Abstract
The management of patients with steroid-dependent nephrotic syndrome (SDNS) refractory to treatment with long-term steroids, levamisole and cyclophosphamide is difficult. We report our experience on long-term treatment with mycophenolate mofetil (MMF) and alternate-day prednisolone in 42 patients with SDNS previously treated with levamisole (n = 35) and/or cyclophosphamide (n = 37). The mean age (range) at onset of nephrotic syndrome was 37 (13–92) months and at treatment with MMF 104.7 (32–187) months. MMF was administered at a mean daily dose of 26.5 (16.6–31.3) mg/kg for 14.3 (6–45) months. The mean 6-monthly relapse rates decreased from 3.0 episodes before therapy to 0.9 episodes in the first 6 months, 0.7 in next 6 months, and 0.3 in those treated longer than 12 months (P < 0.0001). While on therapy, 32 (76.2%) patients showed 50% or more reduction in relapse rates, and nine (21.4%) had sustained remission. The cumulative dose of prednisolone declined significantly from 0.6 mg/kg per day before to 0.3 mg/kg per day while receiving MMF. Prednisolone requirement was reduced by 50% or more in 16 patients and between 40% and 50% in eight patients. Treatment continuation beyond 12 months resulted in sustained steroid sparing and reduced need for alternative treatments while maintaining low relapse rates. No patients had diarrhea, hematological abnormalities, or impaired renal function. This data confirms the efficacy and safety of treatment with MMF and tapering doses of alternate-day prednisolone in patients with SDNS and supports its use for longer than 12 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycophenolic acid (MPA) is a highly selective, noncompetitive inhibitor of inosine monophosphate dehydrogenase, the rate-limiting enzyme in de novo biosynthesis of guanosine nucleotides. Mycophenolate mofetil (MMF, the prodrug of MPA) that strongly inhibits both T- and B-lymphocyte proliferation, has been used for prevention and treatment of renal allograft rejection [1] and for glomerulonephritis due to systemic lupus erythematosus [2], vasculitis [3], immunoglobulin-A (IgA) nephropathy [4], and membranous nephropathy [5]. Based on evidence that perturbations of lymphocyte number and function are crucial in the pathogenesis of nephrotic syndrome (NS) [6], a number of medications, including corticosteroids, levamisole, calcineurin inhibitors, and recently, rituximab, have been used for its management. Similarly, agents that inhibit lymphocyte proliferation by inhibiting nucleic acid synthesis or causing DNA alkylation (azathioprine, mizoribine, cyclophosphamide) have been used to treat patients with steroid-sensitive NS [6]. MMF has been found effective in reducing relapse rates and steroid requirement in patients with frequently relapsing and steroid-dependent nephrotic syndrome (SDNS) [7–10]. We previously reported the efficacy of 12 months of therapy with MMF in patients with SDNS but showed that cessation of treatment resulted in increased relapse rates [11].
Here we report our extended experience on the effectiveness of MMF and alternate-day prednisolone in patients with SDNS. We also examined whether continued treatment with MMF beyond 12 months sustained the reduced rates of relapses and ensured steroid sparing, without the risk of adverse effects.
Subjects and methods
Study population
We reviewed the clinical records of all children with SDNS who had received at least 6 months of treatment with MMF at the All India Institute of Medical Sciences, New Delhi, India, between September 1999 and June 2006. This included patients who were prospectively treated with MMF and alternate-day prednisolone between September 1999 and April 2001 [11]. Approval for use of MMF in patients with NS was obtained from the Drug Controller General of India (Government of India) and the Institute Ethics Committee. Informed written consent was obtained from either of the parents before inclusion into the study.
Forty-seven patients were identified as having received this medication for SDNS, including 19 who were studied prospectively. Standard definitions and treatment guidelines were used for the study [12, 13]. Briefly, NS was defined by nephrotic-range proteinuria (3+ or more by dipstick test), hypoalbuminemia (serum albumin < 2.5 g/dl), and edema. Remission was defined as trace or nil proteinuria for 3 consecutive days. The recurrence of nephrotic-range proteinuria for 3 or more days constituted a relapse, which was treated with prednisolone at a daily dose of 2 mg/kg until remission, followed by 1.5 mg/kg on alternate days for 4 weeks [12]. Patients with two consecutive relapses of NS while receiving prednisolone on alternate days or within 15 days of its discontinuation were defined as SDNS. The initial therapy of SDNS at this center comprises prolonged (9–12 months) administration of alternate-day prednisolone; its dose is tapered by 0.25 mg/kg every 4 weeks until 0.5 mg/kg [12]. Patients having two or more relapses despite prednisolone dosage of more than 0.5 mg/kg on alternate days and/or evidence of steroid toxicity (cushingoid features, weight >120% of that expected for age, stage II hypertension, or subcapsular cataract on slit-lamp examination) are treated using alternative medications, including levamisole or cyclophosphamide [12]. Patients who continued to show SDNS despite 6 months of therapy with levamisole [14] and/or 12 weeks of treatment with cyclophosphamide were considered for treatment with MMF at least 3 months after discontinuation of these medications. Patients who had received treatment at any time with calcineurin inhibitors (cyclosporine, tacrolimus) or other immunosuppressive agents, apart from levamisole and cyclophosphamide, were excluded.
Therapy with MMF was started at the time of relapse at a dose of 20–25 mg/kg per day in two divided doses. Prednisolone was administered at a dose of 2 mg/kg per day until remission, followed by 1.5 mg/kg on alternate days for 4 weeks, with subsequent tapering to 0.3–0.5 mg/kg on alternate days. Antacids or ranitidine were administered only to patients with gastrointestinal symptoms. Relapses during this period were treated with daily prednisolone in the aforementioned regimen, followed by tapering. Therapy with MMF was continued during relapses. Patients with hypertension were treated with enalapril at an initial daily dose of 0.2–0.3 mg/kg, which was increased if required to 0.4–0.5 mg/kg in two divided doses. None of the patients received therapy with statins during the study, and all received calcium supplementation at 250–500 mg daily.
Clinical features, renal histology, and details of previous treatment were noted. Height and weight standard deviation scores (SDS) [15] and body mass index (BMI) were calculated at inauguration of treatment with MMF and at last follow-up. Complete blood counts and biochemistry (blood levels of creatinine, albumin, and cholesterol) were repeated at 3- and 6-month intervals, respectively. Urine albumin was monitored and recorded in a diary at home by parents using dipsticks.
Adverse effects of treatment with corticosteroids (cushingoid features, hypertension, cataract, striae, behavioral problems) and MMF (abdominal pain, diarrhea, leukopenia, thrombocytopenia) were enquired for and recorded at monthly visits. Details of all minor infections (diarrhea, upper respiratory tract infections, impetigo), and systemic infections requiring hospitalization (lower respiratory tract infections, peritonitis, cellulitis, osteomyelitis, and meningitis) were recorded in the patient diary and case-report forms. From 1999 to 2001, annual safety reports were sent to the Institute Ethics Committee and the Drug Controller General of India.
Outcomes
The primary outcome was change in 6-monthly relapse rates before and during MMF therapy. Relapses were recorded in the preceding 6 months and through each of the 6 months of MMF treatment unless otherwise specified. Secondary outcome variables were the comparison of cumulative dose of prednisolone (milligrams per kilogram per day) before and during MMF therapy; change in height and weight SDS and BMI; blood levels of creatinine, albumin, and cholesterol; and adverse effects of medications. Relapse rates, prednisolone requirement, and adverse effects of therapy were compared in patients in whom treatment was electively discontinued at 12 months (1999–2001, excluding treatment failures) to those who received longer duration therapy. Patients were categorized as being in sustained remission, infrequent relapse (one relapse in first 6 months, or three or less relapses in 12 months), or treatment failure (two or more relapses in first 6 months or more than 3 relapses in 12 months).
Statistical analysis
Continuous data are represented as mean [95% confidence interval (CI)]. Paired data were compared using one-way analysis of variance (ANOVA) and ordinate variables using the chi-square test; P < 0.05 was considered significant. Results were analyzed using SPSS for Windows version 10 software.
Results
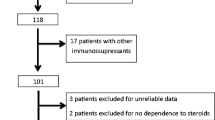
Of 47 patients with SDNS treated with MMF, therapy was discontinued in one at 7 weeks due to persistent abdominal pain. Four patients were excluded, as follow-up was not available after the first 2 months (n = 2) or the duration of MMF therapy was less than 6 months (n = 2), leaving 42 eligible patients (27 boys) whose data was analyzed. Mean age at onset of NS was 37 months (95% CI 29.9, 44.1; range 13–92) and at treatment with MMF 104.7 months (92.1, 117.4; range 32–187). Renal histology in 19 patients showed minimal-change disease in 15 and focal segmental glomerulosclerosis in four. All patients had received treatment with long-term alternate-day prednisolone with unsatisfactory response; additional therapy in 39 patients included levamisole (n = 2), cyclophosphamide (n = 4), and a combination thereof (n = 33). Mean duration of levamisole treatment was 18.0 (13.4, 22.6; range 2.8–52.5) months, and the number of relapses on levamisole were 4.3 (3.2, 5.3; range 1.1 to 11.1) episodes/year. Mean remission duration following 12 weeks of therapy with cyclophosphamide was 6.1 (2.7, 9.5; range 0–59) months. Mean dose of MMF administered was 26.5 (95% CI 25.4, 27.6; range 16.6–31.3) mg/kg per day for 14.3 (12.0, 16.6; range 6–45) months. The number of patients receiving MMF and tapering doses of alternate-day prednisolone at 6, 12, 18, and more than 24 months was 42, 36, 8, and 4, respectively.
Relapse rates
Mean 6-monthly relapse rates decreased significantly from 3.0 episodes before therapy to 0.9 episodes in the first 6 months, 0.7 in next 6 months, and 0.3 in those receiving treatment beyond 12 months (P < 0.0001) (Table 1).
Table 2 shows the clinical and biochemical characteristics before and during MMF therapy. Mean reduction in relapse rates was 3.8 (2.8, 4.6) episodes/year, a reduction of 62.0% (49.1%, 75.0%). Thirty-two (76.2%) patients showed 50% or more reduction in relapse rates. Nine (21.4%) had sustained remission while on MMF, whereas 28 (66.7%) were infrequent relapsers. There were five (11.9%) treatment failures: two patients had two relapses in the first 6 months and three had four to five relapses in 12 months. MMF was discontinued in these patients, and they received cyclosporin (n = 3) or a second course of cyclophosphamide (n = 2) with satisfactory results.
Steroid dose and biochemical features
The cumulative dose of prednisolone declined significantly from 0.6 (0.5, 0.7; range 0.1–1.3) mg/kg per day before to 0.3 (0.3, 0.4; range 0.1–1.2) mg/kg per day during MMF treatment (Table 2), a mean reduction of 40.4%. Prednisolone requirement was reduced by 50% or more in 16 patients and between 40% and 50% in eight patients. Steroid treatment was discontinued in five patients. Despite reduction in prednisolone dosage, no significant changes were found in weight and height SDS and BMI (Table 2). Although plasma albumin levels increased and cholesterol declined, the changes were not significant.
Twelve months vs longer treatment with MMF
Table 3 compares baseline characteristics, relapse rates, and prednisolone dosage in 16 patients who received, as per protocol, 12 months of MMF therapy vs 13 patients in whom treatment continued beyond 12 months. Initial characteristics including age at onset and at MMF therapy, prior treatment, baseline relapse rates, and steroid requirement were similar.
Whereas mean relapse rates declined on treatment, they did not differ significantly in the groups at the end of 12 months. Elective cessation of therapy at 12 months resulted in significant increase in relapse rate from 0.7 to 2 episodes/6 months in the post-MMF phase. The benefits of MMF therapy were sustained in patients who continued therapy beyond 12 months, who showed a mean relapse rate of 0.3 episodes/6 months in this period. Similarly, whereas prednisolone requirement before and during 12-month therapy with MMF was similar in the two groups, this increased to 0.4 mg/kg per day when MMF was stopped at 12 months, compared with 0.1 mg/kg per day if continued (P 0.002). Alternative medications required due to frequent relapses following cessation of MMF included cyclophosphamide in six, levamisole and azathioprine in three each, calcineurin inhibitors in four, and a repeat course of MMF in two.
Adverse effects
All patients had cushingoid features; other features included abdominal-wall striae (9), hypertension and hirsutism (6 each), cataract (4), and open-angle glaucoma (1) prior to MMF treatment. At last follow-up, 16 patients had no features of steroid toxicity, 20 continued to show cushingoid features, and three each had cataract and hypertension. Nine patients had transient abdominal pain, which was relieved following treatment with ranitidine. One patient each had an episode of hepatitis A, peritonitis, cellulitis, and exacerbation of skin lesions of molluscum contagiosum. No patients had diarrhea, vomiting, hematological abnormalities, or impaired renal function.
Discussion
Management of patients with SDNS is challenging, and a number of medications have been used with variable results. The initial choice is long-term treatment with prednisone on alternate days for 9–18 months. Patients having multiple relapses, steroid toxicity, or high steroid threshold require therapy with other agents, including levamisole or alkylating agents, with benefit in about 30–50% patients [6, 12]. Patients who continue to show steroid dependence are considered for long-term treatment with calcineurin inhibitors, either cyclosporin or tacrolimus. Long-term administration of calcineurin inhibitors might, however, be associated with cosmetic and neurological side effects, nephrotoxicity, and glucose intolerance.
Treatment with MMF has recently been used for maintaining remission in these patients, with satisfactory results [7–10]. Reports also indicate a potential for switching to MMF in patients with steroid-dependent or steroid-resistant NS receiving prolonged treatment with cyclosporin [10, 16, 17]. Whereas the mechanism of action of MMF is through inhibition of lymphocyte proliferation, there is evidence that it might also prevent nonimmune renal damage by inhibiting proliferation of smooth muscle, renal tubular, and mesangial cells and fibroblasts, reducing proteinuria and preventing fibrosis [18]. However, the precise indications and duration of treatment with MMF need to be defined.
This report, on 42 patients with difficult SDNS, is the largest single-center experience in patients treated with MMF and alternate-day prednisolone. Our observations confirm the efficacy of this regimen in 88.1% patients, with 62% reduction in relapse frequency. This experience extends and endorses our previous findings on similar treatment in children with SDNS [11]. Reduction of relapse rates was associated with almost 40% reduction in the cumulative administered dose of prednisolone, confirming the steroid-sparing effect of MMF [7, 16].
Table 4 summarizes the results from similar published studies in children with NS [7, 9, 10, 16, 17, 19, 20]. Treatment with MMF in children with frequent relapses and/or SDNS is reported to be successful in 20–80% cases. These case series have included patients with variable severity of steroid dependence, including those with previous or current cyclosporin therapy [7, 10, 16, 17]. Further, MMF dose has been different, ranging from a mean of 26.5 mg/kg per day (approximately 750 mg/m2) in our patients to 1,200 mg/m2 or more per day in other reports [7, 9, 16, 17, 20, 21]. It is interesting that despite including subjects with difficult SDNS, comparatively lower doses of MMF were effective in maintaining remission in our patients. The precise reason why lower doses of MMF were as effective is unclear but might be because most patients continued to be cotreated with alternate-day prednisolone. Secondly, the pharmacological effects of MMF are believed to be related to the concentration of free MPA, which is not bound to albumin [22]. It is possible that hypoalbuminemia in our patients might have resulted in higher levels of free MPA and better medication efficacy.
A multicentric, randomized controlled trial from the Netherlands, published recently as an abstract, compared the efficacy of MMF (1,200 mg/m2 per day) with cyclosporin (4–5 mg/kg per day) in 24 patients with frequently relapsing NS [21]. Whereas relapse rates were higher in those treated with MMF, 58.3% patients had sustained remission during 1 year of follow-up. Patients receiving MMF had a better side-effect profile with stable glomerular filtration rates and normal blood pressure compared with those treated with cyclosporin.
Prolonged use of MMF in patients with frequently relapsing or steroid-dependent NS is considered safe and associated with few adverse effects (Table 4). Side effects in this study were limited to mild abdominal pain that resolved spontaneously. The absence of diarrhea, serious infections, and hematological adverse effects in our study might be attributed to a lower but therapeutically effective dosage of the medication.
MMF treatment duration in previous reports is variable, ranging between 2.4 and 42 months (Table 4). Our findings suggest that prolongation of MMF beyond 12 months is effective in sustaining reduced relapse rates and is steroid sparing, without the risk of additional side effects (Table 3). Whereas the precise treatment duration with MMF is not clear, we propose that extending treatment beyond 12 months should be considered, particularly in patients showing satisfactory response.
The study population in this report was relatively homogenous, comprising patients with severe SDNS who had done poorly despite treatment with levamisole and oral cyclophosphamide. All patients were followed up at a single center with more or less standard practices of management [12]. Therefore, despite being a retrospective report, comparisons between the two groups enrolled at different time intervals (1999–2001 and 2001–2006) was possible, and we believe our conclusions are valid.
An important limitation of this study was the absence of a control group, with patients serving as their own controls. Though the decrease in relapse rates and steroid use during treatment with MMF and alternate-day prednisolone might be attributed to a spontaneous decrease in illness severity, this is unlikely. Most patients had prolonged duration of SDNS, which was previously difficult to manage. Elective discontinuation of MMF, at 1-year in some patients, led to reversal of the benefits and need for alternative therapy, suggesting that MMF was responsible for at least some of the observed effects. Secondly, the study does not address whether the observed reduction in steroid use was due to reduced frequency of relapses (thereby less need for daily treatment) or if treatment with MMF indeed resulted in reduction of maintenance steroid needs (steroid sparing). Thirdly, our patients were highly selected and extremely compliant with therapy. It is not certain whether findings from this study can be generalized to unselected children with SDNS who are likely to be less regular with their medication. Finally, as many patients are still receiving the medication, it is unclear whether the risk of steroid and MMF dependence is diminished with prolonged treatment. Whereas recent evidence suggests that MMF therapy should be monitored pharmacokinetically [10, 16, 21], this was not done in our study.
Data from this and recent studies suggest that MMF offers an effective and safe mode of treatment in patients with SDNS. The lack of renal, hemodynamic, and metabolic toxicity with this agent is an important consideration in its preference over calcineurin inhibitors. Whereas preliminary results from a randomized controlled trial that compared the efficacy of MMF with cyclosporin [21] are promising, a study with adequate power is necessary to clarify the utility of the agent in patients with nephrotic syndrome. Optimum treatment duration and importance of dose adjustment based on pharmacokinetic studies also needs to be examined.
References
Thorban S, Schwarznau A, Huser N, Stangl M (2006) Efficacy of conventional immunosuppressive therapy in related and unrelated living renal transplantation. Clin Transplant 20:284–288
Pisoni CN, Karim Y, Cuadrado MJ (2005) Mycophenolate mofetil and systemic lupus erythematosus: an overview. Lupus 14 (Suppl 1):S9–S11
D’Cruz DP (2005) Mycophenolate mofetil in systemic vasculitis. Lupus 14 (Suppl 1):S55–S57
Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G (2005) Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double blind randomized controlled trial. Nephrol Dial Transplant 20:2139–2145
Cattran DC (2003) Mycophenolate mofetil and cyclosporine therapy in membranous nephropathy. Semin Nephrol 23:272–277
Bagga A, Mantan M (2005) Nephrotic syndrome in children. Indian J Med Res 122:13–28
Mendizabal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J (2005) Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20:914–919
Moudgil A, Bagga A, Jordan SC (2005) Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions. Pediatr Nephrol 20:1376–1381
Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R (2006) Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: A report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1:1173–1178
Gellermann J, Querfeld U (2004) Frequently relapsing nephrotic syndrome: treatment with mycophenolate mofetil. Pediatr Nephrol 19:101–104
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Indian Pediatric Nephrology Group, Indian Academy of Pediatrics (2001) Consensus statement on management of steroid sensitive nephrotic syndrome. Indian Pediatr 38:975–986
Bagga A, Hari P, Srivastava RN (1999) Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 13:824–827
Bagga A, Sharma A, Srivastava RN (1997) Levamisole therapy in corticosteroid-dependent nephrotic syndrome. Pediatr Nephrol 11:415–417
Centers for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts: United States http://www.cdc.gov/growthcharts/May 30, 2000
Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K (2007) A prospective study on the use of MMF in children with cyclosporine dependent nephrotic syndrome. Pediatr Nephrol 22:71–76
Barletta GM, Smoyer WE, Bunchman TE, Flynn JT, Kershaw DB (2003) Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome. Pediatr Nephrol 18:833–837
Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, Zeier M, Muranyi W (2006) Antifibrotic actions of mycophenolic acid. Clin Transplant 20 (Suppl 17):25–29
Al-Akash S, Al-Makdama A (2005) Mycophenolate mofetil in children with steroid-dependent and/or frequently relapsing nephrotic syndrome. Ann Saudi Med 25:380–384
Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H (2005) Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 20:1265–1268
Dorresteijn E, Kist-van Holthe J, Levtchenko E, Nauta J, Hop W, Van der Hejden A (2007) Randomized controlled trial of mycophenolate mofetil versus cyclosporine A in children with frequently relapsing nephrotic syndrome. Pediatr Nephrol 22:1442 (28 FC)
Jeong H, Kaplan B (2007) Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol 2:184–191
Acknowledgements
Kamran Afzal was supported by the International Pediatric Nephrology Association for short-term training at this center.
Collaboration between the All India Institute of Medical Sciences (New Delhi) and Cedars Sinai Medical Center (Los Angeles) was made possible through the Renal Sister Center Program Initiative of the International Society of Nephrology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, K., Bagga, A., Menon, S. et al. Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 22, 2059–2065 (2007). https://doi.org/10.1007/s00467-007-0617-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0617-9