Abstract
Measuring intima–media thickness (IMT) is now a standard diagnostic procedure in assessing cardiovascular risk and hypertensive target-organ damage (TOD) in adults. There is also an increasing number of pediatric publications evaluating IMT in children from high-risk groups, such as those with arterial hypertension, diabetes, chronic kidney disease, obesity, dyslipidemia, and homocystinurias. It has been shown that carotid IMT is strongly related with other markers of TOD in children with arterial hypertension and with metabolic cardiovascular risk factors. In children with coarctation of the aorta, carotid IMT correlated both with blood pressure and even with mild residual aortic gradient. On the other hand, studies in children with high cardiovascular risk have shown that normalization of blood pressure and metabolic abnormalities led to regression of arterial changes and decrease of IMT. Although not yet accepted as standard pediatric procedure, IMT measurement is emerging as a promising method of assessing TOD and cardiovascular risk and monitoring treatment efficacy. From a practical point of view, clinical utility of IMT measurements seems to be similar to use of echocardiography in assessing left ventricular mass. However, IMT measurements in children and adolescents should be standardized to avoid bias caused by the use of different measurement methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is a leading cause of death in the general population [1]. Although with the exception of high-risk groups, such as children with chronic kidney disease (CKD), familial hyperlipidemias, Kawasaki disease, or homocystinurias, CVD is extremely rare in children, but there is no doubt that it already begins in childhood and may be programmed perinatally [2–4].
The pathological basis for CVD is arterial damage in the form of arteriosclerosis. Arteriosclerosis is a broader term that usually describes diffuse thickening and stiffening of mainly large- and medium-sized arteries and is observed in different conditions. The pathological changes include both the media and intima. Atherosclerosis is a form of arteriosclerosis and causes characteristic focal lesions in the intima of large and medium-sized arteries. Despite different underlying pathologies, the main risk factors of arteriosclerosis and atherosclerosis are similar [5, 6]. In adults, both arteriosclerosis and atherosclerosis occur together. The clinical significance of arteriosclerosis and atherosclerosis is related to progressive stiffening of arterial trunks, progressive narrowing at some particular sites, and risk of atherothrombosis. Although the main risk factors are common, these processes have distinct pathogeneses, and it can be said that arteriosclerosis leads to diffuse stiffening of arteries, whereas atherosclerosis leads to focal and patchy narrowing and/or atherothrombosis [7]. The third and, at least in part distinct, form of arteriosclerosis is Moenckeberg’s arteriosclerosis observed in CKD and diabetes and related not only to classic cardiovascular risk factors but also to metabolic abnormalities typical for CKD and diabetes [7, 8]. Prospective population-based studies have enabled identification of risk factors of arterio/atherosclerosis [9]. Among them, arterial hypertension (AH) is the most important modifiable factor.
There are two main theories on the pathogenesis of arterio/atherosclerosis: hemodynamic and metabolic. The hemodynamic theory claims that hemodynamic damage to the arterial wall is the first and most important event initiating arterio/atherosclerotic processes. According to the metabolic theory, the arterial wall is damaged by lipids, oxygen radicals, and mediators of inflammation. Because atherosclerosis develops particularly in sites exposed to the greatest hemodynamic insult, it seems that both hemodynamic and metabolic factors act together.
Until the 1950s, arterio/atherosclerosis was believed to develop only in adulthood. Atherosclerotic lesions were first described in coronary arteries of young men killed during the Korean War, which shed new light on the pathogenesis of CVD [10]. The first reports from large population-based studies from the US published in the subsequent decades identified risk factors of CVD and arterio/atherosclerosis [9, 11]. In general, they include three groups of factors damaging arterial walls: hemodynamic (blood pressure), metabolic (dyslipidemia, insulin resistance, homocysteine), and inflammatory. Soon, it was evident that there was link between all these main groups of risk factors [12].
Because a tendency toward elevated blood pressure develops very early, this risk factor already operates in childhood and correlates with early signs of arterio/atherosclerosis [13]. Introduction into clinical practice of noninvasive methods of arterial imaging caused an eruption of reports on early arterial damage already present in children and caused by elevated blood pressure [14–17]. These noninvasive methods can diagnose both structure and function of the arterial wall, and the results obtained with the use of any of these methods are strictly correlated. The general principle of noninvasive evaluation of arterial structure is based on ultrasound imaging of arterial walls using high-definition imaging (HDI) devices allowing measurement of intima–media thickness (IMT) and for functional studies of elastic properties of the arterial wall. Measurement of flow-mediated dilation (FMD) is also evaluated by ultrasonography. The third method of arterial evaluation is measurement of pulse-wave velocity (PWV) estimated from pulse-wave contour and speed of pulse-wave propagation [18–21]. All these methods are used in clinical practice, and guidelines for managing hypertensive adults recommend measuring both IMT and PWV to assess subclinical target organ damage (TOD) and predict risk of stroke and cardiac events [22]. Despite increasing amounts of pediatric data, IMT, FMD, and PWV measurements have not yet been recommended for routine use in evaluating hypertensive children [23]. It should be stressed that both FMD and PWV give insight into the functional properties of arteries but cannot determine the etiology of arterial disease. IMT is also a nonspecific marker of arterial injury, and only the presence of plaque is a direct sign of atherosclerosis. However, it has been shown in adults that carotid IMT >0.9 mm is a marker of significant cardiovascular risk caused by atherosclerosis.
Standards of intima–media thickness measurements
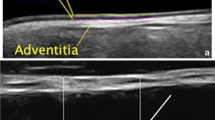
The standard sites of IMT measurement in adults are the common carotid artery (CCA), carotid bulb, and internal carotid artery (ICA). Usually, CCA-IMT is measured 1–2 cm below the bifurcation, which may be easily visualized in most children and adults. The superficial femoral artery is another site of IMT measurement, and with the exception of obese children, it is easy to visualize. In comparison with CCA, the media layer of the superficial femoral artery contains more smooth muscle cells. The standard site of measurement of IMT in the superficial femoral artery is the upper one third of the thigh. Although examination of the CCA gives information on intima–media thickening, more advanced stages of atherosclerosis in the form of plaque usually occur in the carotid bulb and/or ICA. These sites can be visualized from adolescence onward but only rarely in younger children.
The basic measurement includes evaluation of IMT in B-mode presentation. Although some authors measure IMT both on the near and far wall, most often, IMT is measured on the far wall of each CCA. The results are shown as the average value of IMT measurement from the left and right side. When IMT is measured in the ICA and/or bulb, the results are given separately for every segment of artery and/or are averaged.
There are two main methods of measuring IMT. The most frequently used is based on the manual cursor placement technique, with IMT measurement at several points (usually three to six) on each side, and then averaging. The second technique is also based on manual cursor placement, but the investigator draws a line on the upper border of the intima and a second line on the lower border of the media. In this method, IMT is calculated digitally by computer. Results from some analyses suggest that mean and not maximal or minimal IMT values should be used [24, 25].
In addition to IMT measurement, M-mode presentation of an artery allows measurement of internal diameters of an artery in systole and diastole. Knowing IMT enables calculation of other markers of wall structure, such as wall cross-sectional area (WCSA) and lumen cross-sectional area (LCSA) [26]. It is believed that WCSA also gives information on arterial wall remodeling [26]. In addition, the relationship between systolic and diastolic diameter also gives a crude estimate of elastic properties. More reliable and standardized blood pressure indices of functional properties of the arterial wall (distensibility, compliance, stiffness, and elastic modulus) can be calculated when blood pressure is known. From a methodological point of view, local blood pressure using aplanatory tonometry should be used [27]. However, brachial blood pressure is strictly correlated with carotid blood pressure, and in studies of carotid elasticity when blood pressure was measured on the brachial artery, the results were concordant [28, 29].
Recently, the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences issued the updated Mannheim Carotid Intima–Media Thickness Consensus describing standards of IMT measurements in adults [30]. The most important points of consensus are shown in Table 1.
IMT measurements as a marker of target-organ damage
The clinical significance of AH depends not on blood pressure value by itself but on the effects that blood pressure exerts on the cardiovascular system. Thus, clinically evident cardiovascular events are preceded by development of TOD assessed as left ventricular hypertrophy, retinal arteriopathy, microalbuminuria, and increased IMT. The physiological reaction of the cardiovascular system to hemodynamic insult is an adaptive increase in both arterial/ventricular lumen and wall thickness [26]. However, long-acting and/or sufficiently severe pressure overload causes pathological remodeling of the arterial wall, i.e. a disproportionate increase in arterial wall thickness. Both the media and intima participate in this adaptive response. On the level of small-resistance arterial vessels, this reactive response of the arterial wall may present as benign hyaline arteriolosclerosis and/or malignant, hyperplastic sclerosis. These changes usually occur in adults with long-standing hypertension (HT), as in benign arteriolosclerosis, as well as in hypertensive crisis, e.g. in malignant arteriolosclerosis. The consequence of arteriolosclerosis is decreased arteriolar lumens and reduced blood flow with diminished perfusion of vital organs, i.e. kidneys, and increased peripheral resistance. In contrast, although hemodynamic insult to the aorta and other large and medium-sized arteries also increases arterial wall thickness, it does not cause hemodynamically significant narrowing of the arterial lumen. Increased IMT of large arteries does, however, cause stiffening of the arterial wall and increases afterload, with its cardiac consequences. Another consequence of hemodynamic insult to large artery walls is promotion of atherosclerosis. Pathological remodeling of the arterial wall, presenting as an increase of CCA-IMT and WCSA, is preceded by generalized impaired endothelial function, which can be assessed by FMD and nitrite-mediated dilation (NMD) in brachial arteries [31, 32].
As mentioned above, IMT increase is in part a physiological reaction to a physiological rise in blood pressure and can already be observed in aortas of human fetuses [33]. The same biological phenomenon also occurs in venous vessels subjected to higher pressure conditions, as is observed in venous grafts in arterial trees [34]. Thus, in the general pediatric population, IMT also increases with age and correlates with the age-related rise of blood pressure. The most important predictive hemodynamic factor of IMT is the pulsatile characteristics of arterial flow, i.e. systolic and pulse pressure [35, 36]. An important finding is that IMT is related to blood pressure, even in the normal range. The extent of adaptive and pathological response to hemodynamic load is modulated by metabolic and genetic factors. Under physiological conditions, increased blood pressure increases the arterial lumen and causes an adaptive increase of IMT. This reaction is a general characteristic of the cardiovascular system and is also observed in athletes in whom physiological response to hemodynamic load increases both ventricular volume and left ventricular mass, with preserved functional capacity. Observations from studies in the adult population show that there is a steady increase in the diameter of CCA with age, and this phenomenon is followed by increased IMT [37, 38]. In this case, the increase in IMT is an adaptive response to the increased artery diameter, counteracting the increase in intraluminal pressure [26]. The morphological basis of IMT thickening is hypertrophy of smooth muscle cells and increase of extracellular matrix [39]. The second phase of response is pathological remodeling with inappropriate increase of IMT in relation to the increase of the arterial lumen. Because increased IMT is a compensatory reaction to increased artery diameter, IMT should be interpreted in relation to artery diameter, i.e. as WCSA [38]. Ultrastructural studies may show transverse tears in the intima caused by pressure damage [40], and it is believed that such hemodynamic priming allows lipids to invade the intima and to exert toxic effects. However, even normotensive persons exposed to significant metabolic disturbances, as in familial hyperlipidemia or homocystinuria, develop arterio/atherosclerosis.
Although IMT is an unspecific marker of arterial-wall injury, it is closely related to cardiovascular risk in adults. It was shown that CCA-IMT correlates with risk of stroke, coronary heart disease, and acute myocardial infarction [41–43]. Because risk factors for both arteriosclerosis and atherosclerosis are common, and increased IMT is strictly related to disturbed endothelial function and arterial stiffening assessed by decreased FMD and increased PWV, it is generally accepted that increased CCA-IMT is a nonspecific, surrogate marker of generalized atherosclerosis and surrogate marker of endothelial dysfunction.
IMT in healthy children
Although in many studies IMT was measured in healthy children, the largest study to date on normative IMT data in children and adolescents was published by Jourdan and ESCAPE Study investigators [35]. These authors evaluated CCA and the superficial femoral artery IMT in 250 healthy children and adolescents aged 10–19 years and calculated both mean IMT values and percentiles for girls and boys. Moreover, they gave values of standard deviations (SD), allowing calculation of SD scores. The results obtained by the ESCAPE Study Group investigators fit the normative values obtained in the Stanislas Study in young adults and in the study by Denarie et al. [44, 45]. In addition to IMT, Jourdan et al. provided data on normative values for WCSA, LCSA, and functional parameters of the carotid artery, including distensibility, compliance, and stiffness coefficient. The main result of the study was that IMT significantly increases during adolescence, is related to height and body mass index (BMI), systolic blood pressure (SBP) and pulse pressure (PP). An additional important and practical finding was that the subgroup of children who were smokers also had greater carotid IMT. The relation between IMT and anthropometric parameters and IMT and SBP and PP indicates that taller children who also had higher SBP should have a greater IMT than shorter children. This question has not, however, been resolved.
Although the relation between exposure to biochemical risk factors and IMT was already reported in adults with cardiovascular disease and children with inherited metabolic diseases causing premature arterio/atherosclerosis, the same relationships are already operating in the general pediatric population. It was shown that in healthy and normotensive children, carotid IMT correlates negatively with serum levels of high-density lipoprotein cholesterol (HDL-c) and apoprotein A1, and superficial femoral artery IMT correlates with homocysteine and negatively with apoprotein A1. The same was found for the carotid artery stiffness coefficient [16, 46]. In other studies of healthy children, a relationship between markers of inflammation, i.e. high-sensitivity C reactive protein (hs-CRP) and CCA-IMT, was found [47]. It was also found that acute upper respiratory tract infection increased CCA-IMT. Although CCA-IMT decreased after the acute period, it was still increased compared with controls, even 3 months after the acute episode, and this arterial wall reaction was related to oxidative stress expressed as increased generation of oxidized low-density-lipoprotein cholesterol (LDL-c) [48]. The second finding of the study was that children in whom a bacterial cause was suspected and who received antibiotics had a lower CCA-IMT than children who did not receive antibiotics.
In large longitudinal observations of the general pediatric population done in the Muscatine Study and Bogalusa Heart Study, it was found that exposure to traditional anthropometric, hemodynamic, and biochemical cardiovascular risk factors during childhood and adolescence is strictly related to greater values of carotid IMT and cardiovascular events in the fourth decade of life [49–54]. The important finding is that exposure to cardiovascular risk factors, even a in range regarded as normal, is related with vascular damage. Moreover, because cardiovascular risk factors tend to cluster together, obesity and central fat distribution in childhood predicted CCA-IMT in adulthood [51].
Subclinical arterial injury in hypertensive children
Primary hypertension
Because secondary HT usually presents as severe, stage-2 HT, TOD is an obvious complication. However, children with primary hypertension (PH) also suffer from significant subclinical TOD already detected at diagnosis of HT. It was found that about 40% of children with newly diagnosed PH have a left ventricular mass index (LVMi) >95th percentile, and in 12–14%, significant left ventricular hypertrophy (LVH), i.e. LVMi >51 g/m height2.7 is present [46, 55, 56]. IMT thickening is also a constant finding and parallels LVH [14, 46]. As in the general population, in children with PH, the main hemodynamic predictors of IMT were SBP and PP [14, 15, 46].
PH in childhood is a syndrome not only of elevated blood pressure but also of the typical intermediate phenotype of characteristic anthropometric and metabolic abnormalities. It was shown that the increase in populational values of BMI observed since 1980 was paralleled by an increase in populational values of blood pressure [57, 58]. Thus, the prevalence of PH also seems to increase already in childhood. It parallels the prevalence of type 2 diabetes in childhood [59]. In fact, children and adolescents with PH have the same intermediate phenotype as young type 2 diabetics, and overweight and obesity are the typical phenotypes of adolescents with PH [16, 46, 60–62]. In children with PH, both elevated blood pressure and metabolic abnormalities therefore cluster together as cardiovascular risk factors, and it is difficult to analyze the effects of only elevated blood pressure separately from other accompanying metabolic abnormalities [63]. On average, children with PH have significantly greater values of CCA-IMT and superficial femoral artery IMT [14, 16, 46, 64]. Increased CCA-IMT was also closely related to markers of generalized dysfunction of the endothelium [65] and decreased functional properties of CCA [16]. When analyzing the effects of obesity on arterial damage in PH children, it was found that the most important factor predicting IMT increase was elevated blood pressure and not obesity [16, 64]. Because it is not obesity expressed as BMI but disturbed distribution of fat tissue that causes metabolic disturbances, it seems that more detailed measurements of fat tissue may explain the relations between excessive fat tissue and TOD, including arterial wall damage. Indeed, when distribution of fat tissue was analyzed by magnetic resonance imaging and waist-to-hip ratio in a group of adolescent boys with PH, it was found that boys who had central fat tissue distribution had the greater CCA-IMT [66].
The close relationship between obesity and PH is why adolescents with PH are a special group at high risk for metabolic syndrome (MS). It was found that MS is present in more than 20% of adolescents with PH compared with 2–3% in the general pediatric population [62]. When exposure to MS criteria and TOD, including CCA-IMT, was evaluated, it was found that there was a clear correlation between exposure to the number of MS criteria and TOD. In addition, concentrations of more specific markers of MS, such as adiponectin, significantly correlated with CCA-IMT [62].
Because there is a worldwide increase in BMI and blood pressure values, the populational percentile range of blood pressure is also increasing, i.e. the absolute values for a given percentile are now higher than a few decades ago. This raises an important question as to whether pediatricians should use absolute values of anthropometric parameters, such as BMI, waist circumference, or blood pressure above which cardiovascular risk is increased significantly. Recently, Katzmarzyk et al. proposed the use in the general pediatric population of age-, gender- and race-adjusted, absolute cutoff values of BMI and waist circumference above which cardiovascular risk factors rise significantly [63]. We found that in adolescents with PH, a significant risk of TOD, such as LVH and increased CCA-IMT, is already present when BMI is above average threshold values for the 60–65th percentile [67].
IMT in children with secondary hypertension
IMT in children with chronic kidney disease
IMT is uniformly increased in children with CKD in comparison with healthy counterparts and correlates negatively with glomerular filtration rate [68–70]. It was found that IMT is already increased in children with CKD stage 2 [70], and young adults who developed CKD in childhood had increased carotid IMT in comparison with a healthy control group [69]. In contrast, Groothoff et al. found that adult patients suffering from CKD since childhood and who survived on dialysis since young adulthood did not differ in terms of carotid IMT compared with healthy adults, but they had increased stiffness of carotid arteries [68]. However, one may argue that these results may also suggest that those who survived until adulthood on dialysis were those who had the lowest IMT. In a study by Mitsnefes et al., it was found that children after renal transplantation (Rtx) also had significantly greater carotid IMT than healthy children [71].
As mentioned above, arteriopathy in CKD has its distinct pathological characteristics known as Moenckeberg’s sclerosis and is strictly related with calcium and phosphate disturbances. However, the main predictive factors for IMT increase in children with CKD, besides calcium and phosphate disturbances, treatment with calcium-containing phosphate binders, parathormone, and calcitriol dose, was blood pressure [70–74]. The same relationships were found in children after Rtx, in whom blood pressure was one of the most important factors predicting carotid IMT [70].
IMT in children with coarctation of aorta
AH is the leading clinical sign of coarctation of aorta (CoA). In contrast to PH and HT in CKD, patients with CoA are submitted solely to hemodynamic insult and not to accompanying metabolic abnormalities. It was shown that even after coarctation repair, children with CoA had significantly greater CCA-IMT [75]. In some studies, it was found that carotid IMT is related to the degree of postoperative stenosis expressed as pressure gradient [75, 76]. Because stenosis of the aorta diminishes the pulsatile component of the pulse wave below the stenosis, and because blood pressure in the lower parts of the body is lower than above the stenosis, one should expect that IMT in superficial femoral arteries should be lower than in healthy children. However, data exist showing that in patients with CoA, IMT was uniformly increased in both the carotid and the femoral arteries [77]. Our own unpublished experience is that in children with CoA, IMT increases in parallel to local blood pressure, i.e. it is greater where blood pressure is higher (carotid arteries) and is decreased where blood pressure is lower (superficial femoral arteries). The same was also observed by Vriend et al., who found increased carotid IMT and decreased femoral IMT in CoA patients compared with normotensive controls and did not differ between normotensive and hypertensive CoA patients [78]. In a such perspective, both increased carotid IMT and lowered IMT in superficial femoral arteries should be a marker of cardiovascular risk in CoA patients. To date, there are no data from a prospective study evaluating the cardiovascular risk in CoA patients in relation to IMT measurements. However, the significant increase of carotid IMT in the presence of even mild aortic narrowing and blood pressure gradient is a strong argument for earlier treatment of patients with residual gradient [76].
IMT in children with obesity and other metabolic diseases
There is a relatively large amount of data on carotid IMT in children with obesity. Some authors found that obese children have significantly increased CCA-IMT in comparison with healthy controls [79–81]. However, it should be stressed that obesity usually is related not only to characteristic metabolic abnormalities, which are known cardiovascular risk factors, but there is also an intermediate phenotype in adolescents with primary HT. Second, BMI values above the cardiovascular risk threshold are strictly linked with exposure to a cluster of risk factors, including elevated blood pressure [63]. Thus, increased CCA-IMT in obese children may be caused not only by obesity and its metabolic complications but by elevated blood pressure. Indeed, Tounian et al. showed that when normotensive obese children were matched with healthy controls, there were no significant differences in CCA-IMT between groups [82]. On the other hand, when children with PH were divided according to BMI, no difference between obese hypertensive and nonobese hypertensive children was found regarding CCA-IMT [16, 64]. However, because obesity may precede development and diagnosis of PH [83], obese children are a high-risk group for future cardiovascular complications. In the studies by Pilz et al. and Beauloye et al., it was found that in obese children, CCA-IMT correlated with BMI-SDS, SBP, fasting insulin levels, insulin resistance expressed as homeostatic model assessment (HOMA) index, fasting insulin, resistin, and low adiponectin. However, when controlled for gender, maturity, and BMI, only adiponectin concentrations were an independent predictor of IMT [84, 85].
Increased CCA-IMT is significantly increased in children with metabolic diseases leading to premature atherosclerosis, such as familial hyperlipidemia and homocystinurias [86–89]. On the other hand, intensive and early treatment with statins caused a significant decline in CCA-IMT [90].
Although clinically evident macroangiopathic complications of type 1 diabetes infrequently occur in children, increased CCA-IMT have been found in many studies [91, 92]. Because nocturnal HT and nondipping pattern of blood pressure rhythm occur early in the course of type 1 diabetes, it is difficult to separate effects related to hemodynamic alterations from metabolic disturbances [93]. It was also found that in children with type 1 diabetes, carotid IMT was related to blood pressure, even in the absence of dyslipidemia [94]. However, hyperglycemia expressed as concentrations of glycated hemoglobin and oxidative stress are related to CCA-IMT in diabetic adolescents, and increased CCA-IMT was found even in normotensive children with type 1 diabetes [95–97]. This may indicate that when exposure to metabolic abnormalities is sufficiently severe, arterial wall damage may develop, even in normotensive individuals.
Is intima–media thickening reversible?
The problem of regression of arterial damage in terms of reversal of both anatomical and functional properties is a very important issue. It is known from studies in adults with cardiovascular disease that both regression of LVH in patients with HT and regression of atherosclerotic plaque in carotid artery decrease the risk of cardiac death and stroke [98, 99]. Because long-term exposure to hemodynamic and metabolic factors may lead to irreversible remodeling of arterial walls, therapeutic interventions should be introduced as early as possible. It was found that the age of treatment initiation with pravastatin in children with familial hypercholesterolemia (FH) was the main predictor of carotid IMT regression [90]. The effects of pharmacological therapy with statins in children with FH has been evaluated in a few randomized, controlled studies. Wiegman et al. randomly assigned 106 children with FH to treatment with pravastatin and 108 children with FH to placebo group [100]. After 2 years of treatment, there was statistically significant decrease of absolute values of IMT in carotid arteries (the mean of CCA, bulb, and ICA IMT) in children treated with statins. The reversibility of CCA-IMT thickening during treatment with statins is strictly related to lowering of LDL-c and the type of LDL receptor mutation. In a substudy of the above-mentioned trial with pravastatin, it was found that children with null mutation had greater mean carotid artery IMT compared with children with undetermined mutation and receptor-defective mutations. Although mean carotid artery IMT decreased in all groups during treatment, children with null alleles still had greater carotid IMT [101].
The reversibility of carotid thickening and impaired endothelial function assessed as FMD in obese children was also evaluated in randomized controlled trials. Meyer at al. randomly assigned 33 obese children to an intervention group of a 6-month therapeutic program of physical exercise and 34 to a control group [102]. After 6 months, it was shown that only in obese children from the intervention group was there significant decrease of anthropometric parameters, insulin resistance, LDL-c, hs-CRP, blood pressure, and CCA-IMT and an improvement of FMD. In another randomized, controlled study by Woo et al., it was shown that shorter, 6-week program of diet and exercise did not influence IMT but led to significant improvement in FMD. However, after 1 year, there was also a significant decrease in CCA-IMT in children who continued to exercise [103]. This indicates that improvement in endothelial function precedes regression of CCA-IMT thickening. This conclusion is supported by Watts et al., who found in a small, randomized, crossover protocol study of 19 obese children that even a short 8-week program of circuit training led to significant improvement of FMD and the beneficial vascular effects correlated with decrease of abdominal fat [104].
Although blood pressure is the most important factor predicting CCA-IMT, there are no published data, except in abstract form, on reversal of subclinical arterial injury in children with PH. However, it was found in an prospective, observational study of 57 children and adolescents with PH that after 1 year of combined nonpharmacological and pharmacological treatment, there was significant lowering of blood pressure; improved distribution of fat tissue as expressed by significant decrease of WHR; decrease of lipoprotein (a), LDL-c, and hs-CRP; and increase in HDL2-c concentrations. This was accompanied by a significant decrease in left ventricular mass, carotid IMT, and WCSA [105].
Rtx can reverse the uremic state and so enables evaluation of the effects of both metabolic and hemodynamic improvement on arterial structure and function in children with CKD. In cross-sectional studies, it was found that children after Rtx had lower values of carotid IMT in comparison with children with CKD stage 2–4 and children on dialysis [70]. However, carotid IMT was still greater than in healthy children [70, 71]. In a prospective study of 32 children on dialysis, among whom 19 were transplanted and 13 remained on dialysis, it was found that after 12 months, carotid IMT and WCSA significantly decreased in children who received a kidney graft and increased further significantly in those who remained on dialysis. In addition, in a subgroup of 24 patients with CKD stage 2–4, there was a significant increase in carotid IMT and WCSA [106]. The only significant predictor of carotid IMT after Rtx was systolic blood pressure. In another prospective study of 31 children after Rtx, it was found that CCA-IMT did not change in children after mean of 4 years after Rtx despite good blood pressure control and relatively good graft function [107]. However, when interpreting this data, it should kept in mind that CCA-IMT increases with age at a rate of 0.01–0.005 mm per year. Stabilization of CCA-IMT after Rtx therefore indicates regression rather than lack of improvement.
What is the role of IMT measurement in assessment of hypertensive children?
The increasing amount of data from studies on IMT in children with high cardiovascular risk poses an important question as to whether this method should be introduced as a routine tool in assessing and monitoring TOD in children. It seems that the problem is similar to the use of echocardiography in assessing left ventricular mass in children with AH and other chronic diseases causing increased cardiovascular risk, i.e. CKD. The large amount of data from both cross-sectional, observational, and the few prospective studies in children with AH and/or other cardiovascular risk factors cannot be ignored. Evaluation of IMT and its relationship to the arterial lumen is an unspecific marker of arterial wall injury, it is strictly correlated with other cardiovascular risk factors and other markers of TOD, and should be used both for basal description of TOD and for monitoring the course of disease and treatment. Thus, from a practical point of view, clinical utility of IMT measurements seems to be similar to use of echocardiography in assessing left ventricular mass.
As with other measurements of biological variables, the basic problem is with a clear definition of a cutoff value between physiology and pathology. In analogy to studies in adults, such a definition could be created on the basis of a hypothetical prospective, observational study evaluating the risk of cardiovascular events in relation to IMT. Obviously, such a study would last for many years. From both the practical and ethical points of view, waiting for the results of such a study is not prudent. However, pediatricians are used to defining abnormality based on statistical distributions of values using percentiles or SD scores. The best example is the definition of elevated blood pressure defined as ≥95th percentile for age, gender, and height. Similarly, definitions of LVH in childhood are also based on percentile values. However, as with echocardiographic assessment of left ventricular mass, to avoid any bias, IMT should be measured in a standardized manner [108, 109]. Table 2 summarizes the results of a number of IMT studies in children.
At the moment, until the methodology of measurements of IMT and internal dimensions of arteries are unified, it seems that IMT measurement should be used at least as a tool for monitoring (using the same methodology) TOD and the effects of treatment in children with cardiovascular risk.
References
McTigue K, Kuller L (2008) Cardiovascular risk factors, mortality, and overweight. JAMA 299:1260–1263
Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth MEJ (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298:564–567
Barker DJP (1998) In utero programming of chronic disease. Clin Sci 95:115–128
Vehaskari VM, Woods LL (2005) Prenatal programming of hypertension: Lessons from Experimental model. J Am Soc Nephrol 16:2545–2556
Verdecchia P, Angeli F, Taddei S (2006) At the beginning of stiffening. Endothelial dysfunction meets “Pulsology”. Hypertension 48:541–542
Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Person G, O’Leary DH, Bluemke DA, Lima JAC (2008) Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the multi-ethnic study of atherosclerosis. Clinical and population studies. Arterioscler Thromb Vasc Biol 28:194–201
Schoen FJ, Cotran RS (1999) Blood vessels. In: Cotran RS, Kumar V, Collins T (eds) Pathologic basis of disease, 6th edn. W.B. Saunders, Philadelphia
Wessels S, Amman K, Toring J, Ritz E (1999) Cardiovascular structural changes in uremia: Implications for cardiovascular function. Semin Dial 12:288–292
McMahan CA, Gidding SS, Viikari JSA, Juonala M, Kahonen M, Hutri-Kahonen N, Jokinen E, Taittonen L, Pietikainen M, McGill HC Jr, Raitakari OT (2007) Association of pathobiologic determinants of atherosclerosis in youth risk score and 15-year change in risk score with carotid artery infima-media thickness in young adults (from the cardiovascscular risk in young finns study). Am J Cardiol 100:1124–1129
Virmani R, Robinowitz M, Geer JC, Breslin PP, Beyer JC, McAlister HA (1987) Coronary artery atherosclerosis revisited in Korean war combat casualties. Arch Pathol Lab Med 111:972–976
Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS (2003) Childhood cardiovascular risk factors and carotid vascular changes in adulthood. JAMA 290:2271–2276
Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340:115–126
McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill HC (2005) Risk scores predict atherosclerotic lesions in young people. Arch Intern Med 165:883–890
Sorof JM Alexandrov AV, Cardwell NG, Portman RJ (2003) Carotid intima-media thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics 111:61–66
Sorof JM, Alexandrov AV, Garami Z, Turner JL, Grafe RE, Lai D, Portman RJ (2003) Carotid ultrasonography for detection of vascular abnormalities in hypertensive children. Pediatr Nephrol 18:1020–1024
Litwin M, Trelewicz J, Wawer Z, Antoniewicz J, Wierzbicka A, Rajszys P, Grenda R (2004) Intima-media thickness and arterial elasticity in hypertensive children: controlled study. Pediatr Nephrol 19:767–774
Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, Bonaa KH (1996) Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol 16:984–991
Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R (1986) Intima plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 74:1399–1406
Gariepy J, Simon A, Chironi G, Moyse D, Levenson J (2004) Large artery wall thickening and its determinants under antihypertensive treatment: The IMT-INSIGHT Study. J Hypertens 22:137–143
Pyke K, Tschakovsky M (2005) The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 2:357–369
Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI (1995) Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension 26:485–496
The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (2007) Guidelines for the management of arterial hypertension. J Hypertens 25:1105–1187
National high blood pressure education program working group on high blood pressure in children and adolescents (2004) The fourth report on diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Kanters SDJM, Algra A, van Leewen MS, Banga J-D (1997) Reproducibility of in vivo carotid intima-media thickness measurements. A review. Stroke 28:665–671
Seçil M, Altay C, Gülcü C, Çeçe H, Göktay A, Dicle O (2005) Automated measurement of intima–media thickness of carotid arteries in ultrasonography by computer software. Diagn Interv Radiol 11:105–108
Bots ML, Hofman A, Grobbe DE (1997) Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam study. Stroke 28:2442–2447
Tanaka H, Dinenno TH, Monahan KD, DeSouza CA, Seals DR (2001) Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vasc Biol 21:82–87
Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JSA, Raitakari OT (2005) Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: The cardiovascular risk in young finns study. Circulation 112:1486–1493
Juonala M, Kähönen M, Laitinen T, Hutri-Kahonen N, Jokinen E, Taittonen L, Pietikainen M, Helenius H, Viikari JSA, Raitakari OT (2008) Effect of age and sex on carotid intima–media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in young finns study. Eur Heart J 29:1198–1206
Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif J-C, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M (2007) Mannheim intima-media thickness Consensus (2004–2006). Cerebrovasc Dis 23:75–80
Yeboah J, Burke GL, Crouse JR, Herrington DM (2008) Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: the cardiovascular health study. Atherosclerosis 197:840–845
Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E (2005) Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol 45:1980–1986
Martyn CE, Greenwald SE (2001) A hypothesis about mechanism for the programming of blood pressure and vascular disease in early life. Clin Exp Pharm Physiol 28:948–951
Gurne O, Chenu P, Buche M, Louagie Y, Eucher P, Marchandise B, Rombaut A, Blommaert D, Schroeder E (1999) Adaptive mechanisms of arterial and venous coronary bypass grafts to an increase in flow demand. Heart 82:336–342
Jourdan C, Wühl E, Litwin M, Fahr K, Trelewicz J, Jobs K, Schenk JP, Grenda R, Mehls O, Tröger J, Schaefer F (2005) Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens 23:1707–1715
Zureik M, Touboul P-J, Bonithon-Kopp C, Courbon D, Berr C, Leroux C, Ducimetiere P (1999) Cross-sectional and 4-year longitudinal associations between brachial pulse pressure and common carotid intima-media thickness in a general population. The EVA study. J Hypertens 30:550–555
Stein JH, Douglas PS, Srinivasan SR, Bond GM, Tang R, Li S, Chen W, Berenson GS (2004) Distribution and cross-sectional age-related increases of carotid artery intima-media thickness in young adults. The Bogalusa heart study. Stroke 35:2782–2787
Bots ML, Grobbee DE, Hofman A, Witteman JCM (2005) Common carotid intima-media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke 36:762–767
Polak JF, Kronmal RA, Tell GS, O’Leary DH, Savage PJ, Gardin JM, Rutan GH, Borhani NO (1996) Compensatory increase in common carotid artery diameter. Relation to blood pressure and artery intima-media thickness in older adults. Stroke 27:2012–2015
Scuteri A, Manolio TA, Marino EK, Arnold AM, Lakatta EG (2004) Prevalence of specific variant carotid geometric patterns and incidence of cardiovascular events in older persons. The cardiovascular health study. J Am Coll Cardiol 43:187–193
Zielinski T, Dzielinska A, Januszewicz A, Rynkun D, Makowiecka-Ciesla M, Tyczyński P, Prejbisz A, Demkow M, Kadziela J, Naruszewicz M, Januszewicz M, Juraszynski Z, Korewicki J, Rużyłło W (2007) Carotid intima-media thickness as a marker of cardiovascular risk in hypertensive patients with coronary disease. Am J Hypertens 20:1058–1064
Sacco RL (2007) The 2006 William Feinberg lecture: shifting the paradigm from stroke to global vascular risk estimation. Stroke 38:1980–1987
Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Azen SP (1998) The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 128:262–269
Sass C, Herbeth B, Chapel O, Siest G, Visvikis S, Zannad F (1998) Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens 16:1593–1602
Denarie N, Gariepy J, Chironi G, Massonneau M, Laskri F, Salomon R, Levenson J, Simon A (2000) Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis 148:297–302
Litwin M, Niemirska A, Sladowska J, Antoniewicz J, Daszkowska J, Wierzbicka A, Wawer ZT, Grenda R (2006) Left ventricular hypertrophy and arterial wall thickening in children with essential hypertension. Pediatr Nephrol 21:811–819
Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, Lehtimaki T, Simell O, Raitakari OT (2002) Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol 22:1323–1328
Liuba P, Persson J, Luoma J, Yla-Herttuala S, Pesonen E (2003) Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL, and are followed by thickening of carotid intima-media. Eur Heart J 24:515–521
Berenson GS (2002) Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa heart study. Am J Cardiol 90:3L–7L
Davis PH, Dawson JD, Riley WA, Lauer RM (2001) Carotid intima-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine study. Circulation 104:2815–2819
Johnson HM, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS, Stein JH (2007) Predictors of carotid intima-media thickness progression in young adults. The Bogalusa heart study. Stroke 38:900–905
Li S, Chen W, Srinivasan SR, Berenson GS (2004) Childhood blood pressure as a predictor of arterial stiffness in young adults. The Bogalusa heart study. Hypertension 43:541–546
Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS (2003) Childhood cardiovascular risk actors and carotid vascular changes in adulthood. The Bogalusa heart study. JAMA 290:2271–2276
Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torko N, Jarvisilo MJ, Uhari M, Jokinen E, Ronemaa T, Akerblom HK, Viikari JSA (2003) Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood. The cardiovascular risk in young finns study. JAMA 290:2277–2283
Daniels SR, Loggie JM, Khoury P, Kimball TR (1998) Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 97:1907–1911
Hanevold C, Waller J, Daniels S, Portman R, Sorof J, International Pediatric Hypertension Association (2004) The effects of obesity, gender and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics 113:328–333
Ogden DL, Kuczmarski RJ, Flegal KM, Mel Z, Guo S, Wel R, Grummer- Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:46–60
Muntner P, He J, Cutler JA, Wildman RP, Whelton PK (2004) Trends in blood pressure among children and adolescents. JAMA 291:2107–2113
Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P (1996) Increased incidence of non–insulin-dependent diabetes mellitus among adolescents. J Pediatr 128:608–615
Robinson RF, Batisky DL, Hayes JR, Nahata MC, Mahan JD (2004) Body mass index in primary and secondary hypertension. Pediatr Nephrol 19:1379–1384
Flynn JT, Alderman MH (2005) Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol 20:961–966
Litwin M, Śladowska J, Antoniewicz J, Niemirska A, Wierzbicka A, Daszkowska J, Wawer ZT, Janas R, Grenda R (2007) Metabolic abnormalities, insulin resistance and metabolic syndrome in children with primary hypertension. Am J Hypertens 20:875–882
Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS (2004) Body mass index, waist circumference and clustering of cardiovascular risk factors in a biracial sample of children and adolescents. Pediatrics 144:198–205
Lande MB, Carson NL, Roy J, Meagher CC (2006) Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension 48:40–44
Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio ABR, Beghetti M (2008) Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressur. Eur Heart J 29:792–799
Niemirska A, Litwin M, Antoniewicz J, Jurkiewicz E, Kosciesza I, Sladowska J, Janas R, Wawer ZT (2006) Fat tissue distribution and metabolic alterations in boys with primary hypertension. Przegl Lek 63(suppl 3):49–53
Litwin M, Sladowska J, Syczewska M, Niemirska A, Daszkowska J, Antoniewicz J, Wierzbicka A, Wawer ZT (2008) Different BMI cardiovascular risk thresholds as markers of organ damage and metabolic syndrome in primary hypertension. Pediatr Nephrol 23:787–796
Groothoff JW, Gruppen MP, Offringa M, De Groot E, Stok W, Bos WJ, Davin JC, Lilien MR, Van De Kar NC, Wollf ED Heymans HS (2002) Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol 13:2953–2961
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F (2005) Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16:1494–1500
Mitsnefes MM, Kimball TR, Witt SA, Glassock BJ, Khoury PR, Daniels SR (2004) Abnormal carotid artery structure and function in children and adolescents after successful renal transplantation. Circulation 110:97–101
Mitsnefess MM, Kimball TR, Kartel J, Witt SA, Glassock BJ, Khoury PR, Daniels SR (2005) Cardiac and vascular adaptation in pediatric patients with chronic renal diseases: role of calcium-phosphorus metabolism. J Am Soc Nephrol 16:2799–2803
Poyrazoglu HM, Dusunsel R, Yilmaz A, Narin N, Anarat R, Gunduz Z, Coskun A, Baykan A, Ozturk A (2007) Carotid artery thickness in children and young adults with end stage renal disease. Pediatr Nephrol 22:109–116
Schroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield C, Rees L (2007) Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18:2996–3003
Meyer AA, Joharchi MS, Kundt G, Schuff-Werner P, Steinhoff G, Kienast W (2005) Predicting the risk of early atherosclerotic disease development in children after repair of aortic coarctation. Eur Heart J 26:617–622
Vriend JWJ, Zwinderman AH, de Groot E, Kastelein JJP, Bouma BJ, Mulder JM (2005) Predictive value of mild, residual descending aortic narrowing for blood pressure and vascular damage in patients after repair of aortic coarctation. Eur Heart J 26:84–90
Vriend JW, de Groot E, de Waal TT, Zijta FM, Kastelein JJ, Mulder BJ (2006) Increased carotid and femoral intima-media thickness in patients after repair of aortic coarctation: influence of early repair. Am Heart J 151:242–247
Vriend JJ, de Groot E, Kastelein JJ, Mulder BJ (2004) Carotid and femoral B-mode ultrasound intima-media thickness measurements in adults post-coarctectomy patients. Int Angiol 23:41–46
Retnakaran R, Zinman B, Connelly PA, Harris SB, Hanley AJ (2006) Non-traditional cardiovascular risk factors in pediatric metabolic syndrome. J Pediatr 148:176–182
Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W (2006) Impaired flow-mediated vasodilation, carotid intima-media thickening and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics 117:1560–1567
Roh EJ, Lim JW, Ko KO, Cheon EJ (2007) A useful predictor of early atherosclerosis in obese children: serum high-sensitivity C-reactive protein. J Korean Med Sci 22:192–197
Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet J-P, Bonnet D (2001) Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358:1400–1404
Sakarcan A, Jerrell J (2007) Population-based examination of the interaction of primary hypertension and obesity in South Carolina. Am J Hypertens 20:6–10
Pilz S, Horejsi R, Moller R, Almar G, Scharnagl H, Stojakovic T, Dimitrore R, Weihrauch G, Borkenstein M, Maerz W, Schanenstein K, Mering H, Mangee H (2005) Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J Clin Endocrinol Metab 90:4792–4796
Beauloye V, Zech F, Tran HT, Lapuyt P, Maes M, Brichard SM (2007) Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab 92:3053–3032
Alagona C, Soro A, Ylitalo K, Salonen R, Salonene JT, Taskinen MR (2002) A low high density lipoprotein level is associated with carotid artery intima-media thickness in asymptomatic members of low HDl families. Atherosclerosis 165:309–316
Wiegman A, de Groot E, Hutten BA, Rodenburg J, Gort J, Bakker HD, Sijbrands EJG, Kastelein JJP (2004) Arterial intima-media thickness in children heterozygous for familial hypercholesterolemia. Lancet 363:369–370
Rubba P, Mercuri M, Faccenda F, Iannuzzi A, Irace C, Strisciuglio P, Gnasso A, Tang R, Andria G, Bond MG (1994) Premature carotid atherosclerosis: does it occur in both familial hypercholesterolemia and homocystinuria ? Ultrasound assessment of aterial intima-media thickness and blood flow velocity. Stroke 25:943–950
Megnien JL, Gariepy J, Saudubray JM, Nuoffer JM, Denarie N, Levenson J, Simon A (1998) Evidence of carotid artery wall hypertrophy in homozygous homocystinuria. Circulation 98:2276–2281
Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graf A, de Groot E, Wijburg A, Kastelein JJ, Hutten BA (2007) Statin `treatment in children with familial hypercholesterolemia: the younger the better. Circulation 116:664–668
Jarvisalo MJ, Jartti L, Nanoto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, Celermajer DS, Raitakari OT (2001) Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high risk children. Circulation 104:2943–2947
Jarvisalo MJ, Putto-Laurila A, Jartti L, Lehtimaki T, Solakivi T, Ronnemaa T, Raitakari OT (2002) Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes 51:493–498
Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Battle D (2002) Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347:797–805
Schwab KO, Doerfer J, Krebs A, Krebs K, Schorb E, Hallerman K, Superti-Furga A, Zieger B, Marz W, Schmodt-Trucksass A, Winkler K (2007) Early atherosclerosis in childhood type 1 diabetes: role of raised blood pressure in the absence of dyslipidemia. Eur J Pediatr 166:541–548
Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH Kaufmann FR (2004) Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr 145:452–457
Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimaki T, Ronnemaa T, Viikari J, Raittakari OT (2004) Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 109:1750–1755
Singh TP, Groehn H, Kazmers A (2003) Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol 19:661–665
Spence JD, Hegele RA (2004) Noninvasive phenotypes of atherosclerosis. Arterioscler Thromb Vasc Biol 24:e188
Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thvgesen K (2007) Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE study. Circulation 116:700–705
Wiegman A, Hutten BA, Groot de E, Rodenburg J, Bakker HD, Büller HR, Sijbrands EJG, Kastelein JJP (2004) Efficacy and safety of statin therapy in children with familial hypercholesterolemia. A randomized controlled trial. JAMA 292:331–337
Koeijvoets KCMC, Rodenburg J, Hutten BA, Wiegman A, Kastelein JJP, Sijbrands EJG (2005) Low density lipoprotein receptor genotype and response to pravastatin in children with familial hypercholesterolemia. Substudy of an intima-media thickness trial. Circulation 112:3168–3173
Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W (2006) Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol 48:1865–1870
Woo KS, Chook P, Yu CW, Sung RYT, Qiao M, Leung SSF, Lam CWK, Metreweli C, Celermajer DS (2004) Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 109:1981–1986
Watts K, Beye P, Siafarikas A, Davis E, Jones TW, O’Driscoll G, Green DJ (2004) Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 10:1823–1827
Litwin M, Sladowska J, Niemirska A (2008) Regression of target organ damage and metabolic abnormalities in children with primary hypertension – prospective study Proceedings of World Congress of Hypertension, Berlin 2008 (Abstract)
Litwin M, Wuhl E, Jourdan C, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F (2008) Evolution of large vessel arteriopathy in pediatric patients with chronic kidney disease. Nephrol Dial Transplant 23:2522–2527. doi:https://doi.org/10.1093/ndt/gfn083
Krmar RT, Balzano R, Jogestrand T, Cedano-Minguez A, Englund MS, Berg U (2008) Prospective analysis of carotid wall structure in pediatric renal transplants with ambulatory normotension an in treated hypertensive recipients. Pediatr Transplant 12:412–419
De Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260
De Simone G, Deveraux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH (1995) Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25:1056–1062
Stein JH, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS (2004) Distribution and cross-sectional age-related increases of carotid artery intima-media thickness in young adults. The Bogalusa heart study. Stroke 35:2782–2787
Wunsch R, de Sousa G, Reinehr T (2005) Intima-media thickness in obesity: relation to hypertension and dyslipidemia. Arch Dis Child 90:1097–1136
Reinher T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R (2006) Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 55:113–118
Zhu W, Huang X, He J, Li M, Neubauer H (2005) Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr 164:337–344
Pacifico L, Antisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C (2008) Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res 63:423–427
Schiel R, Beltschikow W, Radon S, Kramer G, Perenthaler T, Stein G (2007) Increased carotid intima-media thickness and associations with cardiovascular risk factors in obese and overweight children and adolescents. Eur J Med Res 12:503–508
Juonala M, Ritakari M, Viikari J, Raitakari OT (2006) Obesity in youth is not an independent predictor of carotid IMT in adulthood. The cardiovascular risk in young finns study. Atherosclerosis 185:388–393
Di Salvo G, Pacileo G, del Giudice FM, Limonelli G (2006) Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J 27:2689–2695
Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B (2006) A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol 26:2541–2546
Yamasaki Y, Kawamori R, Matsuchima H, Nishizawa H, Kodama M (1994) Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes 43:634–639
Pozza DR, Bechtold S, Bonfig W, Putzker S, Kozlik-Feldmann R, Netz H, Schwarz HP (2007) Age of onset of type 1 diabetes in children and carotid intima medial thickness. J Clin Endocrinol Metab 92:2053–2057
Pauciullo P, Iannuzzi A, Sartorio R, Irace C, Covetti G, Di Costanzo A, Rubba P (1994) Increased intima-media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb 14:1075–1079
Lavrencic A, Kosmina B, Keber I, Videcnik V, Keber D (1996) Carotid intima-media thickness in young patients with familial hypercholesterolaemia. Heart 76:321–325
Aggoun Y, Sidi D, Bonnet D (2001) Arterial dysfunction after treatment of coarctation of the aorta. Arch Mal Coeur Vaiss 94:785–789
Bilginer Y, Ozaltin F, Basaran C, Aki TF, Karabulut E, Duzova A, Besbas N, Topaloglu R, Ozen S, Bakkaloglu M, Bakkaloglu A (2007) Carotid intima-media thickness in children and young adults with renal transplant: Internal carotid artery vs. common carotid artery. Pediatr Transplant 11:888–894
Töz H, Duman S, Altunel E, Seziş M, Ozbek S, Ozkahya M, Aşci G, Ok E, Başci A (2004) Intima media thickness as a predictor of atherosclerosis in renal transplantation. Transplant Proc 36:156–158
Acknowledgement
This work was supported by grants N N40742533 and N N402226835 funded by the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Litwin, M., Niemirska, A. Intima–media thickness measurements in children with cardiovascular risk factors. Pediatr Nephrol 24, 707–719 (2009). https://doi.org/10.1007/s00467-008-0962-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0962-3