Abstract
Treating children with steroid-resistant nephrotic syndrome (SRNS) has been a clinical challenge for pediatricians. We recruited 24 children (18 boys and six girls) with steroid-resistant idiopathic nephrotic syndrome (SRINS) who were <2 years. All patients were administered prednisone 2 mg/kg per day prior to mycophenolate mofetil (MMF). By the end of the eighth week, MMF was initiated at 25–30 mg/kg daily for 6− 12 months. Prednisone dose was reduced stepwise. Biochemical assays were performed every 2 months. Complete remission was achieved in 15 patients, partial remission in six, and no response to MMF was noted in three. With MMF treatment, the levels of urinary protein and serum cholesterol decreased and that of serum albumin increased in a time-dependant manner. We demonstrated the MMF could reduce proteinuria in SRINS children <2 years. Our study suggests that MMF therapy might be an effective strategy for treating SRINS in children <2 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with steroid-resistant idiopathic nephrotic syndrome (SRINS) often have poor prognosis, and some patients progress to end-stage renal disease (ESRD) [1–3]. In recent years, cyclosporine (CsA) has been used to treat SRINS, making it possible to reduce steroid dosage and alleviate proteinuria [4]. However, CsA is associated with nephrotoxicity in some patients, especially in young children.
Mycophenolate mofetil (MMF) is a newly developed immunosuppressant drug that suppresses acute host rejection of renal grafts. MMF is a derivative of the active substance mycophenolic acid. It antagonizes purine metabolism and selectively suppresses T- and B lymphocytes dependent on de novo synthesis of purines, thus exerting immunosuppressive effects [5]. Therefore, MMF has little effect on other cells and is unlikely to cause severe adverse reactions such as bone marrow suppression. This drug has recently been used to treat nephrotic syndrome in adults and children [6–19]. However, in reviewing the literature, we were unable to determine the benefits of MMF treatment in children with SRINS, especially those <2 years. We report our clinical experience on the effectiveness of MMF treatment in children <2 years of age with SRNS.
Patients and methods
General study outline
This was a prospective, single-center trial aimed at studying the efficacy and side effects of MMF in children <2 years of age with SRINS. Patients were enrolled between January 2005 and September 2008 in Hunan Children’s Hospital, Changsha, China. The ethics committees of the hospitals approved the study protocol. Consent was obtained from a parent or guardian after written and oral information was given.
Patients
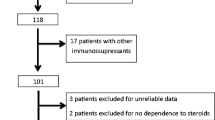
We recruited 24 children <2 years of age with SRINS. The definition and criteria for nephrotic syndrome were the same as those used in the International Study of Kidney Disease in Children (ISKDC) [20]: edema, serum albumin <2.5 g/dl, and proteinuria >40 mg/m2/h. Patients had not responded to the 8-week course of prednisone (2 mg/kg daily), serum creatinine was ≤1.5 mg/dl and hemoglobin ≥90 g/L, they were negative for hepatitis C, aged <2 years, and kidney pathology was confirmed by renal biopsy. Exclusion criteria were as follows: possible presence of secondary nephrotic syndrome, diagnosis of congenital nephrotic syndrome, presence of hereditary kidney disease, and the presence of severe infection. The patient population comprised 18 boys and six girls (Table 1). The starting median age of nephrotic syndrome was 1 year and 7 months (range 8-24 months). Median duration of illness was 4.2 (range 2− 12) months. Initial steroid resistance was present from disease outset in eight children and appeared later, during one of the relapses, after 3−10 months of NS evolution in 16 (late steroid resistance). Thirteen of 24 patients had associated microscopic hematuria, two (case 6 and case 18) had associated macroscopic hematuria, and one (case 6) had associated with hypertension. The pathological findings revealed proliferative and sclerosis glomerulonephritis in two patients, focal segmental glomerulosclerosis (FSGS) in three, immunoglobulin M (IgM) nephropathy in four, severe mesangioproliferative glomerulonephritis in four, middle mesangioproliferative glomerulonephritis in eight, and mild mesangioproliferative glomerulonephritis in three.
Study medication
Prednisone was administered at 2 mg/kg of bodyweight per day, which was continued for 8 weeks prior to MMF. By the end of the eighth week, MMF was initiated at a daily dose of 25−30 mg/kg per day and administered as two divided doses for 6−12 months. Prednisone dose was reduced stepwise by 5 mg/day every 4 weeks, when down to a maintenance dose of 1 mg/kg of bodyweight per day, which was continued for 8 weeks, followed by 1 mg/kg on alternate days for 4 weeks and then tapered to zero. Every 2 months, biochemical assays were performed for 24-h urinary protein content, blood creatinine, liver functions, blood albumin, cholesterol, urine retinol-binding protein (RBP), and β2-microglobulin (β2-M). During the treatment period, adverse effects such as infection, abnormal peripheral white blood cell count, and abnormal gastrointestinal manifestations (vomiting, diarrhea, and abdominal pain) were observed. Complete remission was defined as a reduction in 24-h urinary protein excretion to <100 mg/m2 per day and normal levels of serum albumin, whereas partial remission was defined as a 50% reduction of initial proteinuria and <40 mg/m2 per hour. Reduction of 24h total protein excretion to <50% and >40 mg/m2 per hour was indicated as no remission.
Biochemical measurements
Twenty-four-hour urinary protein excretion, serum creatinine, albumin, cholesterol, and electrolytes were measured with an automatic biochemistry analyzer. Urine volume in 24 h was collected via a collection bag in boys and an indwelling bladder catheter in girls.
Statistical analysis
All values are expressed as medians and ranges. Wilcoxon rank-sum test was used to determine the significance of differences. A value of P < 0.05 was considered to be statistically significant.
Results
After 2 months of MMF treatment, nine patients (37.5%) exhibited complete remission and seven (31.2%) partial remission. After 4 months, 13 patients (56.3%) exhibited complete remission and six (25.0%) partial remission. After 6 months, 15 patients (62.5%) exhibited complete remission, six (25.0%) partial remission, and three (12.5%) did not respond (Table 1). Median values of 24-h urinary protein levels of the 24 patients before MMF treatment and after 2, 4, and 6 months of treatment were 1.68 g, 0.76 g, 0.32 g, and 0.16 g, respectively. Differences between pre- and the posttreatment urine protein levels were significant at each time point (P < 0.01). Along with a decrease in the 24-h urinary protein levels, patients’ serum albumin levels exhibited a gradual increase and serum cholesterol levels gradually returned to normal (Table 2).
Follow-up
After 6 months for MMF treatment, 15 patients (62.5%) exhibited complete remission; by the end of the sixth month, MMF dosage was reduced by 50% of the initial dose, which was continued for 3 months, and then to zero. Two patients (case 5 and case 9) were lost to follow-up, four patients relapsed at the fourth (case 3), sixth (case 7), eighth (case 17), and ninth month (case 10), respectively, after cessation of MMF treatment. Three patients (cases 1, 11, and 15) exhibited complete remission >10 months after treatment cessation, six (cases 2, 12, 19, 20, 21, and 23) achieved remission >12 months. Six (25.0%) patients exhibited partial remission. Case 8 discontinued MMF treatment after 6 months. Case 16 persisted with MMF for 8 months, two (cases 4 and 14) persisted with MMF for 10 months, and two (cases 22 and 24) persisted with MMF for 12 months; however, none of them achieved complete remission. Three patients (12.5%) (cases 6, 13, and 18) did not respond to 6 months of MMF treatment, and by the end of 6 months, MMF treatment was discontinued: case 6 was lost to follow-up, case 13 died of a serious infection after 3 months of stopping MMF therapy, and case 18 had been treated using traditional Chinese medicine.
Side effects
Three patients experienced mild nausea and vomiting; two developed mild diarrhea in the initial stage of the treatment, but the symptoms disappeared 1 week later; and three patients demonstrated a slight decrease in peripheral white blood cell count (3 × 109 cells/L–4 × 109 cells/L), which recovered over the following 4 weeks. Hepatic and renal functions and blood electrolyte levels during treatment were within the normal range.
Discussion
The percentage of children with SRINS is estimated to range from 7% to 18%, with the possibility of increasing frequency in recent years [20–22]. Recently, Huan et al. conducted clinical research on the effects of steroid therapy in patients <3 years of age with idiopathic nephrotic syndrome. In their study, 15.4−25.8% of cases demonstrated steroid-sensitive nephrotic syndrome, whereas 74.2−84.6% of these cases demonstrated SRNS, among which SRINS accounted for 19.4−65.4% of cases [23–27]. Since 2005, of the 136 patients <3 years admitted to our center for idiopathic nephrotic syndrome, 37 (27%) developed steroid-sensitive nephrotic syndrome, 60 (44%) demonstrated steroid dependence or frequent relapses, and 39 (29%) demonstrated steroid resistance: 24 of 39 SRINS patients were <2 years.
Children with SRINS pose the most difficult therapeutic challenge. These children are at risk for complications of unremitting nephrotic syndrome and developing ESRD. A number of medications such as methylprednisolone [28, 29], cyclophosphamide [23, 25, 26], vincristine [30, 31], azathioprine [25, 26], nitrogen mustard [27], and cyclosporine A [23, 26, 32–34] have been used with varying result. These drugs for treating SRINS have total remission rates ranging from 20% to 70%, but they also cause serious side effects such as nephrotoxicity, hypertension, hirsutism, arrhythmias, psychosis, and severe infections. Hence, an alternative is welcome.
In recent years, MMF has become a widely used immunosuppressant. Due to a lack of side effects such as liver and kidney toxicity and myelosuppression, its efficacy and safety in treating childhood nephrotic syndrome of various etiologies [6–19], severe purpuric nephritis [35, 36], lupus nephritis [37, 38], and other glomerular diseases [38, 39] has been confirmed and publicly accepted. MMF therapy was shown to be useful in managing children with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome [6–17]. With regard to its efficacy in children with SRINS, study results are variable. Mendizábal et al. reported that in five patients with SRINS whose renal histopathological pattern was FSGS, only one achieved complete remission and one partial remission. Of the three MMF-resistant patients, one progressed to chronic renal failure (CRF) and two with SRINS were continued on antiproteinuric therapy [14]. Ulinski et al. reported that two patients with secondary SRNS to MMF therapy presented a decrease in residual proteinuria over the follow-up period of 261 days; serum protein levels did not significantly change significantly [15]. Here we report that 24 children with SRINS were treated with the combination of MMF and prednisone. This report is the largest single-center experience in SRINS children <2 years of age treated with MMF. Results showed 15 patients (62.5%) exhibited complete remission, and six (25.0%) exhibited partial remission. This study revealed that MMF treatment for SRINS children has a higher rate of complete remission than other reports. However, this may be related to the group patients in our study. Eight children showed initial steroid resistance and 16 late steroid resistance. Pathological findings were proliferative and sclerotic glomerulonephritis in two, FSGS in three, IgM nephropathy in four, severe mesangioproliferative glomerulonephritis in four, moderate mesangioproliferative glomerulonephritis in eight, and mild mesangioproliferative glomerulonephritis in three. In contrast, patients had initial steroid resistance, and the histopathological pattern was usually FSGS in other reports.
Studies showed that the side effects of MMF therapy are generally mild, and most patients tolerated MMF well. Little is known about whether children <2 years of age with SRINS can tolerate MMF therapy. Gastrointestinal disorders, widely known to be an adverse reaction to MMF, were noted in five patients, and three patients demonstrated a slight decrease in peripheral white blood cell count in this study. Though these disorders were mild and transient, it is possible that more risks for gastrointestinal-related side effects may develop over time. We found one patient died of severe infection after 3 months of stopping MMF therapy; the severe event may have been related to MMF treatment. Also, infectious risks of long-term MMF in small children are still unknown. These risks need to be studied.
In summary, this study observed the effects of MMF on urinary protein excretion and side effects in children with SRINS <2 years of age. Results demonstrated that MMF significantly decreased urinary protein excretion in this population. However, our findings are from a single center and only a small number of cases. A larger randomized controlled trial of this therapy is needed.
References
El-Husseini A, El-Basuony F, Mahmoud I, Donia A (2004) Co-administration of cyclosporine and ketoconazole in idiopathic childhood nephrosis. Pediatr Nephrol 19:976–981
Ejaz I, Khan HI, Javaid BK, Rasool G (2004) Histopathological diagnosis and outcome of paediatric nephrotic syndrome. J Coll Physicians Surg Pak 14:229–233
Sorof JM, Hawkins EP, Brewer ED, Boydstun II, Kale AS, Powell DR (1998) Age and ethnicity affect the risk and outcome of focal segmental glomeruloslcerosis. Pediatr Neprhol 12:764–768
Okada M, Hino S, Takemura T, Fukushima K (1999) Cyclosporin therapy in children with steroid-resistant nephrotic syndrome. Clin Exp Nephrol 3:S34–S39
Allison AC, Eugui EM (1993) Immunosuppressive and other effects of mycophenolic acid ester prodrug, Mycophenolate mofetil. Immunol Rev 136:5–28
Barletta G, Smoyer W, Bunchman T, Flynn J, Kershaw D (2003) Use of Mycophenolate Mofetil in steroid-dependent and –resistant nephrotic syndrome. Pediatr Nephrol 18:833–837
Okada M, Sugimoto K, Yagi K, Yanagida H, Tabata N, Takemura T (2007) Mycophenolate mofetil therapy for children with intractable nephrotic syndrome. Pediatr Int 49:933–937
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Afzal K, Bagga A, Menon S, Hari P, Jordan SC (2007) Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 22:2059–2065
Moudgil A, Bagga A, Jordan SC (2005) Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions. Pediatr Nephrol 20:1376–1381
Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ (2008) Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23:2013–2020
Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K (2007) A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol 22:71–76
Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H (2005) Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 20:1265–1268
Mendizábal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J (2005) Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20:914–919
Ulinski T, Dubourg L, Saïd MH, Parchoux B, Ranchin B, Cochat P (2005) Switch from cyclosporine A to mycophenolate mofetil in nephrotic children. Pediatr Nephrol 20:482–485
Gellermann J, Querfeld U (2004) Frequently relapsing nephrotic syndrome: treatment with mycophenolate mofetil. Pediatr Nephrol 19:101–104
Chandra M, Susin M, Abitbol C (2000) Remission of relapsing childhood nephrotic syndrome with mycophenolate mofetil. Pediatr Nephrol 14:224–226
Peña A, Bravo J, Melgosa M, Fernandez C, Meseguer C, Espinosa L, Alonso A, Picazo ML, Navarro M (2007) Steroid-resistant nephrotic syndrome: long-term evolution after sequential therapy. Pediatr Nephrol 22:1875–1888
Montané B, Abitbol C, Chandar J, Strauss J, Zilleruelo G (2003) Novel therapy of focal glomerulosclerosis with mycophenolate and angiotensin blockade. Pediatr Nephrol 18:772–777
International Study of Kidney Disease in Children (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 98:561–564
Srivastava T, Simon SD, Alon US (1999) High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol 13:13–18
McBryde KD, Kershaw DB, Smoyer WE (2001) Pediatric steroid-resistant nephrotic syndrome. Curr Probl Pediatr Adolesc Health Care 31:280–307
Feng SP, Huang JP, Xu M, Li S, Luo W, Lai XJ (2007) Clinical presentations and pathologic features of 25 cases of infant nephrotic syndrome. Zhongguo Dang Dai Er Ke Za Zhi 9:151–152
Yu L, Wen ZY, Hua SD, Zhang YX, Huang YH, Meiying Z, Xiaoshu Y (2002) Analysis of clinical characteristics in infantile nephrotic syndrome. Chinese Journal of Practical Pediatrics 17:553–555
Wenyan H, Mingchang H, Xingyou J, Yi C (1995) Nephrotic syndrome in infants below 3-year-old. Acta Universitatis Medicinalis Nanjing 15:326–327
Fangyun S, Xingyu H, Yingqing G, Moyi G, Xiurong Z (1994) Long term follow-up of nephrotic syndrome in children under 2 years. Chin J Pediatr 32:276–278
Wang JG, Zhou ZL, Pan T, Li CC, Yang Q (2002) Integrated traditional Chinese and Western medicine for infantile nephrotic syndrome-150 cases report. Shanghai Journal of Traditional Chinese Medicine 2:27–29
Tune BM, Kirpekar R, Sibley RK, Reznik VM, Griswold WR, Mendoza SA (1995) Intravenous methylprednisolone and oral alkylating agent therapy of prednisone-resistant pediatric focal segmental glomerulosclerosis: a long-term follow-up. Clin Nephrol 43:84–88
Griswold WR, Tune BM, Reznik VM, Vazquez M, Prime DJ, Brock P, Mendoza SA (1987) Treatment of childhood prednisone-resistant nephrotic syndrome and focal segmental glomerulosclerosis with intravenous methylprednisolone and oral alkylating agents. Nephron 46:73–77
Goonasekera CD, Koziell AB, Hulton SA, Dillon MJ (1998) Vincristine and focal segmental sclerosis: do we need a multicentre trial? Pediatr Nephrol 12:284–289
Almeida MP, Almeida HA, Rosa FC (1994) Vincristine in steroid-resistant nephrotic syndrome. Pediatr Nephrol 8:79–80
Gregory MJ, Smoyer WE, Sedman A, Kershaw DB, Valentini RP, Johnson K, Bunchman TE (1996) Long-term cyclosporine therapy for pediatric nephrotic syndrome: a clinical and histologic analysis. J Am Soc Nephrol 7:543–549
Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group (2001) Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int 59:1484–1490
Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL (1999) A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int 56:2220–2226
Zaffanello M, Brugnara M, Franchini M (2007) Therapy for children with henoch-schonlein purpura nephritis: a systematic review. ScientificWorldJournal 7:20–30
Algoet C, Proesmans W (2003) Renal biopsy 2–9 years after Henoch Schönlein purpura. Pediatr Nephrol 18:471–473
Liu HM, Xu H, Zhou LJ, Zhang J (2007) Efficacy of mycophenolate mofetil and methylprednisolone alternate-day therapy in childhood lupus nephritis. Chinese Journal of Practical Pediatrics 22:137–138
Appel AS, Appel GB (2009) An update on the use of mycophenolate mofetil in lupus nephritis and other primary glomerular diseases. Nat Clin Pract Nephrol 5:132–142
Xu G, Tu W, Jiang D, Xu C (2009) Mycophenolate mofetil treatment for IgA nephropathy: a meta-analysis. Am J Nephrol 29:362–367
Acknowledgement
This work was supported by the State of Health Science Foundation of Hunan province (B2005149), State of Science and Technology Science Foundation of Hunan province (06SK3198), State Administration of Traditional Chinese Medicine of Hunan province (6301) in China. The authors thank all colleagues who took medical records, managed patients, did renal biopsies, and assisted with follow-up.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Duan, C., He, J. et al. Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 25, 883–888 (2010). https://doi.org/10.1007/s00467-009-1375-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1375-7