Tylavsky F, Kroger H, Karkkainen M, Lyytikainen A, Koistinen A, Mahonen A, Alen M, Halleen J, Vaananen K, Lamberg-Allardt C (2003) Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr 78:485–492
Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ (2004) Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537
Greer FR (2003) Vitamin D deficiency—it’s more than rickets. J Pediatr 143:422–423
Parfitt AM, Gallagher JC, Heaney RP, Johnston CC, Neer R, Whedon GD (1982) Vitamin D and bone health in the elderly. Am J Clin Nutr 36:1014–1031
Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA (2004) Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875
Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371
Acknowledgments
We greatly appreciate the dedication and enthusiasm of the children and their families who participated in this study. This study was supported in part by grants F32-DK062580 (F.L.W.), K08-DK002523 (M.B.L.), and M01-RR000240–390465 (The Children’s Hospital of Philadelphia General Clinical Research Center) from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weng, F.L., Shults, J., Herskovitz, R.M. et al. Vitamin D insufficiency in steroid-sensitive nephrotic syndrome in remission. Pediatr Nephrol 20, 56–63 (2005). https://doi.org/10.1007/s00467-004-1694-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1694-7