Abstract
Background and purpose
Venous thromboembolism (VTE) is a serious complication encountered in surgical practice. The purpose of this study was to identify changes in coagulation status and deep vein flow parameters, within 24 h postoperatively, for patients undergoing laparoscopic total extraperitoneal inguinal hernia repair (TEP).
Methods
For 144 patients undergoing TEP, coagulation markers including prothrombin time (PT), partial thromboplastin time, thrombin time, D-dimer, fibrinogen, fibrin degradation products (FDP), and international normalized ratio (INR) were monitored preoperatively and in the first morning postoperatively. Echo-Doppler recordings preoperatively and again within 24 h postoperatively were completed for 23 patients to monitor lower extremity deep vein flow parameters including speed of flow (cm/s), diameter (cm), and cross-sectional area (cm2). Clinically significant VTE and other complications were recorded.
Results
No significant VTE were diagnosed. Significant increases were seen in the first morning postoperatively for PT, D-dimer, FDP, and INR (P < 0.05). Stratified by age, except for those < 50 years, the ratio of value-outside-the-normal-range (VONR) for D-dimer and FDP increased significantly postoperatively for all age groups. Stratified by operation duration, a significant difference in the ratio of VONR in D-dimer was identified postoperatively between those with an operation duration < 60 min and ≥ 60 min. There were significant decreases in the iliac and common femoral vein flow velocity of the ipsilateral extremity postoperatively (P < 0.05).
Conclusions
Activated hypercoagulability and hampered lower extremity deep vein flow were observed immediately after TEP. DVT formation was more pronounced in older patients and for those with operation duration ≥ 60 min. Proper VTE risk stratification for laparoscopic inguinal hernia repair (LIHR) and prophylaxis early after LIHR should be important clinical considerations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venous thromboembolism (VTE) is a serious complication, frequently encountered in surgical practice that comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE). Approximately 50% of DVT cases are asymptomatic, and the first sign of the disease may be a fatal PE presented as sudden death with no opportunity for medical intervention [1]. The incidence of fatal PE ranges from 0.1 to 0.8% in patients undergoing general elective surgical procedures [2]. Inguinal hernia repair is the most commonly planned general surgical procedure [3, 4], and laparoscopic repair of inguinal hernia has become an increasingly popular method of herniorrhaphy, with the advantages of lower wound infection rates, faster recovery times, and less postoperative pain compared to open procedures [5,6,7]. However, current hernia guidelines provide no details on incidence of VTE following laparoscopic inguinal hernia repair (LIHR) or regimens for VTE prophylaxis [8]. Reviews of VTE complications are often based on collections of individual cases in randomized controlled trials (RCTs), retrospective follow-up cohorts, and case reports. Mayer et al. reported seven cases of thrombosis and five cases of PE in an analysis of 24,571 primary inguinal hernia patients treated by laparoscopic approaches [9]. Neumayer et al. reported one death on postoperative day 3 caused by PE after LIHR [10]. Nilsson et al. identified 73 cases of PE within 30 days of groin hernia surgery in 143,042 registered patients, with incidence of 0.03% and 0.08%, respectively, in men and women undergoing an elective operation. However, their data did not further stratify the PE incidence for LIHR or for open repair [11]. Currently available comprehensive guidelines that address VTE prophylaxis for non-orthopedic surgery patients were released by the American College of Chest Physicians (ACCP) in 2012 (9th edition) [12]. However, due to the paucity of data on VTE prophylaxis specific to the LIHR population, recommendations derived from other surgical populations including gastrointestinal, urological, and vascular procedures have been generalized to the laparoscopic inguinal hernia population. Recently, Humes et al. reported a 0.53% incidence of VTE in the 90 days following surgery in 28,782 men who underwent an inguinal hernia repair with the risk of VTE highest during the first month following the surgery [13]. However, that study did not stratify the individual risk of VTE in patients undertaking LIHR or the timing of the earliest onset of VTE. Previously, we reported a sudden death due to acute PE in the 20 h after a laparoscopic total extraperitoneal inguinal hernia repair (TEP) [14]. The development of VTE is linked to at least one of the three Virchow’s triad: vascular injury, venous stasis, and/or hypercoagulability [15]. The status of venous stasis and hypercoagulability in the very early postoperative stage in patients who undergo a LIHR has not been well defined. Therefore, we sought to determine change in coagulation markers and deep vein flow parameters within 24 h postoperatively in patients undergoing a TEP procedure.
Patients and methods
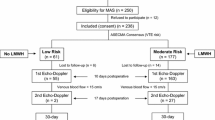
The study was performed as a prospective non-randomized study from August 2016 to January 2018 in patients undergoing LIHR with a TEP approach. Altogether 150 consecutive patients were enrolled into the study. Six patients were excluded due to incomplete laboratory tests or Echo-Doppler examinations. Thus, 144 patients were assessed who underwent an elective TEP procedure for inguinal hernia. Inclusion criterion was elective laparoscopic surgery for symptomatic inguinal hernia. Exclusion criteria were refusal of consent; incarcerated inguinal hernia; female; age < 18 or > 90 years; conversion to open procedure; VTE in previous 6 months; unsuitable for general anesthesia due to chronic obstructive pulmonary disease or pneumonia; body mass index > 25; sepsis < 1 month; currently at bed rest; trauma or recent surgery; malignancy; varicose veins; swollen legs; grade > 2 according to the American Society of Anesthesiologists grading system; on medication affecting coagulation (anticoagulants, antiaggregants, non-steroidal anti-inflammatory drugs, or steroids); generalized bleeding disorders; renal or hepatic diseases; congestive heart failure; stroke; or history of inflammatory bowel disease. The Caprini risk assessment model was used to assess the VTE risk in every patient preoperatively and postoperatively [16]. Each patient was classified into one of the four risk groups: low, moderate, high, and highest risk. The duration of operation was estimated to 1 h in the preoperative risk calculation. VTE prophylaxis comprised early ambulation and intermittent pneumatic compression (IPC). No low-molecular-weight heparin (LMWH) or low-dose unfractionated heparin (LDUH) was administrated in this cohort study. In the postoperative period, complications and clinical apparent VTE in 30 days following operation were observed. Clinical assessment of DVT risk was performed according to the Wells score system [17]. Coagulation markers including prothrombin time (PT), partial thromboplastin time (PTT), thrombin time (TT), D-dimer, fibrinogen, fibrin degradation products (FDP), and international normalized ratio (INR) were monitored preoperatively and in the first morning postoperatively. Echo-Doppler recordings were made preoperatively and repeated within 24 h postoperatively by one specified sonographer in patients who had unilateral inguinal hernia and gave consent for repeated examination. The lower extremities deep vein flow parameters including speed of flow (cm/s), diameter (cm), and cross-sectional area (cm2) were monitored. The study was approved by the ethics committee of the hospital. Each patient gave written informed consent to be included in the study and with the anonymous publication of data.
Statistical analysis
Values for all continuous variables were expressed as means ± standard deviation (SD). Data were analyzed with Origin 8.0 software (Origin Lab Corp. Northampton, MA, USA). Data were evaluated by two-sample t test for continuous variables and Chi-square test for categorical variables. Vascular diameter, speed of flow, and cross-sectional area were found to vary greatly pre- and postoperatively, hence adjustments were made to the data to insure comparability. The relative values (postoperative data vs preoperative data and preoperative radices were regarded as 1) were used for comparative analysis in this study. P < 0.05 was interpreted as significantly different.
Results
Of the 150 eligible patients, 144 (96%) completed the study, of whom 23 had Echo-Doppler preoperatively with repeat 24 h postoperatively. All patients underwent TEP under general anesthesia without events. Patient characteristics are shown in Table 1. The range in age was 25–88 years with a median of 64 years. VTE risk assessment was done according to the Caprini risk scoring model both preoperatively and postoperatively. For the preoperative assessment, the duration of operation was assumed to be 1 h based on our routine experience with the postoperative assessment risk score adjusted based on the actual duration of operation. Risk scores ranged from 2 to 5 points. The preoperative and postoperative VTE scores showed no statistical difference (P > 0.05), 19 patients were considered at high risk for VTE with a score of 5 points. All patients underwent IPC immediately after operation. A urine catheter was not applied, and patients urinated by themselves in the postoperative period and were mandated to take gentle walks after they recovered from anesthesia. No clinically significant VTE or PE was found 30 days following operation. Eight patients presented with a swollen operated inguinal area postoperatively, due to seroma, all diminished spontaneously in 1–2 months. No infection or recurrence was observed in the follow-up period. All patients were discharged within 1–2 days after operation.
Significant increases were observed in the first morning, postoperatively for PT, D-dimer, FDP, and INR (P < 0.05). No significant changes in PTT, TT, or fibrinogen were seen (P > 0.05; Fig. 1). A further stratification by age is shown in Fig. 2, where postoperatively, a significantly high ratio of value-outside-the-normal-range (VONR) in D-dimer and FDP was observed when compared to the preoperative period, in all patients except those < 50 years of age (P < 0.05). Preoperatively, no significant differences in the ratio of VONR in D-dimer and FDP were observed between the various age groups (P > 0.05), while in the postoperative period, those 70–79 years of age demonstrated a significantly higher ratio of VONR for D-dimer compared to the other age groups. By stratification, the change in ratio of VONR in PT between the pre- and postoperative periods was significant only in the population < 70 years of age, while no significant difference was observed postoperatively. Stratification by operation duration is shown in Fig. 3. All groups showed a significant increase in the ratio of VONR for D-dimer, and FDP postoperatively when compared to the preoperative period. A significant difference in the ratio of VONR for D-dimer was found between operation duration < 60 min and ≥ 60 min in the postoperative period.
Twenty-three patients with unilateral inguinal hernia underwent Echo-Doppler of the lower extremities preoperatively with repeat within the first 24 h, postoperatively. No symptomatic or asymptomatic DVT was observed. There were significant decreases in the iliac vein flow and common femoral vein flow velocity, with decreased cross-sectional area of the iliac vein of the ipsilateral extremity (that of the operated groin) (P < 0.05). Significant decreases in the diameter of the common femoral vein and the cross-sectional area for both the common femoral vein and the iliac vein of the contralateral extremity were observed (P < 0.05) (Fig. 4). No significant difference in deep vein flow parameters was observed between ipsilateral and contralateral side, either pre- or postoperatively.
Discussion
Inguinal herniorrhaphy is the most commonly planned general surgical procedure and is generally categorized as a low-risk VTE procedure. Laparoscopic surgeries are also a low VTE risk compared to open procedures [18]. However, the real VTE risk for laparoscopic inguinal hernia is not known in that there is a paucity of VTE incidence data in patients undergoing LIHR. Currently, VTE complications associated with LIHR are found in literature citations that are case reports, collections of individual cases in RCTs, or registry databases. These databases have practical limitations on reporting of the details of VTE such as earliest onset time, duration, mortality, and possible data omissions due to poor report compliance [19].
We have encountered a sudden death due to acute PE in the first day following a TEP repair [14]. The patient presented with no obvious preoperative high VTE risk, which suggests that lethal VTE can develop unexpectedly very early postoperatively. Understanding changes in coagulability and deep vein flow during the early phase following LIHR may be crucial to the proper prophylaxis and treatment of DVT. In this study, we monitored the following coagulation markers: PT, PTT, TT, D-dimer, fibrinogen, FDP, and INR perioperatively, which are routine laboratory tests for all surgical patients in our hospital. PT, serum D-dimer, FDP, and INR increased significantly during the first day after TEP. Among them, serum D-dimer appeared to be the most remarkable parameter when stratified for age and duration of operation. In general, a secondary postoperative elevation of D-dimer, fibrinogen, and FDP can be induced by surgical trauma [20], where fibrinogen is a marker of activated coagulation and FDP corresponds to the formed volume of thrombin, which is regarded as a coagulation activity marker [21]. D-dimer forms during the plasmin-induced break down of fibrin in the fibrinolytic pathway. Increases in D-dimer indicate increased formation and breakdown of fibrin, and D-dimer has been used as a marker of intravascular clot formation [22]. In this study, we found that 8.3% of patients had serum D-dimer concentrations outside the normal range (≤ 0.5 mg/L) prior to operation, which dramatically elevated to 61.1% during the first day after TEP procedure. This significant postoperative elevation of serum D-dimer, in the absence of clinically significant DVT, confirmed the notion that D-dimer levels are useful as a means to rule out thrombosis. D-dimers are highly sensitive as a biomarker for VTE at a cut-off value of ≤ 0.5 mg/L, while the converse is not true given its low positive predictive value [23, 24]. However, elevated D-dimer levels may be an indication that some of these patients were vulnerable to the development of DVT. Shigemi et al. reported in gynecologic patients that those with low preoperative D-dimer levels (0.6–0.9 µg/mL) had a 2.7% incidence of VTE compared to an incidence of 0 in patients with D-dimer levels ≤ 0.5 µg/mL and 23.7% in patients with D-dimer levels ≥ 1.0 µg/mL [25]. Kimura et al. found that maintenance of higher plasma D-dimer levels 7 days after surgery was found in patients with DVT but not without [26]. Moreover, we found that the elderly population had a more pronounced elevation of D-dimer levels. In those < 70 years of age, the percentage of people with serum D-dimer levels outside the normal range was approximately 50%, while for those 70–79 years and ≥ 80 years, the ratio was as high as 82.76% and 75%, respectively. Older patients had a more intense activation of the fibrinolysis cascade, which is in accordance with the widely accepted notion that the risk of DVT increases with age. Recently, Osaki et al. argued that the optimal cut-off D-dimer level was 1.4 µg/mL in an analysis of risk and incidence of perioperative DVT in patients undergoing gastric cancer surgery [27]. Another important finding in this study was a significant difference in the D-dimer VONR ratio postoperatively, between the population with an operation duration < 60 min and a duration of ≥ 60 min. By the Caprini risk assessment model, laparoscopic surgery > 45 min receives two points, no matter how long the duration is beyond 45 min and regardless of the type of specific procedure. Our findings suggest that TEP duration ≥ 60 min corresponds to a more pronounced possibility for elevated D-dimer levels. For aged patients or for those with operation duration ≥ 60 min, close observation is required to rule out the possibility of DVT and proper prophylaxis should be considered.
Venous stasis is one of the three key factors for the development of DVT. At one time there was a concern that insertion of a prosthetic mesh into the preperitoneal space would compress the femoral vein, possibly influencing the flow of the vein, increasing risk for venous stasis and DVT. With a 6-month follow-up, Ozmen et al. found that neither mean diameter nor mean flow velocity of the femoral vein was changed by the insertion of the mesh during TEP procedures with no patients developing clinically significant DVT [28]. However, an inevitably adverse physiological impact was induced by pneumoperitoneum during laparoscopic procedures. The mean pressure in the iliac vein and inferior vena cava is normally 2–5 mmHg [29, 30]. The pneumoperitoneum during the TEP procedure increased intraabdominal pressure to a level of 12–14 mmHg, which had a direct compressive effect on the inferior vena cava and iliac veins and decreased lower extremity venous flow. There were few patients in this study that permitted a repeat Echo-Doppler for the following reasons: (1) Patients discharged earlier before Echo-Doppler could be arranged in the postoperative period. (2) The absence of the specified sonographer assigned to the study. (3) No consent for a repeat examination was permitted by the patient. We measured the change in flow parameters in both the iliac and femoral veins and observed a significant decrease in the iliac vein and common femoral vein flow velocity but not with increases in the vein cross-sectional area of the ipsilateral extremity. Our data are not completely in accordance with results of other laparoscopic procedures. Nguyen et al. reported that increased intraabdominal pressure during laparoscopic gastric bypass reduced peak femoral systolic velocity by 43% and increased the femoral cross-sectional area by 52% [31]. However, we observed morphologic change of the iliac vein during TEP procedure as illustrated in Fig. 5. The iliac vein was almost completely collapsed, which not only impaired venous return from the lower extremities but also caused vein distention. The acute distention caused vessel wall damage due to mechanical disruption of the endothelial lining. Vessel wall damage is one of Virchow’s triad in the pathogenesis of thrombosis [32, 33].
In this study, activated coagulation and impaired deep venous flow implied that the TEP procedure had a certain degree of potential risk for DVT during the early postoperative period. Zaghiyan et al. reported that the majority of early postoperative VTE occurred intraoperatively in patients undergoing major colorectal surgery [34]. Such a high risk for VTE, during operation and immediately following operation, may be similar for patients undergoing TEP, especially in the aged people. However, no VTE prophylaxis consensus for laparoscopic inguinal hernia has been identified. Generally, inguinal herniorrhaphy is considered a VTE low-risk procedure, and with the worry of a bleeding risk associated with chemical thromboembolic prophylaxis [35], thromboprophylaxis may be omitted [36]. Interestingly, in a survey of general surgeons practicing in Ontario, 85% did not provide DVT prophylaxis in open inguinal hernia repair. This percentage dropped to 63.7% in LIHR, reflecting a more aggressive approach to DVT prophylaxis when compared to the open procedure [37]. In this cohort, an absence of clinical VTE, in spite of a dramatic change in coagulation and venous flow velocity, may be partially explained by mandatory early ambulation and IPC following operation. Milic et al. suggested that with regard to postoperative development of DVT of the calf, ambulation activates the fibrinolytic system, preventing the thrombi from increasing in size and proximal propagation [38].
ACCP guidelines recommend the endorsement of the Caprini VTE scoring model for calculation of overall risk. According to ACCP guidelines, patients with 1–2 points are at low risk. Mechanical prophylaxis, preferably with IPC, is recommended over no prophylaxis. Patients with 3–4 points are at moderate risk for VTE. LMWH, LDUH, or mechanical prophylaxis, preferably with IPC, are recommended over no prophylaxis. For patients at high risk for major bleeding complications, mechanical prophylaxis like IPC is recommended. For patients at high risk for VTE (points ≥ 5) who are not at high risk for major bleeding complications, pharmacologic prophylaxis combined with mechanical prophylaxis like elastic stockings or IPC is recommended. For patients at high risk for major bleeding complications, guidelines suggest the use of mechanical prophylaxis, preferably with IPC, over no prophylaxis until the risk of bleeding diminishes and pharmacologic prophylaxis can be initiated. In this study, we excluded all variables but age and duration of operation, which balanced the data for statistical analysis and provided a range of two to five points. However, all patients enrolled in this study were not tested for thrombophilia (not available in our hospital’s laboratory), which is a study weakness that may have underestimated actual VTE risk. Hypercoagulability may link to certain genetic traits. More than half of all patients with juvenile or idiopathic VTE have been identified to have an inherited thrombophilic condition [15]. Deficiencies of antithrombin III, protein C, protein S, mutation of factor V Leiden, and factor II (prothrombin) G20210A genes leads to hypercoagulability. Some of these inherited deficiencies such as presence of factor V Leiden, positive prothrombin 20210A represents three points each in Caprini risk model. We believe that in our cohort, more people would be categorized into high-risk group if the scores of hereditary thrombophilia factors were counted. However, in this study, all patients with a 5-point VTE risk score did not develop VTE with early ambulation and IPC, early administration of prophylaxis following operation proved to be essential. Coleman et al. recommended repeated risk assessment to facilitate appropriate VTE prophylaxis [39]. We believe that close observation is crucial for VTE prevention during the perioperative period. Risk scoring models may not have adequate precision and VTE risk may not be static during the perioperative period, which requires reconsideration of prophylaxis. In our previously case report, a 60-year-old patient, bedbound for over 20 h following TEP, unexpectedly developed a fatal PE. Bed rest longer than 72 h is weighted at two points by the Caprini risk scoring model. No other VTE risk factors were recorded. Hence, this patient only had a risk score of 3, not getting necessary prophylaxis. An unexpectedly prolonged recovery from anesthesia or an unusually prolonged bed rest should be considered factors that increase VTE risk in individual patients, although no specific recommendations are provided in current guidelines. A condition-specific VTE risk assessment tool for ventral hernia repair does exist [40], in which a 25-fold variability in VTE risk was found among the overall population. Risk assessment for LIHR should include the specific type of laparoscopic procedure as well as operation-related parameters such as mechanical ventilation [41], anesthesia, and pneumoperitoneum duration for VTE. More detailed information for vulnerable subgroups is desirable, with dynamic stratification of the risk for VTE before and after operation.
The incidence of VTE following LIHR is low. Activated coagulation and hampered lower extremity deep venous flow immediately following TEP represent potential risk factors for VTE. Hence, LIHR may be associated with VTE events. Considering the large number of inguinal hernia surgeries and their importance to patient quality-of-life, VTE, although rare, should be carefully considered. Proper VTE risk stratification for LIHR and prophylaxis early after operation are essential and should always be considered by clinicians.
References
Stein PD, Henry JW (1995) Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 108(4):978–981
Colditz GA, Tuden RL, Oster G (1986) Rates of venous thrombosis after general surgery: combined results of randomised clinical trials. Lancet 2(8499):143–146
Rutkow IM (2003) Demographic and socioeconomic aspects of hernia in the United States in 2003. Surg Clin North Am 83(5):1045–1051
Bittner R, Schwarz J (2012) Inguinal hernia repair: current surgical techniques. Langenbecks Arch Surg 397(2):271–282. https://doi.org/10.1007/s00423-011-0875-7
Li J, Wang X, Feng X, Gu Y, Tang R (2013) Comparison of open and laparoscopic preperitoneal repair of groin hernia. Surg Endosc 27(12):4702–4710. https://doi.org/10.1007/s00464-013-3118-x
Karthikesalingam A, Markar SR, Holt PJ, Praseedom RK (2010) Meta-analysis of randomized controlled trials comparing laparoscopic with open mesh repair of recurrent inguinal hernia. Br J Surg 97(1):4–11. https://doi.org/10.1002/bjs.6902
Eker HH, Langeveld HR, Klitsie PJ, van’t Riet M, Stassen LP, Weidema WF, Steyerberg EW, Lange JF, Bonjer HJ, Jeekel J (2012) Randomized clinical trial of total extraperitoneal inguinal hernioplasty vs Lichtenstein repair: a long-term follow-up study. Arch Surg 147(3):256–260. https://doi.org/10.1001/archsurg.2011.2023
HerniaSurge Group (2018) International guidelines for groin hernia management. Hernia 22(1):1–165. https://doi.org/10.1007/s10029-017-1668-x
Mayer F, Lechner M, Adolf D, Öfner D, Köhler G, Fortelny R, Bittner R, Köckerling F (2016) Is the age of>65 years a risk factor for endoscopic treatment of primary inguinal hernia? Analysis of 24,571 patients from the Herniamed Registry. Surg Endosc 30(1):296–306. https://doi.org/10.1007/s00464-015-4209-7
Neumayer L, Giobbie-Hurder A, Jonasson O, Fitzgibbons R Jr, Dunlop D, Gibbs J, Reda D, Henderson W, Veterans Affairs Cooperative Studies Program 456 Investigators (2004) Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med 350(18):1819–1827
Nilsson H, Angerås U, Sandblom G, Nordin P (2016) Serious adverse events within 30 days of groin hernia surgery. Hernia 20(3):377–385. https://doi.org/10.1007/s10029-016-1476-8
Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM (2012) Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e227S–e277S. https://doi.org/10.1378/chest.11-2297
Humes DJ, Abdul-Sultan A, Walker AJ, Ludvigsson JF, West J (2018) Duration and magnitude of postoperative risk of venous thromboembolism after planned inguinal hernia repair in men: a population-based cohort study. Hernia 22(3):447–453. https://doi.org/10.1007/s10029-017-1716-6
Yang C, Zhu L (2017) Sudden death caused by acute pulmonary embolism after laparoscopic total extraperitoneal inguinal hernia repair: a case report and literature review. Hernia 21(3):481–486. https://doi.org/10.1007/s10029-017-1587-x
Anderson FA Jr, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107(23 Suppl 1):I9–I16
Caprini JA (2005) Thrombosis risk assessment as a guide to quality patient care. Dis Mon 51(2–3):70–78
Wells PS (2006) Advances in the diagnosis of venous thromboembolism. J Thromb Thrombolysis 21(1):31–40
Nguyen NT, Hinojosa MW, Fayad C, Varela E, Konyalian V, Stamos MJ, Wilson SE (2007) Laparoscopic surgery is associated with a lower incidence of venous thromboembolism compared with open surgery. Ann Surg 246(6):1021–1027
Alizadeh RF, Sujatha-Bhaskar S, Li S, Stamos MJ, Nguyen NT (2017) Venous thromboembolism in common laparoscopic abdominal surgical operations. Am J Surg 214(6):1127–1132. https://doi.org/10.1016/j.amjsurg.2017.08.032
Schietroma M, Carlei F, Mownah A, Franchi L, Mazzotta C, Sozio A, Amicucci G (2004) Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc 18(7):1090–1096
Boisclair MD, Ireland H, Lane DA (1990) Assessment of hypercoagulable states by measurement of activation fragments and peptides. Blood Rev 4(1):25–40
Owings JT, Gosselin RC, Battistella FD, Anderson JT, Petrich M, Larkin EC (2000) Whole blood D-dimer assay: an effective noninvasive method to rule out pulmonary embolism. J Trauma 48(5):795–799 (discussion 799–800)
Wakefield TW, Myers DD, Henke PK (2008) Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 28(3):387–391. https://doi.org/10.1161/ATVBAHA.108.162289
Taira T, Taira BR, Carmen M, Chohan J, Singer AJ (2010) Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med 28(4):450–453. https://doi.org/10.1016/j.ajem.2009.01.023
Shigemi D, Matsuhashi T, Yamada T, Kamoi S, Takeshita T (2017) Preoperative screening of thromboembolism using plasma D-dimer test and proximal vein compression ultrasonography in Japanese gynecologic patients. Ann Med Surg (Lond) 15:52–55. https://doi.org/10.1016/j.amsu.2017.02.003
Kimura Y, Oki E, Ando K, Saeki H, Kusumoto T, Maehara Y (2016) Incidence of venous thromboembolism following laparoscopic surgery for gastrointestinal cancer: a single-center, prospective cohort study. World J Surg 40(2):309–314. https://doi.org/10.1007/s00268-015-3234-y
Osaki T, Saito H, Fukumoto Y, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Sato K, Hirooka Y, Fujiwara Y (2018) Risk and incidence of perioperative deep vein thrombosis in patients undergoing gastric cancer surgery. Surg Today 48(5):525–533. https://doi.org/10.1007/s00595-017-1617-4
Ozmen M, Zulfikaroglu B, Ozalp N, Moran M, Soydinc P, Ziraman I (2010) Femoral vessel blood flow dynamics following totally extraperitoneal vs Stoppa procedure in bilateral inguinal hernias. Am J Surg 199(6):741–745. https://doi.org/10.1016/j.amjsurg.2009.03.025
Bais JE, Schiereck J, Banga JD, van Vroonhoven TJ (1998) Resistance to venous outflow during laparoscopic cholecystectomy and laparoscopic herniorrhaphy. Surg Laparosc Endosc 8(2):102–107
Jorgensen JO, Lalak NJ, North L, Hanel K, Hunt DR, Morris DL (1994) Venous stasis during laparoscopic cholecystectomy. Surg Laparosc Endosc 4(2):128–133
Nguyen NT, Cronan M, Braley S, Rivers R, Wolfe BM (2003) Duplex ultrasound asseseement of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc 17(2):285–290
Mammen EF (1992) Pathogenesis of venous thrombosis. Chest 102:640 s–644 s
Comerota AJ, Stewart GJ, Alburger PD, Smalley K, White JV (1989) Operative venodilation: a previously unsuspected factor in the cause of postoperative deep vein thrombosis. Surgery 106(2):301–308 (discussion 308–309)
Zaghiyan KN, Sax HC, Miraflor E, Cossman D, Wagner W, Mirocha J, Gewertz B, Fleshner P, Cedars-Sinai DVT Study Group (2016) Timing of chemical thromboprophylaxis and deep vein thrombosis in major colorectal surgery: a randomized clinical trial. Ann Surg 264(4):632–639. https://doi.org/10.1097/SLA.0000000000001856
Persson G, Strömberg J, Svennblad B, Sandblom G (2012) Risk of bleeding associated with use of systemic thromboembolic prophylaxis during laparoscopic cholecystectomy. Br J Surg 99(7):979–986. https://doi.org/10.1002/bjs.8786
Enoch S, Woon E, Blair SD (2003) Thromboprophylaxis can be omitted in selected patients undergoing varicose vein surgery and hernia repair. Br J Surg 90(7):818–820
Beekman R, Crowther M, Farrokhyar F, Birch DW (2006) Practice patterns for deep vein thrombosis prophylaxis in minimal-access surgery. Can J Surg 49(3):197–202
Milic DJ, Pejcic VD, Zivic SS, Jovanovic SZ, Stanojkovic ZA, Jankovic RJ, Pecic VM, Nestorovic MD, Jankovic ID (2007) Coagulation status and the presence of postoperative deep vein thrombosis in patients undergoing laparoscopic cholecystectomy. Surg Endosc 21(9):1588–1592
Coleman DM, Obi A, Henke PK (2015) Update in venous thromboembolism pathophysiology, diagnosis, and treatment for surgical patients. Curr Probl Surg 52(6):233–259. https://doi.org/10.1067/j.cpsurg.2015.04.003 doi
Pannucci CJ, Basta MN, Fischer JP, Kovach SJ (2015) Creation and validation of a condition-specific venous thromboembolism risk assessment tool for ventral hernia repair. Surgery 158(5):1304–1313. https://doi.org/10.1016/j.surg.2015.04.001
Rothberg MB, Lindenauer PK, Lahti M, Pekow PS, Selker HP (2011) Risk factor model to predict venous thromboembolism in hospitalized medical patients. J Hosp Med 6(4):202–209. https://doi.org/10.1002/jhm.888
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Chengguang Yang and Leiming Zhu have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Zhu, L. Coagulation and deep vein flow changes following laparoscopic total extraperitoneal inguinal hernia repair: a single-center, prospective cohort study. Surg Endosc 33, 4057–4065 (2019). https://doi.org/10.1007/s00464-019-06700-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06700-6