Abstract
Background
There is limited and conflicting data on the optimal intervention for the treatment of achalasia in adolescents and young adults (AYA), Heller myotomy (HM), esophageal dilation (ED) or botulinum toxin injection (botox). The goal of this study is to determine the most appropriate index intervention for achalasia in the AYA population.
Methods
We completed a longitudinal, population-based analysis of the California (2005–2010) and New York (1999–2014) statewide databases. We included patients 9–25 years old with achalasia who underwent HM, ED or botox. Comparisons were made based on the patients’ index procedure. Rates of 30-day complications, long-term complications, and re-intervention up to 14 years were calculated. Cox regression was performed to determine the risk of re-intervention, adjusting for patient demographics.
Results
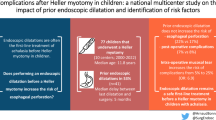
A total of 442 AYAs were analyzed, representing the largest cohort of young patients with this disease studied to date. Median follow-up was 5.2 years (IQR 1.8–8.0). The overall rate of re-intervention was 29.3%. Rates of re-intervention for ED and botox were equivalent and higher than HM (65.0% for ED, 47.4% for botox and 16.4% for HM, p < 0.001). Ultimately, 46.9% of ED and botox patients underwent HM. The overall short-term complication rate was 4.3% and long-term, 1.9%. There was no difference in the short-term and long-term complication rates between intervention groups (p > 0.05). On adjusted analysis, ED and botox were associated with increased risks of re-intervention when compared to HM (HR 5.9, HR 4.8, respectively, p < 0.01). Black patients were found to have a risk of re-intervention twice that of white patients (HR 2.0, p = 0.05).
Conclusions
HM has a similar risk of complications but a significantly lower risk of re-intervention when compared to ED and botox. Based on our findings, we recommend HM as the optimal index procedure for AYAs with achalasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Achalasia is an idiopathic disease of esophageal motility that remains rare in adolescents and young adults (AYA). The incidence rate ranges between 0.1–1.8/100,000 children/year [1,2,3], with 0.25/100,000 children hospitalized annually [4]. The most common symptoms of achalasia are regurgitation and dysphagia [5]. Almost all AYA patients with achalasia will eventually undergo either Heller myotomy (HM), esophageal dilation (ED) or botulinum toxin injection (botox) [2, 6,7,8].
The limited available literature suggests that HM is superior to ED and botox for the treatment of achalasia in AYAs [7, 9,10,11]. However, these studies are single-institutional with small sample sizes thus making their generalizability unclear. Indeed, a systematic review of the literature on this topic covering the last 30 years included only 164 patients less than 20 years old and showed mixed conclusions, with one favoring ED, two favoring HM, and four reporting inconclusive findings [9]. Two recently published population-based studies suggest that fewer re-interventions are required after HM than ED or botox in adults, however AYAs were excluded from both studies [12, 13]. A large randomized controlled trial focusing on therapeutic success using Eckardt and quality of life scores showed equivalent therapeutic success with HM and ED, but this paper also excluded AYA patients [14]. Additionally, systematic reviews in the adult literature also have reached conflicting conclusions regarding optimal treatment for achalasia [15,16,17]. Given the paucity of data in AYAs, and conflicting data in adults, it remains unclear if there is a superior index treatment for achalasia in AYAs.
The goal of this study was to determine the most appropriate index intervention for achalasia in AYAs at a population level. We assessed the re-intervention rates and both short-term and long-term complications rates after Heller myotomy, esophageal dilation, or botox injection.
Materials and methods
A retrospective longitudinal analysis was performed using the California Office of Statewide Health Planning and Development (OSHPD) database from 2005 to 2010 and the New York Statewide Planning and Research Cooperative System (SPARCS) from 1999 to 2014. OSHPD and SPARCS contain all inpatient admissions and outpatient procedures performed in public and private hospitals in the state of California (n = 70) and New York (n = 66), excluding US military and Veterans Affairs hospitals. Both inpatient admissions and outpatient procedures were queried. In both databases, all patient data were de-identified with a unique record linkage ID that allows patients to be tracked longitudinally through time and in all in-state hospitals.
The inclusion criteria were patients between age 9 and 25 with a diagnosis of achalasia who underwent HM, ED or botox. To ensure that the index procedure we identified was the first that the patient received, we included a run-in period of at least one year. This method ensures that the patient has not undergone any procedure within one year prior to our index intervention. We excluded patients who had an esophagectomy or esophageal cancer prior to the index procedure.
Achalasia was defined by International Classification of Procedures, Ninth Revision, Clinical Modification (ICD-9) diagnosis code 530.0. HM included ICD-9 procedural codes of esophagomyotomy (42.7) and Current Procedural Terminology (CPT) codes 43330, 43279, 43331, 32665 and S2079. ED included ICD-9 procedural code 42.92 and CPT codes 43220, 43226, 43458, 43453, and 43233. Botox was defined by ICD procedural code 99.29 and CPT code 43236.
Re-intervention for recurrent symptoms of achalasia was defined as a second HM (with or without fundoplication), ED, botox or fundoplication after the index operation. Fundoplication was defined as a fundoplication without a concurrent HM and included ICD-9 codes 44.66 and 44.67, and CPT codes 43280 and 43327. All subsequent HM, ED or botox were considered re-interventions despite the understanding that some may be part of a planned series of treatments [14]. We included both planned or unplanned treatment because either reasons contributes to the morbidity experienced by AYAs with achalasia and thus is not clinically insignificant.
Short-term, 30 day post-operative complications, were captured for the index intervention only and included operative repair for surgical complications, intraoperative iatrogenic injury, infection, wound complication (including non-healing wound), hemorrhage and esophagectomy. Long-term complications included operative repair for surgical complications and esophagectomy between 30-days and up to 14 years after the index procedure.
Operative repair for surgical complications was defined by the following ICD-9 codes: insertion of permanent tube into esophagus (42.81), suture of laceration of esophagus (42.82), closure of esophagostomy (42.83), repair of esophageal fistula, not elsewhere classified (42.84), repair of esophageal stricture (42.85), production of subcutaneous tunnel without esophageal anastomosis (42.86), other graft of esophagus (42.87), and other repair of esophagus (42.89). Intraoperative iatrogenic injury included ICD-9 diagnosis codes of accidental puncture or laceration during a procedure (998.2). Infection was defined by the following ICD-9 diagnosis codes: infected postoperative seroma (998.51), other postoperative infection (998.59), mediastinitis (519.2), empyema with fistula (510.0), empyema without fistula (510.9). Wound complications included ICD-9 diagnosis codes of, disruption of wound, unspecified (998.30), disruption of internal operation (surgical) wound (998.31), disruption of external operation (surgical) wound (998.32), non-healing surgical wound (998.83), and seroma complicating a procedure (998.13). Hemorrhage included ICD-9 diagnosis codes of hemorrhage complicating a procedure (998.11), hematoma complicating a procedure (998.12) and ICD-9 procedural codes of suture of unspecified blood vessel (39.30), suture of artery (39.31), suture of vein (39.32), and hemorrhage control (39.98). Esophagectomy was defined by ICD-9 codes 42.40, 42.41, 42.42, 42.51, 42.52, 42.53, 43.54, 42.55, 42.56, 42.58, 42.59, 42.61, 42.62, 42.63, 42.64, 42.65, 42.66, 42.68, and 42.69.
Re-intervention and complication rates were calculated using the Kaplan Meier failure function. Unadjusted analysis included Student’s t test, Chi square test and log-rank test. Adjusted analysis was performed using Cox proportional hazards regression to predict risk of re-intervention, adjusting for index intervention, age, race, operative setting and insurance types. Statistical analysis was performed with Stata SE statistical software, version 13.1 (StataCorp LP, College Station, TX). IRB approval was granted from the state of California (IRB identifying number: 16-05-2558) and from the state of New York (IRB identifying number: 2017P001211).
Results
We identified a total of 442 AYAs with achalasia who had surgical interventions. Approximately three-fourths of the index procedures were HM (75.8%) with one-fifth of patients undergoing ED (18.3%), and far fewer undergoing botox (5.9%). The average age at index intervention was 19.1 (± 4.3) years old (Table 1).
On unadjusted comparison, there was no difference in age or gender between intervention groups (p > 0.05). However, there was a significant difference in the index intervention between races. Black patients underwent HM less frequently (60.4%) when compared to other races (p < 0.01). In addition, compared to patients with private insurance, patients with Medicaid or who were self-pay had HM less frequently (67.6% for Medicaid, 60.7% for Self-pay versus 80.5% for private insurance; p < 0.01) (Table 2).
The median follow-up time was 5.2 years (IQR 1.8–8.0). Overall re-intervention rate was 29.3%. The re-intervention rates for ED and botox were similar (p = 0.43) but significantly higher than HM (p < 0.001) (Fig. 1). The rate of repeat ED within 6 months was 13.9%.
The overall unadjusted 30-day complication rate was 4.3%, (HM 4.5%, ED 3.7%, and botox 3.9%) and there was no difference in the 30-day complication rates between index procedures (p = 0.95). The most common complications were operative repair of surgical complications (2.7%), followed by iatrogenic injuries (2.3%). The long-term complication rate was 1.9% with 1.3% of patients requiring operative repair of surgical complications and two patients (0.6%) ultimately undergoing esophagectomy. Similar to short term complication rates, there is no difference in the long-term complication rates between index procedures (p = 0.64) (Table 3). Notably, the complication rate of Botox may be elevated because of its small sample size (n = 26). The 3.9% complication rate of Botox represents one patient who developed a short-term complication, and similarly one who developed a long-term complication (these complications occurred in two different patients).
On adjusted analysis, ED and botox had a higher risk of re-intervention when compared to HM (HR 5.9, HR 4.8, respectively, p < 0.01). In addition, the risk of re-intervention in black patients was two times that of white patients, even after adjusting for index intervention, age, operative settings and insurance types (OR 2.0, p = 0.05) (Table 4).
Discussion
This study reports a large, multi-institutional population review of re-intervention rates and complication rates in AYA patients with achalasia. The overall re-intervention rate was 29.3%. Overall short-term and long-term complication rates were low (4.3% and 1.9% respectively) and no difference was found in the short-term and long-term complication rates between different index interventions (all p > 0.05). The high rates of patients requiring HM after ED (23.0%) and botox (23.9%) suggest that the longevity of therapeutic success for ED and botox is limited. This study shows that the risk–benefit ratio of HM is superior to ED and botox, and thus should be recommended as the index procedure for AYA patients with achalasia.
In this study, HM was found to have the lowest re-intervention rate. Our findings are consistent with prior literature in both adults and children [2, 7, 12, 13, 15, 17, 18], and similar to a large systematic review of pediatric HM (16.4% vs. 15%) [19]. In the calculation of re-intervention rate for ED, some clinicians may argue that planned sequential ED should not be counted as re-interventions. However, we argue that sequential ED repeatedly exposes patients to the morbidity of anesthetic exposures and invasive procedures whether the procedure is planned or unplanned. Notwithstanding, if all dilations occurring within 6 months of the index procedure are excluded, the overall rate of re-intervention for ED remains higher than that of HM (51.1% vs. 16.4%). In addition, anecdotal observations have suggested that botox may increase the technical difficulty of subsequent HM, despite conflicting results in the literature [20,21,22,23]. However, given the poor performance and high complications rates of botox seen in this cohort, physicians should only consider botox for AYA patients that have strong medical contraindications to HM. The rates of severe complications, such as esophageal perforation or esophagectomy, were extremely low in our study and in the literature [12, 14]. Overall, our complication rates may be more representative of the AYA population when compared to the current literature given our large sample size, multi-institutional data and long-term follow up [2, 7,8,9, 24,25,26,27].
Interestingly, we found that both the black and Medicaid populations had higher rates of index ED and botox compared to patients with private insurance. Additionally, after accounting for differences in index interventions and insurance types, we found that only black patients had a significantly higher risk of receiving a second intervention, while the effect of insurance was no longer significant. Often these types of disparities are attributable to lack of access to care, provider treatment preferences, or cultural perceptions of medical interventions. However, assessing the contribution of these factors in the outcomes of AYA patients with achalasia was beyond the scope of the study.
Our study has several strengths. First, it is generalizable and less biased than single institution studies as we included a heterogeneous population across multiple socioeconomic, ethnic and geographic domains. As our study tracked patients up to 14 years and across hospitals, it is also more representative of the true rates of re-interventions and complications than the current literature. Furthermore, while most literature only reported major complications such as esophageal perforation, we reported a more granular list of complications. The use of large population datasets introduces some limitations to our analysis, such as the accuracy of the coding of complications. Although data relying on pre-determined codes can potentially underestimate true complication rates because of variance in physician reporting, the alternative approach of chart review also introduces some bias. For example, chart review can be influenced by a single person who reviews the charts, while a population database is collected by people from multiple institutions and thus mitigates the bias introduced by a single reviewer. In addition, people may be concerned that our definition of ED includes a mixture of pneumatic dilation, endoscopic balloon dilation or bougies and thus may affect rates of complication and re-intervention. As pneumatic dilation is the routine method for endoscopic treatment of achalasia, it would be unexpected for other treatments to comprise a significant proportion of the captured ED procedures. However, there are no ICD-9 codes to differentiate methods of dilation, therefore it is possible that the ED re-intervention and complication rates might change if pneumatic dilation could be examined alone. If a large portion of the ED patients do not undergo pneumatic dilation, then it is possible that we could have over-estimated the re-intervention rate but under-estimated that complication rates. However, given that the hazard ratio for re-intervention for the ED group relative to HM group is 5.9, unless the re-intervention rate for ED decreases by more than 80%, then HM would still have a more favorable risk benefit ratio. Lastly, neither SPARCS nor OSHPD captured patients who received procedures in surgi-centers or outpatient offices and this may contribute to the low number of Botox patients. However, we do not expect a difference in outcomes between those who are captured versus who are not captured.
In conclusion, we provide important data for physicians and families regarding the risks and benefits of the treatment options for achalasia in AYAs. The outcomes of this study support HM as the initial intervention for AYAs with achalasia.
References
Marlais M et al., UK incidence of achalasia: an 11-year national epidemiological study. Arch Dis Child, 2010: p. archdischild171975
Smits M et al (2016) Pediatric achalasia in the Netherlands: incidence, clinical course, and quality of life. J Pediatr 169:110–115.e3
Mayberry J (2001) Epidemiology and demographics of achalasia. Gastrointest Endosc Clin N Am. 11(2): 235–248
Sonnenberg A (2009) Hospitalization for achalasia in the United States 1997–2006. Dig Dis Sci 54(8):1680–1685
Hallal C et al (2012) Diagnosis, misdiagnosis, and associated diseases of achalasia in children and adolescents: a twelve-year single center experience. Pediatr Surg Int 28(12):1211–1217
Molena D et al (2015) Hospitalization for esophageal achalasia in the United States. World J Gastrointest Endosc 7(13):1096–1102
Lee CW et al (2010) Outcomes of treatment of childhood achalasia. J Pediatr Surg 45(6):1173–1177
Hussain SZ, Thomas R, Tolia V (2002) A review of achalasia in 33 children. Dig Dis Sci 47(11):2538–2543
Sharp NE, St Peter SD (2016) Treatment of idiopathic achalasia in the pediatric population: a systematic review. Eur J Pediatr Surg 26(2):143–149
Zhang Y et al (2009) Diagnosis and management of esophageal achalasia in children: analysis of 13 cases. World J Pediatr 5(1):56–59
Grabowski A et al (2017) Pediatric achalasia. Single-center study of interventional treatment. Prz Gastroenterol 12(2):98
Markar SR et al (2018) Population-based cohort study of surgical myotomy and pneumatic dilatation as primary interventions for oesophageal achalasia. Br J Surg 105(8):1028–1035
Ehlers AP et al, Treatment A (2017) Outcomes, utilization, and costs: a population-based study from the United States. J Am Coll Surg 225(3):380–386
Boeckxstaens GE et al (2011) Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 364(19):1807–1816
Campos GM et al (2009) Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 249:45–47
Cheng J-W et al. (2017) Laparoscopic Heller myotomy is not superior to pneumatic dilation in the management of primary achalasia: conclusions of a systematic review and meta-analysis of randomized controlled trials. Medicine. 96(7): e5525
Schoenberg MB et al (2013) Laparoscopic Heller myotomy versus endoscopic balloon dilatation for the treatment of achalasia: a network meta-analysis. Ann Surg 258(6):943–952
Persson J et al (2015) Treatment of achalasia with laparoscopic myotomy or pneumatic dilatation: long-term results of a prospective, randomized study. World J Surg 39(3):713–720
Pacilli M, Davenport M (2017) Results of laparoscopic Heller’s myotomy for achalasia in children: a systematic review of the literature. J Laparoendosc Adv Surg Tech 27(1):82–90
Cowgill SM et al (2007) Difficult myotomy is not determined by preoperative therapy and does not impact outcome. JSLS 11(3):336
Patti MG et al (1999) Effects of previous treatment on results of laparoscopic Heller myotomy for achalasia. Dig Dis Sci 44(11):2270–2276
Bloomston M et al (2003) Preoperative intervention does not affect esophageal muscle histology or patient outcomes in patients undergoing laparoscopic Heller myotomy. J Gastrointest Surg 7(2):181–190
Smith CD et al., Endoscopic therapy for achalasia before Heller myotomy results in worse outcomes than Heller myotomy alone. Ann Surg 2006. 243(5):579
Berquist WE et al (1983) Achalasia: diagnosis, management, and clinical course in 16 children. Pediatrics 71(5):798–805
Franklin AL, Petrosyan M, Kane TD (2014) Childhood achalasia: a comprehensive review of disease, diagnosis and therapeutic management. World J Gastrointest Endosc 6(4):105
Corda L et al (2010) Laparoscopic oesophageal cardiomyotomy without fundoplication in children with achalasia: a 10-year experience. Surg Endosc 24(1):40–44
Di Nardo G et al (2012) Pneumatic balloon dilation in pediatric achalasia: efficacy and factors predicting outcome at a single tertiary pediatric gastroenterology center. Gastrointest Endosc 76(5):927–932
Author information
Authors and Affiliations
Contributions
Dr. Kelleher conceptualized the study, critically reviewed the data and drafted the initial manuscript, and reviewed and revised the final manuscript. Dr. Hung and Dr. Chang conceptualized the study, collected the data and carried out the initial data analysis, critically reviewed the data and drafted the initial manuscript, and reviewed and revised the final manuscript. Dr. Westfal critically reviewed the data, reviewed and revised the initial and final manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Disclosures
ML Westfal is financially supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Award Number: T32 DK007754) and by the Massachusetts General Hospital Department of Surgery Marshall K. Bartlett Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Y-C Hung, DC Chang, CM Kelleher have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hung, YC., Westfal, M.L., Chang, D.C. et al. Heller myotomy is the optimal index procedure for esophageal achalasia in adolescents and young adults. Surg Endosc 33, 3355–3360 (2019). https://doi.org/10.1007/s00464-018-06625-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-06625-6