Abstract
Achalasia is a rare esophageal motility disorder: its optimal treatment in children is still a matter of debate. Records of children treated for achalasia, over an 18-year period, were reviewed.
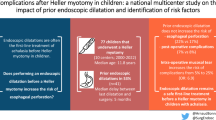
Forty-eight children (median age at diagnosis 10 years; range 3–17 years) were identified. Twenty-eight patients were initially treated with Heller’s myotomy (HM) and 20 with balloon dilatation (BD). At last follow-up (median 3 years; range 1–5.5 years), 43.8% (21/48) of children were symptom free. The number of asymptomatic children was significantly higher among those treated initially with HM compared to BD (HM 15/28, 53.6% BD 6/20, 30%, p < 0.05). All children who underwent BD required HM due to symptom recurrence. The median (range) total number of procedures was significantly higher in the BD group (BD 3 (1–7); HM 1 (1–5); p < 0.05) with a shorter time to the second intervention (BD 14 months, 95%CI 4–24; HM 58 months, 95%CI 38–79; p < 0.05). Of 108 procedures, esophageal perforation occurred in two children after HM (two out of 48 HM procedures in total, 4%) and one child after BD (1/60, 1.7%).
Conclusion: Less than half of children with achalasia are symptom free after initial treatment with either BD or HM. HM, however, when performed as first procedure, provided longer symptom-free period and reduced need for subsequent intervention.
What is Known: • Balloon dilatation (BD) and Heller’s myotomy (HM) are safe and effective treatment options for achalasia. • Controversy, however, exists regarding the most effective initial therapeutic approach. |
What is New: • HM with or without fundoplication may represent the initial therapeutic approach of choice. • Initial BD may negatively affect the outcome of a subsequent HM. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achalasia is an esophageal motility disorder characterized by defective relaxation of the lower esophageal sphincter (LES) during swallowing along with various degrees of esophageal body motility impairment [5, 43]. It is commonly accepted that the degeneration and dysfunction of the nitrinergic inhibitory neurons in the esophageal myenteric plexus results in an imbalance between the excitatory and inhibitory motor inputs, which in turn leads to eventual aperistalsis of the esophageal body and impaired LES relaxation [16, 19].
In the pediatric age group, achalasia is extremely rare, with an estimated incidence of 0.18/100.000 children per year in the UK [32, 33]. Progressive dysphagia for solids and liquids, chest pain, regurgitation of undigested food, cough and weight loss represent the most frequently occurring symptoms [36]. The specific diagnostic work-up includes imaging studies to rule out obstruction at the level of esophagogastric junction (EGJ), esophagoscopy with biopsy to rule out inflammatory pathologies and esophageal manometry to confirm the presence of impaired LES relaxation and define the patterns of esophageal body dysmotility [52].
The arsenal available for the management of childhood achalasia comprises a range of treatments including pharmacologic therapies, balloon dilatation (BD), Heller’s myotomy (HM) and the novel technique of peroral endoscopic myotomy (POEM) [5, 15, 18, 31]. None of the aforementioned treatments are curative, but rather provide symptomatic relief by reducing LES pressure and, consequently, the EGJ outflow obstruction. Whilst the long-term pharmacologic treatments are not generally recommended [3, 6, 28, 51, 57], HM and BD are considered the main procedures for treating achalasia in both adults and children [1, 2, 4, 8, 10, 13, 14, 17, 21, 24, 27, 30, 35, 39, 42, 45,46,47, 52, 54, 56, 57]. In the absence of randomized controlled trials, limited data from retrospective reviews are available in children comparing the long-term outcome between the two therapeutic approaches [34, 49, 55].
The aims of the present study were to review our experience in the treatment of achalasia in a large cohort of children and compare the long-term outcomes of BD and HM.
Methods
A retrospective review of the medical records of children diagnosed and treated for esophageal achalasia at our institution (January 1995 to December 2012) was performed. Patients were identified from the Hospital’s database and information collected included gender, age at the time of diagnosis, presenting symptoms, duration of symptoms, diagnostic modalities, initial and subsequent treatments (BD, HM), peri- and post-interventional/operative complications and outcomes. The diagnosis of achalasia was suspected on the basis of clinical history and barium contrast study showing the characteristic “bird’s beak” appearance of the distal esophagus. The diagnosis was then confirmed with esophageal manometry (conventional or high resolution) showing defective LES relaxation with different degrees of esophageal body dysmotility. All patients had also undergone upper gastrointestinal endoscopy and biopsy to rule out other causes of dysphagia.
The study was approved by the Institutional Review Board of Great Ormond Street Hospital (registration number 13GA09).
Clinical assessment and therapeutic management
Our institution has not implemented a standardized protocol for the diagnosis, treatment and follow-up for patients with esophageal achalasia. After the initial evaluation and confirmation of the diagnosis, the decision for the most appropriate management was mainly gastroenterologist and surgeon specific. It was based upon the presenting symptoms, the state of patient’s health and the preferences of the child and its family. Follow-up was performed by either a gastroenterologist and/or surgeon, and treatment outcomes were assessed by utilizing Eckardt score as published elsewhere [10, 11]; standard post surgery/intervention follow-up appointments were organized initially at 1, 6 and 12 months and afterwards on a yearly basis or upon symptom recurrence. Eckardt score classified the clinical outcome as follows: stage 0 (scores 0–1), stage I (scores 2–3), stage II (scores 4–6), stage III (score >6). Children in stages 0 and I were considered to be in clinical remission whereas these in stages II and III were classified as treatment failure [10, 11].
Balloon dilatation
BD was performed under general anesthesia with endotracheal intubation. The balloon catheter was positioned fluoroscopically by an interventional radiologist over a guide wire at the level of LES. When no single balloon catheter was available in an appropriate size, two (or on one occasion three) balloons were simultaneously inflated (with dilute radiographic contrast, using 10-ml syringes) across the EGJ. The balloon was inflated until its walls appeared parallel on fluoroscopy (i.e. until any “waist” was abolished), left inflated for a maximum of 30 s and then deflated and removed. The effective diameter of the inflated balloon(s) was chosen according to the child’s size and ranged from 14 to 39 mm. No routine post-interventional esophagograms were performed. Patients were treated as day cases as long as the procedure was uneventful.
Over the years, the balloon catheters used were Cristal (Balt Extrusion, Montmorency, France), Accent, Valvuloplasty and Omega NV (William Cook Europe, Bjaeversko, Denmark), XXL (Boston Scientific, Galway, Ireland), Maxi LD (Cordis, Cashel, Ireland) and Atlas (Bard Peripheral Vascular Inc., Tempe, AZ, USA).
Surgical technique
The institution’s approach to the surgical esophagomyotomy (HM) has evolved over the years from an open abdominal procedure to the current laparoscopic approach. In the majority of cases, the HM was combined with an antireflux procedure. Details of the laparoscopic technique can be found in the article published by Pachl et al. in which the authors explored the outcome of HM in a group of children initially treated with surgery [37]. This group of patients is a subset of the cohort analyzed in the present study.
Statistical analysis
Patient and disease characteristics are expressed as mean ± standard deviation and as median (range) dependent on the normality of the distribution as assessed with Kolmogorov-Smirnov test. Patients were divided according to the type of initial treatment into two groups (HM, BD). Comparisons between groups for continuous variables were performed using t test or Mann-Whitney test as appropriate. Categorical variables were compared using chi-square or Fisher’s exact test as appropriate. Furthermore, the adjusted effect of different factors (gender, age at onset of symptoms, time from onset of symptoms until initial intervention, age at first procedure and type of initial treatment) on the outcome (need for additional treatment after the initial management) was explored using logistic regression and Cox regression analysis. Kaplan-Meier curves were also derived by the type of initial management with mean times to second intervention and p values based on the log-rank test. Statistical analysis was performed with SPSS version 17.0 software (SPSS Inc., Chicago, IL). Statistical significance was set at the p < 0.05 level.
Results
Forty-eight children (21 male, 43.8%) with a diagnosis of esophageal achalasia were identified and included in the analysis. Four children were diagnosed as Allgrove syndrome (alacrima, achalasia, adrenal insufficiency). Dysphagia to either solids or liquids was the chief symptom prior to treatment. The median age at diagnosis was 10 years (range 3.2–17.4 years) with a median time (range) from the occurrence of initial symptoms suggestive of achalasia to initial treatment of 13 (7–22) months. The median follow-up duration after initial treatment was 3 years (range 1–5.5 years).
There were no statistically significant differences between the age at diagnosis, time from diagnosis to treatment, duration of follow-up and the choice of initial treatment, i.e. HM or BD. Baseline disease characteristics of the study population are summarized in Table 1.
Sixteen children were treated with calcium channel blockers or nitrates before surgery or balloon dilatation; two children had a single uneventful intrasphincteric (LES) endoscopical botulinum toxin injection as an interim measure for symptom alleviation prior to the definitive therapeutic procedure (HM and BD, respectively).
Twenty-eight patients (58%) were initially treated with HM and 20 with BD. Amongst children who underwent BD, two were under the age of 6 years (4 and 5 years old). Sixteen out of 18 children who underwent open HM also received concomitant antireflux procedures, whereas 10 out of 30 children had a combined HM with fundoplication in the laparoscopic approach. Noteworthily, in two children, an antireflux procedure (one Nissen and one Thal, respectively) was performed independent of HM for the treatment of gastro-esophageal reflux (GER) which was refractory to medical treatment.
At the last follow-up, 21 children in total (44%) were asymptomatic (Eckardt clinical stage: 0), within which there was a statistically significant higher proportion of those who underwent HM as the initial intervention (HM 15/28, 54% BD 6/20, 30%, p < 0.05). Subsequent procedures were required in 28 children, of whom 13 underwent HM and 15 BD as a second intervention. Twenty-two patients had more than three procedures. The median (range) number of total interventions was significantly higher in children initially managed with BD compared to the HM group (BD 3 (1–7); HM 1 (1–5); p < 0.05). Table 2 presents the outcome at last follow-up compared to the initial treatment. A subgroup analysis revealed that the proportion of symptom-free children was significantly lower in the group that had undergone BD as first and HM as subsequent (second) treatment (6/16, 37%) as compared to patients who had HM as first procedure (15/28, 54%) (p < 0.01). Patient flow through the study is shown in Fig. 1, which summarizes the initial and subsequent treatments.
Logistic regression analysis was performed to assess the effect of various factors on the need for a second intervention. The type of first procedure was the only factor found to significantly determine the outcome (HR 0.08, 95%CI 0.01–0.40, p < 0.01) (Table 3). Survival analysis with log-rank test demonstrated that patients undergoing BD as first procedure had a higher rate of second intervention (BD 17/20, 85%; HM 11/28 39%; p < 0.01) with shorter time to it (mean time BD 14 months, 95%CI 4–24; HM 58 months, 95%CI 38–79; p < 0.001) (Fig. 2).
Peri- and immediate post-operative/interventional complication rate was 2.7% overall. Specifically of a total of 108 procedures, esophageal perforation occurred in two children after HM (2/48) and in one child after BD (1/60).
After treatment, 11 patients (11/48, 23%) reported symptoms suggestive of GER, which was objectively proven by either esophagogastroduodenoscopy (EGD), pH-metry or pH-Impedance in five of them. There were no deaths.
Discussion
Current treatments available for childhood achalasia rely on improvement of symptoms by relieving the EGJ outflow obstruction induced by the defective LES relaxation during swallowing, rather than establishing esophageal peristaltic motor sequences [5, 16].
The optimal initial procedure for childhood achalasia remains a matter of debate and controversy with several studies reporting conflicting results [1, 10, 25, 26, 29, 37, 38]. However, whilst BD achieves short-term symptomatic improvement, there is a high requirement for subsequent interventions [40]. This finding was supported in our cohort too, as the total number of procedures was significantly higher in children initially managed with BD. Of children who underwent HM as initial treatment, 53.6% were asymptomatic requiring no further intervention whereas 85% of patients who had BD as the initial procedures required subsequent treatment. These results are in agreement with other published studies although the recurrence of symptoms in our cohort was higher for the BD group [1, 29, 37, 38]. Our findings contradict two recent studies in children, which report a long-term remission rate of 66.7% after a single BD and a complete remission of 65% after a 2-year follow-up period after repeated BD [10, 26]. Furthermore, our data do not compare favourably with the long-term results of the European achalasia trial, which, after at least 5 years of follow-up, revealed comparable success rate for both HM and BD [35]. Differences in the dilatation protocol (e.g. implementation of a graded BD with re-assessment of the patient at regular intervals to decide whether an additional BD is required) and the dilatation technique per se (e.g. size of the balloon, number of dilatations per session, maximum balloon pressure, duration of dilation at maximum balloon pressure) between various centers could explain these discrepancies as they may account for variable effect on the disruption of the LES fibers during the procedure [12, 48]. Clearly, the HM group had the most favourable outcome with the highest remission rate and a longer period before a subsequent intervention compared to BD. The therapeutic efficacy of HM in the present cohort is in agreement with that shown in other studies in both pediatric and adult populations [1, 8, 17, 29, 37, 46, 55]. HM’s superiority in terms of favourable outcome compared to BD could be explained by the fact that it offers a more complete dissection of the LES fibers and, consequently, a more efficient esophageal clearance and relief of dysphagia [8, 50]. In this series, 16 patients underwent HM after a non-relieving initial BD. At the last follow-up, 6/16 (37%) were at Eckardt clinical stage 0, 5/16 (31%) were at stage I and 5/16 (32%) at stage II. Children who underwent HM as their initial procedure were asymptomatic in a significantly higher percent (15/28 Eckardt clinical stage 0, 12/28 stage I, 1/28 stage II) (Fisher’s exact test, p < 0.01). Our data raise the possibility that a history of a previous BD may negatively affect the success of a future HM and do not demonstrate the favourable outcome shown by others [20, 22, 29]. It has been speculated that this could be because BD scars the submucosal layers of the esophagus by causing microhemorrhages, inflammation and subsequent formation of fibrotic tissue which may make a future HM technically more difficult, less effective, with an increase in intraoperative complications [23, 41]. Nonetheless, BD may be utilized as an appropriate second-line intervention, especially in symptomatic patients who were initially managed with HM to further widen the already divided esophageal musculature and to distract any fibrotic tissue or adhesions which may have formed post initial HM. The latter was also reported in the study published by Pachl et al. [37].
In the present cohort, more than half of the esophagomyotomies (26 out of 48) were combined with a fundoplication. The majority of the antireflux surgery (16 out of 26 procedures) took place during the open abdominal HM procedure. Eleven out of 48 children reported symptoms indicative of GER, which was objectively demonstrated in five patients (10.4%); the latter is in accordance with the results published by other researchers [24, 38, 53, 57]. However, we did not identify any significant association between the occurrence of post-intervention GER and type of initial treatment (BD, HM), type of HM (open or laparoscopic approach, combined or not with fundoplication) and the type of fundoplication (Nissen or Dor). It has been shown that the dissection of the LES fibers is less extensive during a laparoscopic HM, i.e. the myotomy is adequate to alleviate dysphagia but not so extensive to compromise the physiological barriers against GER (especially the angle of His) which are therefore preserved [44]. Although a recent systematic review with meta-analysis in adults supported HM with fundoplication as the best initial treatment for esophageal achalasia, there are several studies in pediatrics suggesting that HM without fundoplication is an adequate and effective initial treatment [9, 53, 54]. This area is controversial and open to opinion.
We did not find any statistically significant differences between HM and BD in peri- or post-operative adverse events. In our series of patients, three procedural esophageal perforations were observed (three complications out of 108 procedures in total) which accounts for a complication rate of 2.7%. Two perforations occurred after HM (one HM was the primary procedure and the other after a BD) and one after BD (initial procedure). Perforations after HM were diagnosed peri-operatively whereas in BD, this complication was diagnosed within an hour post procedure. All perforations were managed conservatively and the patients recovered without any further events. The adverse events and the complication rates in the present cohort compare favourably with previous published data [29, 40].
Undoubtedly, this study bears certain limitations that affect the significance of our results. Firstly, this is a retrospective study embracing the well-known limitations of this type of methodology, such as incomplete data, and reliance on child and parental recall. However, using different strategies for data extraction, such as a structured form created a priori, we aimed to minimize the limitations and increase the reliability of our data. Secondly, despite describing the largest single-center cohort in pediatric achalasia, the number of patients is relatively small. Thirdly, the surgical approach has changed over the time from an open abdominal procedure to the current laparoscopic approach. Although no differences were found on the outcome between patients treated with different therapeutic techniques, suggesting that different types of surgery do not seem to affect the final outcome, the sample size and potential skewing of the data might be the source of a type II error that has affected our findings. Finally, there was neither randomization of patients to different therapeutic modalities nor a standardized algorithm/protocol for the evaluation, management and follow-up of patients. As a result, a potential bias on the outcomes amongst treatment groups cannot be excluded. However, we do believe that our study adds important data on a rare condition and aids to further enlighten clinicians on the best initial approach of an entity with a continuously evolving management [7].
In conclusion, our data indicate that both HM (with or without an antireflux procedure) and BD are safe and effective for the treatment of childhood esophageal achalasia. However, in the absence of any evidence from randomized controlled trials, HM with or without fundoplication may be the favoured initial therapeutic approach for childhood achalasia, as it may lead to a higher remission rate, a longer symptom-free period and a more favourable long-term outcome. A well-designed multicenter randomized controlled study is, however, needed to definitively confirm our findings.
Abbreviations
- BD:
-
Balloon dilatation
- CI:
-
Confidence interval
- DAS:
-
Discharged to adult services
- EBTI:
-
Endoscopical botulinum toxin injection
- EGD:
-
Esophagogastroduodenoscopy
- EGJ:
-
Esophagogastric junction
- F/U:
-
Follow-up
- GER:
-
Gastro-esophageal reflux
- GT:
-
Gastric transposition
- HM:
-
Heller’s myotomy
- HR:
-
Hazard ratio
- LES:
-
Lower oesophageal sphincter
- SD:
-
Standard deviation
- Tx:
-
Treatment
References
Askegard-Giesmann JR, Grams JM, Hanna AM, Iqbal CW, Teh S, Moir CR (2009) Minimally invasive Heller’s myotomy in children: safe and effective. J Pediatr Surg 44(5):909–911
Babu R, Grier D, Cusick E, Spicer RD (2001) Pneumatic dilatation for childhood achalasia. Pediatr Surg Int 17(7):505–507
Bassotti G, Annese V (1999) Review article: pharmacological options in achalasia. Aliment Pharmacol Ther 13(11):1391–1396
Boeckxstaens GE, Annese V, Des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, Smout AJ, Tack J, Zwinderman AH, Zaninotto G, Busch OR (2011) European Achalasia Trial Investigators.. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 364(19):1807–1816
Boeckxstaens GE, Zaninotto G, Richter JE (2014) Achalasia. Lancet 383(9911):83–93
Bortolotti M (1999) Medical therapy of achalasia: a benefit reserved for few. Digestion 60(1):11–16
Caldaro T, Familiari P, Romeo EF, Gigante G, Marchese M, Contini AC, Federici di Abriola G, Cucchiara S, De Angelis P, Torroni F, Dall’Oglio L, Costamagna G (2015) Treatment of esophageal achalasia in children: today and tomorrow. J Pediatr Surg 50(5):726–730
Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R (2009) Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 249(1):45–57
Corda L, Pacilli M, Clarke S, Fell JM, Rawat D, Haddad M (2010) Laparoscopic oesophageal cardiomyotomy without fundoplication in children with achalasia: a 10-year experience: a retrospective review of the results of laparoscopic oesophageal cardiomyotomy without an anti-reflux procedure in children with achalasia. Surg Endosc 24(1):40–44
Di Nardo G, Rossi P, Oliva S, Aloi M, Cozzi DA, Frediani S, Redler A, Mallardo S, Ferrari F, Cucchiara S (2012) Pneumatic balloon dilation in pediatric achalasia: efficacy and factors predicting outcome at a single tertiary pediatric gastroenterology center. Gastrointest Endosc 76(5):927–932
Eckardt VF, Aignherr C, Bernhard G (1992) Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 103(6):1732–1738
Elliott TR, Wu PI, Fuentealba S, Szczesniak M, de Carle DJ, Cook IJ (2013) Long-term outcome following pneumatic dilatation as initial therapy for idiopathic achalasia: an 18-year single-centre experience. Aliment Pharmacol Ther 37(12):1210–1219
Esposito C, Cucchiara S, Borrelli O, Roblot-Maigret B, Desruelle P, Montupet P (2000) Laparoscopic esophagomyotomy for the treatment of achalasia in children. A preliminary report of eight cases. Surg Endosc 14(2):110–113
Esposito C, Riccipetitoni G, Chiarenza SF, Roberti A, Vella C, Alicchio F, Fava G, Escolino M, De Pascale T, Settimi A (2013) Long-term results of laparoscopic treatment of esophageal achalasia in children: a multicentric survey. J Laparoendosc Adv Surg Tech A 23(11):955–959
Familiari P, Marchese M, Gigante G, Boskoski I, Tringali A, Perri V, Costamagna G (2013) Peroral endoscopic myotomy for the treatment of achalasia in children. J Pediatr Gastroenterol Nutr 57(6):794–797
Francis DL, Katzka DA (2010) Achalasia: update on the disease and its treatment. Gastroenterology 139(2):369–374
Franklin AL, Petrosyan M, Kane TD (2014) Childhood achalasia: a comprehensive review of disease, diagnosis and therapeutic management. World J Gastrointest Endosc 6(4):105–111
Gariepy CE, Mousa H (2009) Clinical management of motility disorders in children. Semin Pediatr Surg 18(4):224–238
Ghoshal UC, Daschakraborty SB, Singh R (2012) Pathogenesis of achalasia cardia. World J Gastroenterol 18(24):3050–3057
Gockel I, Junginger T, Bernhard G, Eckardt VF (2004) Heller myotomy for failed pneumatic dilation in achalasia: how effective is it? Ann Surg 239(3):371–377
Guardino JM, Vela MF, Connor JT, Richter JE (2004) Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol 38(10):855–860
Gutschow CA, Töx U, Leers J, Schäfer H, Prenzel KL, Hölscher AH (2010) Botox, dilation, or myotomy? Clinical outcome of interventional and surgical therapies for achalasia. Langenbeck’s Arch Surg 395(8):1093–1099
Horgan S, Hudda K, Eubanks T, McAllister J, Pellegrini CA (1999) Does botulinum toxin injection make esophagomyotomy a more difficult operation? Surg Endosc 13(6):576–579
Hussain SZ, Thomas R, Tolia V (2002) A review of achalasia in 33 children. Dig Dis Sci 47(11):2538–2543
Ip KS, Cameron DJ, Catto-Smith AG, Hardikar W. Botulinum toxin for achalasia in children. J Gastroenterol Hepatol 2000;15(10):1100–1104.
Jung C, Michaud L, Mougenot JF et al (2010) Treatments for pediatric achalasia: Heller myotomy or pneumatic dilatation? Gastroenterol Clin Biol 34(3):202–208
Karnak I, Senocak ME, Tanyel FC, Büyükpamukçu N (2001) Achalasia in childhood: surgical treatment and outcome. Eur J Pediatr Surg 11(4):223–229
Koumi A, Panos MZ (2010) Oesophageal food impaction in achalasia treated with Coca-Cola and nifedipine. BMJ Case Rep 2010
Lee CW, Kays DW, Chen MK, Islam S (2010) Outcomes of treatment of childhood achalasia. J Pediatr Surg 45(6):1173–1177
Logan MS, Vossoughi F, Watson CM, Amarnath R, Camps JI (2009) A novel technique for the surgical treatment of achalasia in children: evaluated with postoperative esophageal manometry. J Laparoendosc Adv Surg Tech A 19(4):589–593
Maksimak M, Perlmutter DH, Winter HS (1986) The use of nifedipine for the treatment of achalasia in children. J Pediatr Gastroenterol Nutr 5(6):883–886
Marlais M, Fishman JR, Fell JM, Haddad MJ, Rawat DJ (2011) UK incidence of achalasia: an 11-year national epidemiological study. Arch Dis Child 96(2):192–194
Mayberry JF, Mayell MJ (1988) Epidemiological study of achalasia in children. Gut 29(1):90–93
Meyer A, Catto-Smith A, Crameri J, Simpson D, Alex G, Hardikar W, Cameron D, Oliver M. Achalasia: outcome in children. J Gastroenterol Hepatol 2017;32(2):395–400
Moonen A, Annese V, Belmans A, Bredenoord AJ, Bruley des Varannes S, Varannes S, Costantini M, Dousset B, Elizalde JI, Fumagalli U, Gaudric M, Merla A, Smout AJ, Tack J, Zaninotto G, Busch OR, Boeckxstaens GE (2016) Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 65(5):732–739
Myers NA, Jolley SG, Taylor R (1994) Achalasia of the cardia in children: a worldwide survey. J Pediatr Surg 29(10):1375–1379
Pachl MJ, Rex D, Decoppi P, Cross K, Kiely EM, Drake D, Pierro A, Curry JI (2014) Paediatric laparoscopic Heller’s cardiomyotomy: a single centre series. J Pediatr Surg 49(2):289–292 discussion 292
Pastor AC, Mills J, Marcon MA, Himidan S, Kim PC (2009) A single center 26-year experience with treatment of esophageal achalasia: is there an optimal method? J Pediatr Surg 44(7):1349–1354
Patti MG, Albanese CT, Holcomb GW 3rd, Molena D, Fisichella PM, Perretta S, Way LW (2001) Laparoscopic Heller myotomy and Dor fundoplication for esophageal achalasia in children. J Pediatr Surg 36(8):1248–1251
Pineiro-Carrero VM, Sullivan CA, Rogers PL (2001) Etiology and treatment of achalasia in the pediatric age group. Gastrointest Endosc Clin N Am 11(2):387–408 viii
Portale G, Costantini M, Rizzetto C, Guirroli E, Ceolin M, Salvador R, Ancona E, Zaninotto G (2005) Long-term outcome of laparoscopic Heller-Dor surgery for esophageal achalasia: possible detrimental role of previous endoscopic treatment. J Gastrointest Surg 9(9):1332–1339
Richards WO, Torquati A, Holzman MD, Khaitan L, Byrne D, Lutfi R, Sharp KW (2004) Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg 240(3):405–412 discussion 412-5
Richter JE (2001) Oesophageal motility disorders. Lancet 358(9284):823–828
Robert M, Poncet G, Mion F, Boulez J (2008) Results of laparoscopic Heller myotomy without anti-reflux procedure in achalasia. Monocentric prospective study of 106 cases. Surg Endosc 22(4):866–874
Rothenberg SS, Partrick DA, Bealer JF, Chang JH (2001) Evaluation of minimally invasive approaches to achalasia in children. J Pediatr Surg 36(5):808–810
Schoenberg MB, Marx S, Kersten JF, Rösch T, Belle S, Kähler G, Vassiliou MC, Lüth S, von Renteln D (2013) Laparoscopic Heller myotomy versus endoscopic balloon dilatation for the treatment of achalasia: a network meta-analysis. Ann Surg 258(6):943–952
Singh S, Wakhlu A, Pandey A, Kureel SN, Rawat J (2012) Retrospective analysis of paediatric achalasia in India: single centre experience. Afr J Paediatr Surg 9(2):117–121
Smeets FG, Masclee AA, Keszthelyi D, Tjwa ET, Conchillo JM (2015) Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil 27(10):1495–1503
Smits M, van Lennep M, Vrijlandt R, Benninga M, Oors J, Houwen R, Kokke F, van der Zee D, Escher J, van den Neucker A, de Meij T, Bodewes F, Schweizer J, Damen G, Busch O, van Wijk M (2016) Pediatric achalasia in the Netherlands: incidence, clinical course, and quality of life. J Pediatr 169:110–5.e3
Suárez J, Mearin F, Boque R, Zanón V, Armengol JR, Pradell J, Bermejo B, Nadal A (2002) Laparoscopic myotomy vs endoscopic dilation in the treatment of achalasia. Surg Endosc 16(1):75–77
Triadafilopoulos G, Aaronson M, Sackel S, Burakoff R. Medical treatment of esophageal achalasia. Double-blind crossover study with oral nifedipine, verapamil, and placebo. Dig Dis Sci 1991;36(3):260–7
Vaezi MF, Pandolfino JE, Vela MF (2013) ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 108(8):1238–1249 quiz 1250
Vaos G, Demetriou L, Velaoras C, Skondras C (2008) Evaluating long-term results of modified Heller limited esophagomyotomy in children with esophageal achalasia. J Pediatr Surg 43(7):1262–1269
Yaghoobi M, Mayrand S, Martel M, Roshan-Afshar I, Bijarchi R, Barkun A (2013) Laparoscopic Heller’s myotomy versus pneumatic dilation in the treatment of idiopathic achalasia: a meta-analysis of randomized, controlled trials. Gastrointest Endosc 78(3):468–475
Zagory JA, Golden JM, Demeter NE, Nguyen Y, Ford HR, Nguyen NX (2016) Heller myotomy is superior to balloon dilatation or botulinum injection in children with achalasia: a two-center review. J Laparoendosc Adv Surg Tech A 26(6):483–487
Zaninotto G, Costantini M, Molena D, Buin F, Carta A, Nicoletti L, Ancona E (2000) Treatment of esophageal achalasia with laparoscopic Heller myotomy and Dor partial anterior fundoplication: prospective evaluation of 100 consecutive patients. J Gastrointest Surg 4(3):282–289
Zhang Y, Xu CD, Zaouche A, Cai W (2009) Diagnosis and management of esophageal achalasia in children: analysis of 13 cases. World J Pediatr 5(1):56–59
Author information
Authors and Affiliations
Contributions
Efstratios Saliakellis contributed in designing the study; collected and organized the data for statistical analyses; analyzed and interpreted the statistical results and created the manuscript.
Nikhil Thapar contributed in designing the study and performed critical revision of the manuscript.
Derek Roebuck contributed in designing the study and performed critical revision of the manuscript.
Fernanda Cristofori contributed in designing the study, collected the data and critically reviewed the manuscript.
Kate Cross contributed in designing the study and performed critical revision of the manuscript.
Edward Kiely contributed in designing the study and performed critical revision of the manuscript.
Joseph Curry contributed in designing the study and performed critical revision of the manuscript.
Keith Lindley contributed in designing the study and performed critical revision of the manuscript.
Osvaldo Borrelli contributed in designing the study; analysed and interpreted the statistical results and created the manuscript.
Corresponding author
Ethics declarations
Funding
There was no funding received for this study.
Conflict of interest
The authors declare that they no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The present study was a retrospective review of case notes conducted in accordance with the ethical standards of the institutional (Great Ormond Street Hospital and Institute of Child Health, University College London) and national (NHS England) research committees (Institutional Review Board of Great Ormond Street Hospital; Registration number 13GA09).
Additional information
Communicated by Peter de Winter
Revisions received: 8 April 2017 / 24 April 2017
Rights and permissions
About this article
Cite this article
Saliakellis, E., Thapar, N., Roebuck, D. et al. Long-term outcomes of Heller’s myotomy and balloon dilatation in childhood achalasia. Eur J Pediatr 176, 899–907 (2017). https://doi.org/10.1007/s00431-017-2924-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-2924-x