Abstract
Most patients with minimal change nephrotic syndrome are steroid responsive and tolerate this medication. However, a substantial number of patients relapse frequently and become steroid dependent. These patients often require treatment with alternative immunosuppressive drugs to maintain remission and minimize steroid toxicity. Previous studies have suggested that mycophenolate mofetil is effective in treating these patients. However, there are limited data on the effectiveness of this agent in pediatric patients, specifically those with steroid-dependent nephrotic syndrome. The purpose of this study was to assess the efficacy and safety of mycophenolate mofetil therapy in children and adolescents with steroid-dependent nephrotic syndrome who failed other treatments. A retrospective chart review was performed on all patients with steroid-dependent nephrotic syndrome. Clinical characteristics, laboratory data and the relapse rate were assessed prior to and during mycophenolate mofetil treatment. Twenty-one patients, ages 2–17 years, with steroid-dependent nephrotic syndrome who were treated with mycophenolate mofetil between 2001–2005 were included in this review. The indication for mycophenolate mofetil use was steroid dependence in 17 and steroid toxicity in 4 patients. The mean duration of treatment was 1.0±0.5 years (range: 0.2–2.0 years). Patients treated with mycophenolate mofetil had a reduction in relapse rate from 0.80±0.41 to 0.47±0.43 relapses per month ( P <0.02). Side effects were mild and mostly gastrointestinal in nature. In 1 child, mycophenolate mofetil was discontinued due to varicella infection and not restarted. The findings indicate that mycophenolate mofetil is a useful adjunctive therapy in the treatment of patients with steroid-dependent nephrotic syndrome. It lowers the relapse rate by 40% and is well tolerated by patients with steroid-dependent nephrotic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most children who develop idiopathic nephrotic syndrome respond to steroids and are presumed to have minimal change nephrotic syndrome (MCNS) [1]. They can usually be managed effectively with steroids alone; however, up to a third of these patients develops frequent relapses and become steroid dependent [2]. Treatment of these patients is a challenge, because they are at risk for developing complications from prolonged exposure to steroids. Alternative immunosuppressive agents have been tried, including intravenous methylprednisolone, oral and parenteral cyclophosphamide, cyclosporine, tacrolimus and levamisole [3].
Mycophenolate mofetil (MMF) is a selective reversible inhibitor of inosine monophosphate dehydrogenase that inhibits de novo synthesis of purines [4]. Lymphocytes differ from other cells and are unable to use salvage pathways to synthesize purines. Therefore, MMF exerts cytostatic effect specifically on lymphocytes. MMF has an established role as an immunosuppressive agent in solid organ transplantation [5]. Preliminary studies suggest that it has a beneficial effect as adjunctive therapy for nephrotic syndrome [6, 7]. However, there are limited data on the effectiveness of MMF in pediatric patients with steroid-dependent or steroid-resistant nephrotic syndrome. The purpose of this report is to evaluate the efficacy of MMF as a steroid-sparing agent in patients with steroid-dependent nephrotic syndrome (SDNS) who have failed prior immunosuppressive treatments.
Patients and methods
Patients
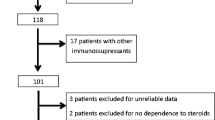
A retrospective chart review was performed on all patients with SDNS who were treated with MMF in the period from 1 June 2001 to 1 March 2005. Nephrotic syndrome was considered steroid dependent if relapse occurred while on alternate day prednisone or within 2 weeks of discontinuation of steroid therapy. Relapses were treated with daily steroids (60 mg/m2/day in divided doses) until the early morning urine specimen was negative or trace by dipstick testing for 3 consecutive days followed by alternate day steroid (40 mg/m2 as a single dose) for 4 weeks. Thus, consistent with standard clinical practice in children with SDNS, the response to therapy was based solely on dipstick testing and measurements of urine protein:creatinine ratio were not performed routinely. Parents monitored their child’s proteinuria daily and recorded the results on charts provided to all patients. The review included patients who had been treated with prednisone alone and patients who were still steroid dependent or steroid toxic despite prior second-line immunosuppressive therapy, such as cyclophosphamide, cyclosporine, and levamisole. Because all of the patients were steroid responsive, none of them underwent a kidney biopsy. This retrospective chart review was approved by the IRB and was considered exempt from continuing review.
Chart review
The following clinical data were tabulated: age at onset of disease, ethnicity, gender, history of prior treatment and age at initiation of MMF therapy. The following laboratory data were recorded at the start and completion of MMF treatment: BUN, creatinine, calculated GFR using the age appropriate length-serum creatine equation, AST, ALT, alkaline phosphatase, total protein, albumin, cholesterol, white blood cell count, absolute neutrophil count, hemoglobin, hematocrit and platelet count. The relapse rate, expressed as the number of relapses per month, was calculated for a period of 12 months prior to initiation of MMF therapy and compared to the relapse rate during MMF therapy. If the onset of disease was less than 12 months prior to the initiation of MMF therapy, then the relapse rate was calculated for the entire period preceding introduction of MMF.
MMF therapy
The indications for the use of MMF were SDNS or steroid toxicity. This included significant behavioral problems in a young child treated for less than 12 months with steroid therapy. MMF was provided orally as a suspension (200 mg/ml) or a capsule (250 mg/capsule). The target dose was 600 mg/m2 per dose, to a maximum of 1 g twice a day. The medication was started at the desired dose and patients were evaluated 2 and 4 weeks after initiation of MMF. They were seen thereafter according to clinical needs.
Statistical analysis
Data are reported as mean ± SD. Inter-group differences were assessed using a t -test and considered significant if P <0.05.
Results
Patients
Twenty-one children, 18 males and 3 females, whose mean age at the onset of the nephrotic syndrome was 3.9±3.1 years, were included in this review. There were 11 white, 2 black and 8 patients of other ethnicities. None of the patients had a diagnostic renal biopsy, because the procedure is not indicated in patients with nephrotic syndrome that responds to steroids [8]. Six patients had been previously treated with cyclophosphamide, 6 with cyclosporine and 6 with levamisole. No patient had received intravenous methylprednisolone or intravenous cyclophosphamide or oral tacrolimus prior to MMF treatment. The indication for MMF was steroid dependence in 17 patients and steroid toxicity in 4. They were started on MMF treatment at a mean age of 8.2±4.4 years. Individual clinical data are reported in Table 1. All patients had a normal calculated GFR at the start of MMF therapy. In addition, except for 1 child who required enalapril therapy, the remaining patients were normotensive and were not given antihypertensive medications. Finally, 4 patients were receiving furosemide to control edema.
MMF therapy
The therapeutic dose given was 600 mg/m2 per dose (maximum 1 g) BID in all patients. The mean duration of MMF treatment was 1.0±0.5 years (range: 0.2–2.0 years) and the total length of follow-up after initiation of the new therapy was 1.9±1.0 years. During the period of MMF treatment, 20 patients received intermittent prednisone therapy for relapses. No other immunosuppressive drugs were administered in conjunction with MMF, except for 1 child who received cyclosporine instead of prednisone because of severe steroid toxicity.
Response to MMF therapy
The overall relapse rate decreased from 0.80±0.41 to 0.47±0.43 relapses per month ( P <0.02). The duration of remission after starting MMF until the first relapse was 3.8±4.2 months. One child was relapse free for a year on MMF therapy. A second child was relapse free for over a year on MMF and remains off all medication for 9 months. A third patient was relapse free during a 6-month course of MMF, remained in remission for 6 months after stopping the drug, and then resumed a steroid-responsive relapsing course. Twelve patients showed a greater than 50% decrease in relapse rate, 4 had a less than 50% decrease, in 1, relapse rate remained unchanged and 4 had an increase in relapse rate while on MMF therapy. Other than a trend towards a better response in female patients, there were no clinical or laboratory features that predicted the degree of response to MMF. In particular, there were no distinguishing characteristics in the 4 children with a worsening course compared to the remaining group of patients. Overall, 76% (16/21) of the patients achieved a therapeutic benefit from MMF therapy.
While the patients were receiving MMF, there were no significant changes in the serum total protein (5.5±0.8 pre- versus 5.7±1.0 g/dl post-MMF), albumin (2.9±0.7 pre- versus 3.2±1.0 g/dl post-MMF) or cholesterol (283±118 pre- versus 279±119 mg/dl post-MMF) concentrations.
The decision to stop MMF and switch to alternative immunosuppressive medications was made based on parental assessment of the efficacy of the new therapy. Thus, at the last follow-up evaluation, 2 patients were in sustained remission off all medications (both achieved remission on MMF alone). Seven were still on MMF (3 were receiving concomitant prednisone, 1 cyclosporine and 3 received no other medications). Of the remaining patients, 3 were being treated with tacrolimus (1 with concomitant prednisone and 2 with no other drugs), 5 were being treated with cyclosporine (3 with concomitant prednisone and 2 with no other drugs), and 4 with prednisone alone. It was not possible to calculate precise steroid dosage while patients were taking MMF or after the drug was discontinued. However, a similar proportion of children received intermittent prednisone therapy while they were on MMF, 3/7, or off the drug, 8/14. Finally, in the group of children in whom MMF was discontinued, the relapse rate over a follow-up period of 1.2±0.5 years was 0.24±0.17, indicating that there was no rebound in disease activity after MMF was stopped.
Safety and tolerability
Side effects were generally mild, and most patients tolerated MMF well. Mild gastrointestinal complaints including stomach upset and diarrhea were reported during the 1st week of MMF therapy in most patients. However, these findings did not necessitate MMF dose modification or discontinuation. One patient developed varicella zoster infection, and MMF was discontinued and not restarted at the request of the parents. One patient had diarrhea, ringworm and thrush, but continued on MMF therapy. In one child, the dose was reduced and discontinued for 1 week because of diarrhea. When gastrointestinal complaints resolved, MMF was resumed at the original dose without further complications.
Discussion
Mycophenolic acid is the pharmacologically active moiety derived from the hydrolysis of the prodrug MMF. Mycophenolic acid is a potent, selective, uncompetitive and reversible inhibitor of inosine monophosphate dehydrogenase and the de novo pathway of guanosine nucleotide synthesis. Because T- and B-lymphocytes depend on de novo synthesis of purines for cell proliferation compared to other cell types that can utilize salvage pathways, mycophenolic acid has selective cytostatic effects on lymphocytes. MPA also suppresses antibody formation by B-lymphocytes. MPA also prevents the glycosylation of lymphocyte and monocyte glycoproteins that are involved in intercellular adhesion to endothelial cells and may inhibit the recruitment of leukocytes into sites of renal inflammation. Finally, it inhibits glycosylation of adhesion molecules and interferes with the binding of activated lymphocytes to activated endothelial cells. There is literature supporting a role for activated monocytes and lymphocytes in the pathogenesis of inflammatory and non-inflammatory glomerular disease.
Recent preliminary studies have suggested that there is a beneficial effect of MMF as adjunctive therapy for childhood nephrotic syndrome [6, 7]. The first study included 14 pediatric patients, 3 with MCNS, 9 with mesangial IgM deposition (IgM nephropathy) and 2 with focal segmental glomerulosclerosis, who were resistant to or dependent on steroids and/or cyclosporine. In the 10 cyclosporine dependent patients, MMF therapy significantly lowered the relapse rate. The beneficial effect on the relapse rate in the 4 children with SDNS failed to reach statistical significance. Bagga and colleagues studied 19 pediatric patients—10 with biopsy proven MCNS and 3 with FSGS. Six children did not have a biopsy-proven diagnosis. The relapse rate decreased more than three fold (6.6 to 2/year) and 14 out of the 19 patients showed a greater than 50% decrease in relapse rate. In contrast to these studies, our report includes a homogeneous cohort of patients with SDNS. Although we observed a significant improvement in the relapse rate, a randomized clinical trial is needed to assess the efficacy of MMF therapy definitely in pediatric patients with SDNS. Interestingly, there was a small number of patients who responded extremely well to MMF, while others showed a moderate decrease in relapse rate. A larger study may elucidate criteria that distinguish responders from non-responders. It is important to emphasize that the decision to discontinue MMF and restart other drugs in 10 patients was based on parental request to stop the drug. Without a controlled clinical trial to guide the use of MMF or data to indicate the safety of long-term administration of MMF, we considered this a reasonable approach. This may account for the nearly 50% failure rate in this study compared to 16% in the report of Bagga et al. [7].
Two drugs commonly used as adjunctive therapy in SDNS are cyclosporine and cyclophosphamide. Both have significant disadvantages with prolonged use. Cyclosporine requires frequent monitoring of drug levels to avoid nephrotoxicity, and cyclophosphamide causes myelosuppression, necessitating dose monitoring for neutropenia. In addition, cyclophosphamide can cause bladder and gonadal injury. Our experience with MMF indicates that it is generally well tolerated. The most common side effect, gastrointestinal discomfort, was usually self limited. The mild side effect profile, the potential for a virtual “cure” in some patients, and the therapeutic benefit in 75% of patients, argue in favor of using MMF as the first line adjunctive therapy for SDNS as a steroid-sparing agent. However, randomized clinical trials are needed to define clinical guidelines for MMF use in children with SDNS, to define optimal dose and duration of therapy, and to confirm the safety and tolerance with more extended courses of treatment.
Conclusion
These uncontrolled data are in agreement with previous reports and indicate that MMF is a useful adjunctive drug for the treatment of pediatric patients with SDNS. Even though complete remission is uncommon and some patients have no beneficial response, the overall relapse rate was reduced by 40%. The low side effect profile argues in favor of earlier use of MMF to decrease steroid-related toxicity in pediatric patients with SDNS, while reserving more toxic drugs if MMF is ineffective.
References
International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13:159–165
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776
Schwarz A (2001) New aspects of the treatment of nephrotic syndrome. J Am Soc Nephrol 12 [Suppl 17]:S44–47
Allison A, Eugui E (1993) Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 125:5–28
Halloran P (1997) Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. Transplantation 63:39–47
Barletta GM, Smoyer WE, Bunchman TE, Flynn JT, Kershaw DB (2003) Use of mycophenolate mofetil in steroid dependent and resistant nephritic syndrome. Pediatr Nephrol 18:833–837
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Stadermann MB, Lilien MR, van de Kar NCAJ, Monnens LAH, Schroder CH (2003) Is biopsy required prior to cyclophosphamide in steroid-sensitive nephrotic syndrome? Clin Nephrol 690:315–317
Acknowledgements
This work was presented in part at the annual meeting of the Society of Pediatric Research, May 2004, San Francisco, CA, and published in abstract form ( Pediatr Res 2004; 55;570A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novak, I., Frank, R., Vento, S. et al. Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 20, 1265–1268 (2005). https://doi.org/10.1007/s00467-005-1957-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1957-y