Abstract
Background
Prosthetic material (mesh) is commonly used to repair inguinal hernias. Its implantation close to the common femoral vein (CFV) can induce slow flow and favor the appearance of venous thromboembolism (VTE) events.
Aim
To investigate the speed of flow, diameter and area of the CFV after inguinal hernioplasty.
Methods
Two hundred and fifty patients receiving open hernioplasty with a non-resorbable mesh for the repair of a unilateral, primary, simple inguinal hernia were prospectively investigated. Patients were stratified, by consensus, into a low or a moderate risk of VTE group. The moderate-risk group (n = 163) received low molecular weight heparin. On day 10 post-operation a blinded Echo-Doppler was carried out, and repeated 7 days later in patients with a venous flow of <15 cm/s. The speed of flow (cm/s), diameter (cm), and area (cm2) of the ipsilateral and contralateral CFV of the groin operated upon were measured.
Results
No event symptomatic of VTE was documented. One case of asymptomatic deep vein thrombosis (1/163, 0.6 %) was found in the moderate-risk group. In 29 patients (2 and 27 in the low- and moderate-risk groups, respectively; p < 0.001) a maximum blood flow velocity of <15 cm/s was found in the ipsilateral CFV; these flows were close to normal in the second measurement. Taking the entire sample into account, the maximum venous blood flow found in the ipsilateral CFV of the operated groin was less than that measured in the contralateral CFV (20.88 vs. 24.01 cm/s; p < 0.001); this difference was significant in both VTE risk groups. The diameter and area of the CFV were both greater in the ipsilateral than the contralateral CFV (p < 0.01); this finding proved to be significant only in hernias of the left groin (p < 0.001).
Conclusions
In the immediate postoperative period, inguinal hernioplasty with mesh induces a temporarily slow venous flow in the ipsilateral CFV. However, this does not lead to an increase in the incidence of VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal hernia surgery is one of the procedures most frequently performed by general surgeons. In the USA, more than 700,000 people undergo this operation annually [1]. More than 70 open and endoscopic surgical techniques have been described for the repair of these hernias [2], although nowadays nobody doubts the greater effectiveness of the “tension-free mesh repair” techniques compared with the traditional method of dealing with tissues [3].
Among the various complications of hernioplasties, one that is often described is the constriction of the femoral vein and the possibility of venous thromboembolism (VTE) [4]. However, the available literature, though scarce, suggests that the frequency of post-inguinal hernia surgery VTE is low [5–8].
Nonetheless, the association of the different individual situations (predisposing or triggering) may increase the risk of VTE, through modification of Virchow’s etiopathogenic triad. Thus, the operative technique, especially the insertion of a foreign body (mesh), may induce a lesion and/or slow flow within the femoral vein. Taylor et al. [9] were the first to study the behavior of blood flow in the femoral veins after tension-free mesh repair hernioplasties. Bearing this knowledge fully in mind, our study centered upon measuring femoral vein blood flow in the period immediately after unilateral, primary hernioplasty (mesh) operations in two groups of patients with different levels of risk of VTE and who were receiving different prophylactic measures.
Materials and methods
This was an observational sub-study from a broader prospective study [10]. All included patients underwent repair of an inguinal hernia in the Major Ambulatory Surgery Unit of the Hospital Universitario de Salamanca, Spain. Exclusion criteria were age <18 years and an ASA risk score ≥3. The study was approved by the Hospital’s Ethical Committee.
Preoperative aspects
For each patient, a clinical history was obtained and the VTE risk stratification was determined according to the Spanish ASECMA consensus ( Table 1) [11], which classifies patients into one of two groups: low or moderate risk of VTE. Low molecular weight heparin (LMWH) (Bemiparin-Hibor®, subcutaneous, 2,500 UI/24 h, starting 6 h after surgery and continuing postoperatively for 7 days) was administered to patients in the moderate-risk group. All patients received written recommendation to do early, sustained gentle walking. Elastic compression stockings (pre- or post-surgery) were not prescribed. All patients signed an informed consent form to participate freely in the study.
Surgical technique
Hernias (direct, indirect or combined, Nyhus types I–III) were all unilateral, primary (non-recurrent) and simple (non-complicated). Repair was open in all cases (i.e., non-laparoscopic). Two techniques were employed [Rutkow–Robbins or Prolene Hernia System (PHS)], all of which had in common the use of a non-resorbable prosthetic mesh (usually made of polypropylene). The mesh was always by using slow-resorption stitches; adhesives were never used for this purpose. For anesthetic induction, the patient received 2 g of cephazoline IV (single dose). All interventions over a period of 6 months were carried out by the same surgical team of two senior surgeons and an anesthetic team.
Follow-up
Clinical evaluation, including a search for events symptomatic of VTE (deep vein thrombosis, DVT, and/or pulmonary embolism, PE), was carried out 10 and 30 days after surgery. Echo-Doppler color and helical computer tomography (CT) (only in the case of suspected PE) were also undertaken. The Echo-Doppler was done on the 10th day post-operation, this time being chosen with the aim of evaluating asymptomatic DVT. A Toshiba Aplio XG® ecograph with a linear multifrequency probe was used at a frequency of 7.5 MHz. Echographs were obtained in a blind manner by two expert sonographers who were unaware of the characteristics of the patient or the group to which they belonged.
Mode B transverse and longitudinal images were obtained of the common femoral vein (CFV) of the two extremities with the patient in a supine position. Since all patients underwent unilateral hernia repair, we compared the two sides. Wherever possible, venous compression was always continued until a complete collapse of the light was achieved. Subsequently, a spectral representation of the flow in both CFVs was obtained, paying particular attention to the morphological aspect of the wave. Flow velocity was measured at that location. To obtain the venous spectrum and the velocity in both CFVs, an attempt was made to modify the angle of incidence of the beam between 30° and 60° and the pulse-repetition frequency was adjusted to the minimum possible to avoid artifacts from “aliasing”. After correcting the angle we obtained a measure from the highest region of the curve that corresponded to the expiratory phase and that favored venous return [12]. Having measured the venous flow and diameter of the CFV, the peak blood velocities (cm/s) and cross-sectional area (cm2) were calculated [13, 14]. These are established parameters for measuring a prethrombotic state characterized by slow venous blood flow venous stasis [15].
A second Echo-Doppler, 7 days after the first, was taken when venous flows <15 cm/s were noted. Patients in the moderate-risk group continued their prophylaxis with LMWH.
Statistical analysis
Data were compiled in a File-Maker ProAdvanced database. PASW version 18 (IBM, New York, USA) was used for statistical analyses, which included summary statistics (mean and standard deviation), Chi square tests, and Student’s paired- and independent-samples t tests to compare groups when comparing flows from both extremities in the same individual, or in one extremity in patients with different risks of DVT, respectively. Statistical significance was concluded for values of p < 0.05.
Results
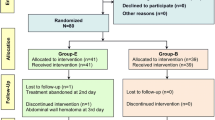
Of the 250 eligible patients, 218 (87.2 %) completed the study. These were split into 55 (25.2 %) and 163 (74.8 %) patients in the low and moderate VTE risk groups, respectively (Fig. 1). Table 2 summarizes the basal data of the two groups. The surgical and anesthetic techniques used were similar in the two groups.
No symptomatic VTE was observed. Only one asymptomatic DVT case was found (in CFV) in the moderate-risk group, representing an incidence of 0.6 % (1 of 163 patients). No helical CT was carried out during follow-up, as no symptoms or signs suggesting PE were noted (Table 3). The other postoperative complications are summarized in Table 3. At the same time, a reduction in venous flow (<15 cm/s) was seen in one of the CFVs examined (always ipsilateral to the surgery) in 29 patients (13.3 %). The proportion was significantly higher in the moderate ETV risk group (16.6 vs. 3.6 %; p < 0.001). Two weeks after surgery (second Echo-Doppler) all patients had femoral venous flows >15 cm/s (Table 4).
Considering all patients, the maximum velocity of venous flow determined in the CFV of the ipsilateral extremity (that of the operated groin), was lower than that measured in the contralateral CFV (20.88 vs. 24.01 cm/s; p < 0.001). This difference proved to be significant in both ETV risk groups (Table 5). The diameter and area of the CFV were both significantly greater in the ipsilateral than in the contralateral leg (p < 0.01). However, this was significant only in hernias arising in the left groin (p < 0.001) (Table 6).
Discussion
The present study shows, in a large group of patients, how inguinal hernioplasty with mesh induces slow venous flow at the level of the CFV ipsilateral to surgery in the immediate postoperative period.
The hypothesis that the reduction of femoral venous flow has repercussions for the incidence of VTE has been investigated, in previous decades, in the context of laparoscopic surgery in which the flow of the CFV could be altered by the pneumoperitoneum and/or the positions of the patient during the procedure. In this context, it was shown that the flow of CFV was significantly reduced during intraperitoneal insufflation, but not during preperitoneal insufflation [16]. Recently, the behavior of femoral venous flow in other types of open surgery has also been investigated [17, 18]. Finally, it is interesting to know how epidural anesthesia compensates for the reduction of femoral flow detected when the laparoscopic technique is carried out under general anesthesia [19].
The introduction of meshes in the repair of inguinal hernias, and their direct contact with the femoral veins and their branches, prompted some researchers to study the effect of the mechanical factor of the prosthesis, their contraction (20–75 % of the original size) and the reaction of the tissue (inflammatory process) to blood flow in the femoral artery and vein. None of the studies of testicular flow have shown negative effects [20–22]. Taylor et al. [9] were the first to study venous flow and found no evidence to support the hypothesis that the contraction of the mesh implanted in the groin had a negative effect on femoral venous flow. More recently, Ozmen et al. [23, 24] have published two studies in the same area. However, several aspects of these studies require comment.
Although our study used similar instrumentation (Echo-Doppler color) and determinations (velocity and diameter of the CFV), it featured some important differences from the aforementioned studies. First, we carried out the determinations in the immediate postoperative period and not 6 months [23, 24] or 3 years [9] after the procedure; second, we always had a control (the contralateral femoral vein) available, since our repairs were always unilateral rather than always bilateral [24]; finally, none of the other studies mentioned considered VTE risk factors or whether they used some type of prophylaxis, although none of the publications of Ozmen et al. [23, 24] reported any symptomatic cases of VTE. The commentary of Mangano et al. [25] concerning these studies is clear: the constriction of the femoral vein by meshes requires further study. In our opinion, late determination (whilst pursuing other objectives) and the lack of VTE risk stratification (which is higher in bilateral than in unilateral hernioplasties) has limited the interest in studying whether venous stasis is induced by the mesh and postoperative VTE.
Sulaimanov et al. [26] experimentally observed the effect of polypropylene mesh on femoral venous flow, 14, 28 and 90 days after implantation and found a significant reduction in flow 14 days after the operation; this reduction was no longer apparent at 28 or 90 days. In our study we observed that the most severe cases of flow reduction (<15 cm/s) recovered after 1 week. The clinical observation of Pannuchi et al. [18] was of a similar nature, whereby the reduction in femoral venous flow after abdominal pressure persisted for 48 h after surgery. However, this recovery of flow did not detract from the importance of the temporary effect of venous stasis and its possible repercussions for VTE during the immediate postoperative period.
To explain our main finding in relation to the mesh, fundamentally with the inflammatory response that occasions its insertion, we have ruled out the influence of local postoperative complications or complications arising from the LMWH (moderate-risk group) on reducing venous flow as a consequence of compression. In four patients with scrotal hematoma, the mean venous flow did not differ significantly from that in their respective risk groups. Hemorrhages from the operation wound, which were more frequent in the LMWH group, were always superficial, as was the presence of abdominal ecchymosis (distant from the area of the incision) consecutive to the application of the LMWH.
In addition, we have compared the blood flow of the patients in this study with two other groups at risk of VTE (surgery for sacrococcygeal pilonidal sinus disease and epigastric or umbilical hernia) and with determinations carried out under the same conditions. The results were significant in showing a mean slowing of blood flow by −7.17 and −4.55 cm/s in the low- and moderate-risk groups of patients who underwent inguinal hernia surgery with mesh (Table 7).
Two other related findings also warrant comment. First, the fact that the group at moderate risk of VTE was significantly older on average suggests that the reduction in blood flow in the CFV may increase with age. Second, since hernias in the left groin presented significantly more frequently than in the right, the greater slowing of venous flow, as demonstrated by a bigger diameter and cross-sectional area of the CFV, may be due to the anatomical fact that the left iliac vein is anteriorly crossed by the iliac artery, leading to the worse emptying of the former.
Although our study shows the temporary presence of ipsilateral femoral venous stasis, this did not result in an increase in VTE, possibly because the group most affected by the reduction in venous flow was classified as being at risk of VTE and received LMWH prophylaxis.
The scarcity of information about VTE after inguinal hernia is surprising; above all when we consider that this is one of the most frequently performed surgical procedures and so some patients must inevitably be at risk of VTE. Few of the many existing consensuses are concerned with the need to identify at risk patients and to determinate the most appropriate prophylaxis for them [27, 28]. At the same time we should not forget that, for many years, VTE has been one of the conditions most frequently associated with problems of medical malpractice and litigation [29].
For these reasons, it is important to note that the few published studies about the frequency of VTE and hernioplasties are all retrospective and do not evaluate the impact of new technical innovations [30]. Furthermore, the even more scarce publications about thromboprophylaxis related to this type of surgery report poorly designed studies [7, 31, 32]. This means that no clear recommendations exist, even in the most recent consensuses [33, 34]. However, surgeons are aware of the increasing number of patients with comorbidity and of young people with VTE risk factors who receive hernia interventions. Given this, the concern expressed in questionnaires carried out in various European and American countries, above all about the variability found when trying to determine risk and propose prophylaxis, is not surprising [35–39]. All these surveys concluded by calling for a consensus for action, prompting a group of Spanish experts to publish one several years ago [11]. This was partially validated by our group [10] and a second version of it was subsequently developed [11].
Despite the findings of our study, there does not appear to be a consequent increase in the risk of VTE, especially for patients who are well stratified (and protected) by their degree of risk. Logically, we do not know the clinical result of the group of with-risk patients with reduced blood flow of <15 cm if they have not received LMWH prophylactically. The meta-analysis of Johnson et al. [40] shows how a first negative Echo-Doppler in patients without antithrombotic treatment reduces the risk of VTE to 0.57 % after 3 months. Nevertheless, patients in our study in whom slow venous flow was detected were subjected to a second Echo-Doppler, which, in all cases, showed values that were closer to normal. In other words, the slow flow was alleviated, without physical measures other than gentle walking, 2 weeks after surgery.
One of the limitations of our study is that two distinct techniques and two anesthetic methods were used, although these were equally distributed across the two risk groups. Another possible limitation of this study was that we did not include Lichtenstein repair (customarily considered as the gold standard), since this technique has for years only been infrequently used in our institution. Recent meta-analyses have compared open hernioplasties with mesh (Lichtenstein type) vs. mesh-plug (Prolene Hernia System type) [41, 42] and found similar results.
In summary, in the immediate postoperative period following a hernioplasty with mesh, patients experience transitory slow femoral venous flow. This represents an additional risk factor for VTE, and means that hernioplasties are not exempt from being a cause of VTE events, for which reason risk must be stratified to make a decision concerning the appropriate actions.
References
Stylopoulos N, Gazelle GS, Rattner DW (2003) A cost-utility analysis of treatment options for inguinal hernia in 1,513,008 adult patients. Surg Endosc 17:180–189
Read RC (2009) Herniology: past, present, and future. Hernia 13:577–580
Amid PK (2005) Groin hernia repair: open techniques. World J Surg 29:1046–1051
Nissen HM (1975) Constriction of the femoral vein following inguinal hernia repair. Acta Chir Scand 141:279–281
Riber C, Alstrup N, Nymann T et al (1996) Postoperative thromboembolism after day-case herniorrhaphy. Br J Surg 83:420–421
Wessel N, Gerner T (1996) Thromboembolic complications in ambulatory surgery. A retrospective study of 1691 patients. Tidsskr Nor Laegeforen 116:615–616
Enoch S, Woon E, Blair SD (2003) Thromboprohylaxis can be omitted in selected patients undergoing varicose vein surgery and hernia repair. Br J Surg 90:818–820
Engbaek J, Bartholdy J, Hjortso NC (2006) Return hospital visits and morbidity within 60 days after day surgery: a retrospective study of 18,736 day surgical procedures. Acta Anaesthesiol Scand 50:911–919
Taylor SG, Hair A, Baxter GM, O’Dwyer PJ (2001) Does contraction of mesh following tension free hernioplasty effect testicular or femoral vessel blood flow? Hernia 5:13–15
Lozano FS, Sánchez J, Santos JA et al (2010) Venous thromboembolism risk stratification and thromboprophylaxis with low molecular weight heparin in patients undergoing major ambulatory surgery: an observational prospective study. Ambul Surg 16:5–12
Raich M, Bustos F, Castellet E et al (2011) Actualización de las recomendaciones de tromboprofilaxis en cirugía mayor ambulatoria. Cir May Amb 16:23–29
Cozcolluela MR, Sarría L, Sanz L et al (2000) Correlation of central venous pressure with Doppler waveform of the common femoral veins. J Ultrasound Med 19:587–592
Beebe DS, McNevin MP, Crain JM et al (1993) Evidence of venous stasis after abdominal insufflation for laparoscopic cholecystectomy. Surg Gynecol Obstet 176:443–447
Ido K, Suzuki T, Kimura K et al (1995) Lower-extremity venous stasis during laparoscopic cholecystectomy as assessed using color Doppler ultrasound. Surg Endosc 9:310–313
Güleç B, Oner K, Yigitler C et al (2006) Lower extremity venous changes in pneumoperitoneum during laparoscopic surgery. ANZ J Surg 76:904–906
Morrison CA, Schreiber MA, Olsen SB et al (1998) Femoral venous flow dynamics during intraperitoneal and preperitoneal laparoscopic insufflation. Surg Endosc 12:1213–1216
Pannucci CJ, Henke PK, Cederna PS et al (2011) The effect of increased hip flexion using stirrups on lower-extremity venous flow: a prospective observational study. Am J Surg 202:427–432
Pannucci CJ, Alderman AK, Brown SL et al (2012) The effect of abdominal wall plication on intra-abdominal pressure and lower extremity venous flow: a case report. J Plast Reconstr Aesthet Surg 65:392–394
Senoglu N, Yuzbasioglu MF, Oksuz H et al (2010) Effects of epidural-and-general anesthesia combined versus general anesthesia alone on femoral venous flow during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 20:219–223
Dilek ON, Yucel A, Akbulut G, Degirmenci B (2005) Are there adverse effects of herniorrhaphy techniques on testicular perfusion? Evaluation by color Doppler ultrasonography. Urol Int 75:167–169
Ramadan SU, Gokharman D, Tuncbilek I et al (2009) Does the presence of a mesh have an effect on the testicular blood flow after surgical repair of indirect inguinal hernia? J Clin Ultrasound 37:78–81
Koksal N, Altinli E, Sumer A et al (2010) Impact of herniorraphy technique on testicular perfusion: results of a prospective study. Surg Laparosc Endosc Percutaneous Tech 20:186–189
Ozmen MM, Ozalp N, Zulfikaroglu B et al (2004) The evaluation of the peak flow velocity and cross-sectional area of the femoral artery and vein following totally extraperitoneal vs preperitoneal open repair of inguinal hernias. Hernia 8:332–335
Ozmen M, Zulfikaroglu B, Ozalp N et al (2010) Femoral vessel blood flow dynamics following totally extraperitoneal vs Stoppa procedure in bilateral inguinal hernias. Am J Surg 199:741–745
Mangano A, Rausei S, Dionigi G (2012) Femoral vessel blood flow dynamics after totally extraperitoneal versus Stoppa procedure in bilateral inguinal hernias: the need for evidence-based surgery. Am J Surg 204:809
Sulaimanov M, Genc V, Cakmak A et al (2010) The effects of polypropylene mesh on femoral artery and femoral vein in mesh repair. Hernia 14:629–634
Sammama CM, Albadalejo P, Benhamou D et al (2006) Venous thromboembolism prevention in surgery and obstetrics: clinical practice guidelines. Eur J Anaesthesiol 23:95–116
Scottish Intercollegiate Guidelines Network (SIGN) (2002) Prophylaxis of venous thromboembolism: a national clinical guideline. Publication No. 62. http://www.sign.ac.uk. Accessed 31 March 2008
Hyers TM (1999) Venous thromboembolism. Am J Respir Crit Care Med 159:1–14
Ahonen J (2007) Day surgery and thromboembolic complications: time for structured assessment and prophylaxis. Curr Opin Anaesthesiol 20:535–559
Wright DM, O’Dwyer PJ, Pateson CR (1998) Influence of injection site for low dose heparin on wound complication rates after inguinal hernia repair. Ann R Coll Surg Engl 80:58–60
Hidalgo M, Figueroa JM (2000) Prophylaxis of venous thromboembolism in abdominal wall surgery. Hernia 4:242–247
Gould MK, Garcia DA, Wren SM et al (2012) Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e227S–277S
Nicolaides AN, Fareed J, Kakkar AK et al (2013) Prevention and treatment of venous thromboembolism. International consensus statement. General, vascular, bariatric and plastic surgical patients. Int Angiol 32:117–128
Anwar S, Scott P (2003) Current practice for anticoagulation prophylaxis in inguinal hernia surgery: a questionnaire survey. NZ Med J 116:U583
Raich M, Martínez J, Bustos F (2004) Encuesta nacional sobre la prevención de la enfermedad tromboembólica venosa en cirugía mayor ambulatoria. Cir May Amb 9:31–36
Beekman R, Crowther M, Farrakhyar F, Birch DW (2006) Practice patterns for deep vein thrombosis prophylaxis in minimal-access surgery. Can J Surg 49:197–202
Shabbir J, Ridgway PF, Shields W et al (2006) Low molecular weight heparin prophylaxis in day case surgery. Ir J Med Sci 175:26–29
Wasowicz-Kemps DK, Biesma DH, Schangen van Leeuwn J, Van Ramshorst B (2006) Prophylaxis of venous thromboembolism in general and gynecological day surgery in the Netherlands. J Thromb Haemost 4:269–271
Johnson SA, Stevens SM, Woller SC et al (2010) Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: a systematic review and meta-analysis. JAMA 303:438–445
Li J, Ji Z, Li Y (2012) Comparison of mesh-plug and Lichtenstein for inguinal hernia repair: a meta-analysis of randomized controlled trials. Hernia 16:541–548
Sanjay P, Watt DG, Ogston SA et al (2012) Meta-analysis of Prolene Hernia System mesh versus Lichtenstein mesh in open inguinal hernia repair. Surgeon 10:283–289
Acknowledgments
We are grateful to S. A. Rovi, for the technical aspects of the project, and to Phil Mason for language assistance.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lozano, F.S., Sánchez-Fernández, J., González-Porras, J.R. et al. Slow femoral venous flow and venous thromboembolism following inguinal hernioplasty in patients without or with low molecular weight heparin prophylaxis. Hernia 19, 901–908 (2015). https://doi.org/10.1007/s10029-015-1353-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-015-1353-x