Abstract
Background
Although the link between achalasia and morbid obesity is unclear, the reported prevalence is 0.5–1% in this population. For bariatric surgery patients, optimal type and timing of achalasia intervention is uncertain.
Methods
Patient charts from a single academic institution were retrospectively reviewed. Between 2012 and 2019, 245 patients were diagnosed with achalasia, 13 of whom underwent bariatric surgery and were included. Patients were divided into two groups depending on the timing of their achalasia diagnosis and bariatric surgery. Groups were compared in terms of type and timing of intervention as well as treatment response.
Results
Group 1 included 4 patients diagnosed with achalasia before bariatric surgery. Three had laparoscopic Heller myotomy (LHM) and 1 had a per oral endoscopic myotomy (POEM). These patients had laparoscopic gastric bypass (LGB) within 5 years of achalasia diagnosis. Postoperatively, 1 had severe reflux with regurgitation necessitating radiofrequency energy application to the lower esophageal sphincter. All had relief from dysphagia.
Group 2 included 9 patients diagnosed with achalasia after bariatric surgery. Achalasia subtypes were evenly distributed. Initial operations were: 5 LGB, 2 laparoscopic sleeve gastrectomy (LSG), 1 duodenal switch (DS), 1 lap band. One LSG patient was converted to LGB concurrently with LHM. On average, achalasia was diagnosed 8.3 years after bariatric surgery. Achalasia interventions included: 1 pneumatic dilation, 1 Botox injection, 1 POEM, 6 LHM. While LHM was the most common procedure, 4 of 6 patients experienced recurrent dysphagia, one of whom required esophagectomy.
Conclusions
Achalasia is a challenging problem in the bariatric surgery population. Recurrent symptoms are common. Patients treated for achalasia after bariatric surgery tended to have worse symptom resolution than those diagnosed prior to bariatric surgery. Additional prospective studies are needed to elucidate whether interventions for achalasia should be performed concurrently or in a particular sequence for optimal results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Esophageal motility disorders are uncommon among the general population but have a relatively high incidence in morbidly obese patients. Obesity is a known independent risk factor for esophageal dysmotility and some estimates of prevalence are over 50% [1,2,3]. With global rates of obesity increasing, a better understanding of the overlap between esophageal dysmotility and obesity will be ever more important to optimally treat patients. One such motility disorder is achalasia, which is characterized by lack of peristalsis of the esophagus as well as a failure of the lower esophageal sphincter (LES) to relax [4]. The incidence of the disorder is reportedly 1.63 in 100,000 [5]. Achalasia has gained attention in recent years due to its connection with morbid obesity, with a prevalence in this specific population of 0.5–1% [1, 6, 7].
Obese patients with achalasia most often present with dysphagia with or without weight loss [1]. However, achalasia is frequently misdiagnosed in this patient population because many of the other associated symptoms such as regurgitation, cough, and aspiration are already common in obesity. Stasis and fermentation in the esophagus can also lead to the sensation of reflux. Patients who pursue bariatric surgery with unrecognized achalasia may have symptoms that masquerade as potential surgical complications which complicate nutritional intake and weight loss postoperatively. The opportunity for a combined treatment of achalasia and morbid obesity may be lost due to a delay in diagnosis of achalasia, necessitating additional surgery in the future. Alternatively, patients can develop achalasia de novo after they have already undergone bariatric surgery. The lack of curative treatment for achalasia to date leaves patients with limited options to treat symptoms, such as a dysphagia. Surgical treatment of achalasia is contingent upon relieving the outflow obstruction with disruption of the LES, easing the passage of esophageal contents. This can be done concurrently with bariatric surgery or at a separate time, before or afterwards. The optimal sequence and types of procedures are currently unknown as there is a paucity of published literature. Various combinations have been attempted. The authors of this study hypothesize that concurrent laparoscopic gastric bypass and Heller myotomy is most optimal to address both achalasia and morbid obesity, eliminating the need for a second procedure. This study details the single-institution outcomes of the treatment of achalasia in bariatric surgery patients.
Materials and methods
This is a retrospective case series from a single academic institution. The study was granted IRB approval by this same institution. Initially, hospital billing records were reviewed for patients who were diagnosed with achalasia between 2012 and 2019. Two hundred forty-five achalasia patients were identified. An electronic medical record review of the 245 patients was performed to identify those patients who also underwent bariatric surgery. Our study included 13 patients during the study period who both had bariatric surgery and were treated for achalasia. Six patients who had procedures solely for morbid obesity or achalasia, but not both, were excluded. Patients were separated into two groups based on the timing of achalasia diagnosis in relation to their bariatric surgery. Individuals who were diagnosed with achalasia prior to undergoing bariatric surgery were designated Group 1. Those with achalasia diagnoses after bariatric surgery were designated Group 2. Data variables collected were: age at achalasia diagnosis, initial symptoms, diagnostic studies, manometric data, type of achalasia (Chicago classification), achalasia treatment method, time interval between achalasia diagnosis and bariatric surgery, type of bariatric surgery, bariatric surgery complications, symptomatic response to treatment, and complications from achalasia treatment. Weight loss was recorded as total weight loss (TWL), denoted by percentage change from pre-bariatric weight. Statistical analysis was limited to simple descriptive analyses due to the small sample size.
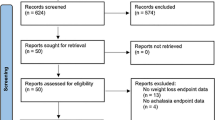
A systematic review was performed to identify comparison publications reporting results in patients having both bariatric surgery and achalasia interventions (Fig. 1). The electronic PubMed database was queried using the terms “achalasia” and “obesity,” yielding 102 results. Results were then filtered for original, English language articles with human subjects. Abstracts were reviewed and studies which included patients with procedural interventions for the treatment of both achalasia and morbid obesity were included. Patients for whom a procedure was intended but not yet completed were excluded. Case reports, series, and prior systematic reviews were included. To capture all pertinent articles, additional search terms were employed, including: Heller, myotomy, POEM, per oral endoscopic myotomy, gastric bypass, and bariatric. With the same inclusion and exclusion parameters, this yielded an additional seven articles. Results were not limited by date, but all publications took place between 2003 and 2019. Data from these publications were compared to our series. As with our series, TWL was preferentially used. When TWL was unable to be calculated, actual weight lost, change in body mass index (BMI), or excess body weight lost (EBW) were provided.
Results
The 4 patients who were diagnosed with achalasia prior to their bariatric surgery were identified as Group 1 (Table 1). All 4 patients received LGB with mean TWL of 30.9%. Mean age was 45 years old. Three of the patients were found to have achalasia during their preoperative evaluation for bariatric surgery (Patients 1, 2, 4). Patient 1 underwent a POEM for type 3 achalasia. This patient had their bypass 4 months later. There was 28.6% TWL and continued absence of achalasia symptoms in the 3-year follow up period. Patient 2 underwent robotic-assisted LHM without fundoplication in anticipation of their bariatric surgery, with LGB 1 month later. The type of achalasia was not documented. Although there was resolution of achalasia symptoms and 51.4% TWL, a gastrojejunostomy stricture developed. There was symptomatic improvement with dilation. Patient 3 had LHM with Dor fundoplication for type 2 achalasia. Again, there was relief of achalasia symptoms but a worsening of reflux symptoms. Approximately 5 years later, this patient had LGB which was converted to an open procedure. TWL afterward was 25.6%. Reflux symptoms remained severe after the procedure, with noted aspiration pneumonia. Reflux was eventually treated with Stretta radiofrequency energy application (Mederi Therapeutics; Norwalk, CT) to the LES. Patient 4 was diagnosed with type 3 achalasia 7 months prior to undergoing concurrent Botox injection and of LGB. The patient had persistent nausea and dysphagia and underwent a repeat Botox injection of the LES and balloon dilation of angulation that appeared to narrow the alimentary limb. No relief was obtained and the patient subsequently underwent a robotic-assisted LHM. There was adhesive disease with possible kinking of the alimentary limb treated by adhesiolysis and pexy. The patient had an improvement in her dysphagia symptoms but had some residual reflux symptoms and nausea. TWL to date was 18% at 8-month follow-up.
Group 2 consisted of 9 patients who had bariatric procedures prior to their diagnosis of achalasia (Table 2). Mean age was 60 years old, and there was an even distribution of achalasia subtypes. The average interval to onset of symptoms was 8.3 years postoperatively, although 2 patients developed achalasia symptoms within 1 year of having bariatric surgery (Patient 8 and 10). There were 5 patients who developed achalasia after LGB. Interval weight loss between LGB and achalasia diagnosis was discernable for 2 patients (Patients 5, 8) with 58.9% and 36.2% TWL, respectively. One of the 5 LGB patients (Patient 5) underwent stenting and dilation followed by POEM with temporary relief of dysphagia, but eventually required dilations of the gastrojejunostomy and gastroesophageal junction at 1 year. Three LGB patients were treated with Heller myotomy (HM). One patient had relief of symptoms (Patient 8), but the other 2 LGB patients (Patient 6,7) experienced recurrent dysphagia and were both ultimately recommended for esophagectomy. Patient 7 went on to esophagectomy, while Patient 6 opted for comfort care measures.
Two LSG patients who developed achalasia were treated with LHM, of which one was combined with conversion to LGB. The other LSG patient (Patient 10) was warned of the likelihood of worsened reflux after myotomy but patient refused conversion to LGB. Subsequent to LHM, this patient experienced minimal relief from dysphagia and suffered ongoing difficulty with regurgitation. The patient was found to have sleeve stenosis requiring gastric dilation. TWL after LSG prior to achalasia diagnosis was 27.8%. The other LSG patient (Patient 11) was concurrently converted to LGB at the time of LHM, with initial improvement of dysphagia that later recurred with associated esophageal spasm. TWL had been 24.3% after LSG, and this increased to 30.2% after conversion to LGB+LHM.
A single patient (Patient 12) had a lap band with development of type 1 achalasia after 8 years. Initially, saline was removed from the lap band port without relief so the patient underwent band removal. Dysphagia symptoms persisted after removal of band, prompting repeat manometry which demonstrated findings consistent with achalasia. The patient was treated with subsequent endoscopic botulinum toxin injection and repeat dilations with mild relief of symptoms. Of note, the patient had documented dysphagia symptoms preceding the original band placement which then progressively worsened after the procedure. As this patient potentially had undiagnosed achalasia prior to band placement, the patient was included for this study. The band placement was performed at another institution and the decision making behind this was unclear. Weight loss for this patient is not able to be determined for the same reasons. There was also 1 patient with a prior duodenal switch (Patient 13). TWL after DS was 57.3%. This patient developed type 2 achalasia 9 years postoperatively and was treated with LHM and concurrent hiatal hernia repair with mesh, resulting in resolution of symptoms. The patient, however, had ongoing chronic malnutrition presumably attributable to the initial bariatric procedure.
Postoperative complications requiring additional procedural interventions were common in the study population. Nine of 13 (69%) patients underwent at least one additional procedure. Endoscopic dilations were performed in 6 of the 13 (46%) patients postoperatively. Three dilations were performed for ongoing achalasia symptoms and 3 for complications of bariatric surgery.
In our series, the most common bariatric procedure was LGB (6 of 13) and the most common therapy for achalasia was LHM (9 of 13). Of the 5 patients in our population who had symptomatic resolution, 4 had LHM and 1 with POEM. This symptom relief was evident at an average follow-up of 2 years; however, one patient within this group (Patient 8) had only 2 months of follow-up.
When categorized by subtype of achalasia, 1 of 4 patients with type 1 achalasia experienced longstanding relief. For type 2 achalasia, relief of dysphagia was noted in 2 of the 3 patients. Of the 5 patients with type 3 achalasia, 1 had symptom resolution, 3 had recurrent symptoms, and 1 was lost to follow up.
Discussion
Achalasia is an uncommon, incurable disease, which is also difficult to definitively manage. Symptomatic control of dysphagia and reflux often remains a persistent challenge. A known association exists between obesity and esophageal motility disorders [1,2,3, 8]. Achalasia in the bariatric patient is a particularly burdensome problem. There are few efficacious medical remedies available to patients and often multiple endoscopic and operative procedures are needed to alleviate dysphagia symptoms.
Much progress has been made in studying the optimal management of achalasia, such as the recent publication by Werner et al., comparing LHM and POEM. Though the authors observed an 80% rate of symptom relief at 2 year follow up, they did not include morbidly obese patients. Average BMI for the LHM and POEM groups was 24.5 kg/m2 and 24.8 kg/m2, respectively [9]. In patients who fail one of these procedures, repeating the procedure or performing the alternate procedure are both feasible options [10,11,12]. Recent publications have also demonstrated favorable outcomes with combined procedures [3, 6, 13, 14]. Our results suggest that achalasia may be even more challenging to manage in bariatric patients than in the non-obese population.
Screening for achalasia, with esophagram or manometry, is not routinely included in the preoperative evaluation of patients anticipating bariatric surgery. The classic symptomatology of achalasia is progressive dysphagia to both solids and liquids [15]. Symptoms can be quantified using the Eckardt score, a 12-point system based on the frequency of dysphagia, regurgitation, chest pain, and weight loss [16]. New-onset dysphagia should prompt screening for malignancy prior to bariatric surgery, as obesity is a known risk factor for esophageal adenocarcinoma [17]. Any such suspicion should be evaluated with upper endoscopy. After malignancy is ruled out, symptoms of dysphagia should ideally be worked up with esophagram and manometry prior to bariatric surgery. Patients diagnosed with achalasia prior to bariatric surgery may be offered a tailored approach to treat both diseases, with potential for improved outcomes. Other diagnoses such as hiatal hernia and other esophageal motility disorders would be identified during this work-up, and contribute to surgical decision making.
Our study suggests that patients diagnosed and treated for achalasia prior to bariatric surgery tended to have a favorable outcome, as compared to patients treated for achalasia after bariatric surgery. The small sample size precluded statistical analysis. Although patients in this series underwent a myriad of different therapeutic interventions, success rates were far lower than in the general achalasia population. Furthermore, there was no clear association with achalasia subtype and treatment success in our series. Classically, treatment is thought to be most efficacious for patients with type 2 achalasia, with decreasing success for type 1 and type 3, respectively [18, 19]. It is possible that there is less correlation between subtype and treatment efficacy in bariatric patients with achalasia.
The effect of achalasia on weight in relation to bariatric surgery is not clear. No weight loss comparison was performed in the study due to small sample size and heterogenous combination of interventions. Many previous studies did not report this data, and those that did provided varied units of measure. In our study, all Group 1 patients underwent bypass with 18.0–51.4% TWL, noting that the patient with the lowest weight loss has had only 8 months of follow up. Patients from Group 2 had substantial weight loss with a myriad of procedures, resulting in a range of 24.3–58.9% TWL. Additional weight loss was seen in some from this group after achalasia intervention, with a final range of 30.4–64.3% TWL. It is possible that weight loss was attenuated by subjects’ dietary choices. Patients with chronic dysphagia may alter their diets, opting for more tolerable liquid foods, which are frequently high in simple carbohydrates. Ultimately, no definite conclusions are able to be drawn regarding the effect of achalasia on postoperative weight loss given the small, retrospective nature of our study. This aspect would be better investigated prospectively to assess the impact of food tolerance.
The patients in our series suffered a high frequency of postoperative complications following their treatment for either obesity or achalasia relative to other papers referenced here. However, when larger series of revisional bariatric and foregut surgery are examined, high complication rates are indeed seen. In one study, that included 84 revisional bariatric procedures, there was a 14.3% complication rate and 5.9% reoperation rate [20]. Even higher rates are reported when bariatric procedures have been combined with revisional foregut surgery. For patients receiving bypass concurrent with revisional paraesophageal hernia repair, a 26.3% complication rate is described [21]. It is also possible that complications were underrepresented in the comparison achalasia studies due to relatively short follow up.
Achalasia diagnosis prior to bariatric surgery
The 4 patients diagnosed with achalasia, and often treated, prior to undergoing bariatric surgery (Group 1) all experienced at least partial relief of dysphagia at 1 year. However, 3 patients required additional procedures. Only two publications were identified in the literature presenting case reports of achalasia treatment prior to bariatric surgery, with contradictory results (Table 3). One patient, from a center in Brazil, had a prior HM and underwent a Ghrondal-Scopinaro procedure (biliopancreatic diversion, cardioplasty, antrectomy, truncal vagotomy) for morbid obesity. There was no resolution of the patient’s symptoms; however, it is worth noting that no relief had been attained after initial HM. TWL was 16.7% for this patient [22]. The other study included two patients who underwent POEM prior to GB. The patient with type 2 achalasia did well with this treatment sequence, experiencing a resolution of their symptoms. Post-treatment Eckardt score was 2. However, the other patient with type 1 achalasia had persistent, albeit improved, symptoms with an Eckardt score of 4 [18]. Similarly, the patient with type 1 achalasia in our series required an additional procedure to obtain symptomatic resolution. Weight loss was not reported in the above studies for comparison. Without additional prospective comparison studies, it is difficult to tailor the optimal treatment for patients diagnosed with achalasia who are planning to undergo bariatric surgery.
Bariatric surgery prior to achalasia
Patients treated for achalasia after their bariatric surgery (Group 2) experienced varied, mostly poor, results in terms of symptomatic resolution in this series. The other identified published case series of similar size report higher success rates for both HM and POEM after GB [18, 23, 24]. The remainder of the previously published literature is comprised of case reports [4, 6, 25,26,27,28,29,30,31,32,33,34,35,36]. These 17 studies, summarized in Table 4, include a total of 37 patients who developed achalasia 2–18 years after their bariatric procedure. Collectively, bariatric surgeries included 31 LGB, 3 vertical banded gastroplasties, 1 LSG, 1 DS, and 1 loop gastrojejunostomy bypass. One vertical banded gastroplasty and LSG were later converted to LGB at time of subsequent operation [25, 33]. The achalasia procedures reported were 30 HM, 7 POEM, and 2 esophagectomies. Postoperatively, virtually all of these patients reported symptomatic relief, with the exception of one type 3 achalasia patient who underwent POEM after gastric bypass [18]. Another patient who had LHM after LGB had initial improvement for 4 months, but then presented 43 months later with recurrent symptoms [35]. Further treatment was only needed in 2 patients, both of whom had HM after GB [23, 29]. Only 2 prior studies reported patients who ultimately required esophagectomies for end-stage achalasia [18, 23].
The results of previous studies contrasted our series, where the majority of patients in Group 2 underwent additional interventions, either from complications of their bariatric surgery or for achalasia symptoms (or both). Only 2 of the 13 patients reported complete resolution of dysphagia (1 LGB+LHM and 1 DS+LHM), and 3 additional reported initial improvement followed by recurrence of dysphagia. Two LGB+LHM patients were offered esophagectomy due to recurrent aspiration pneumonia (Patient 6) and refractory dysphagia (Patient 7). While esophagectomy is a last resort, it is safe and effective for end-stage achalasia when less invasive treatments have failed [37]. The remnant stomach in LGB patients is typically a viable conduit.
Follow up for these previously published studies are short, many with 6 months or less, in the setting of an otherwise chronic incurable disease process. The average follow up for 21 studies reporting was 11.5 months, compared to our study which had an average follow up of 20.5 months. Treatment failure and a need for additional procedures are likely more common than suggested from the numerous published case reports.
Pseudo-achalasia is a known complication of laparoscopic band placement. Often, dysphagia symptoms will resolve after device deflation and removal of the band. However, there are indeed case reports of persistent symptoms, as with the patient in our study [38]. The single lap band patient in our study was managed with removal followed by PD and Botox injection with mild improvement in symptoms over a 4 year follow up period. It is difficult to conclude whether this patient definitively had primary (or secondary) achalasia given the chronicity of symptoms after band removal and only partial response to treatment.
Combined bariatric and achalasia procedures
Five publications were identified in the literature (Table 5) which describe combined bariatric and achalasia operations [1, 3, 6, 13, 14]. Only 1 patient in our series (Patient 4), in Group 1, underwent a combined initial treatment of achalasia (Botox) and primary bariatric procedure (LGB); they subsequently underwent a robotic-assisted LHM. Another patient was considered for a combined approach; however, insurance approval hindered this possibility. There was one combined revision procedure in our series: robotic-assisted conversion of sleeve gastrectomy to gastric bypass with Heller myotomy, which was complicated by anastomotic stricture and treated with EGD and dilation. Four published studies reported postoperative treatment response in 5 total patients, with either resolution of dysphagia or improvement in their prior symptoms [3, 6, 13, 14]. From these very limited results, a combined surgical approach appears to be an appealing approach, although only one of these publications had follow up longer than 6 months [6]. Future work is needed to prospectively compare combined treatment to staged treatment approaches.
The heterogenous nature of populations and treatments described in the literature, as well as the incomplete data, limited any valuable comparative statistical analysis. With the evidence currently available, a treatment algorithm was developed for achalasia in the morbidly obese with or without prior bariatric surgery (Fig. 2). In patients presenting for initial treatment of both achalasia and morbid obesity, a combined approach was recommended, specifically with LHM and LGB. This strategy allows for a single operation with simultaneous procedures, foregoing additional interventions. For patients with prior bariatric surgery, decision making likely depends on the initial procedure. The potential for worsening reflux with LSG coupled with the reflux provoking treatment of achalasia (with myotomy), warrants conversion to LGB. Patients with prior bypass may benefit from either LHM or POEM. Consideration of intervention, POEM vs LHM, may also be given to achalasia subtype. There is evidence to suggest that POEM offers greater myotomy length, which is favorable for type 3 achalasia [39]. The endoscopic nature of the POEM procedure avoids the adhesive hazards of reoperation, and the potential for POEM to worsen reflux is mitigated by the prior bypass. Conversely, there are potential disadvantages to POEM including access to a provider with expertise in this procedure, and coverage by insurance agencies. Formal studies would be needed to effectively evaluate this idea. Although the literature on combined procedures appears favorable and supportive of our hypothesis, this study was not powered to detect a difference in treatment options.
Limitations
The results of this article are limited both by the nature of achalasia as a chronic disease process, and in the study’s inherent design. The low overall prevalence of achalasia in bariatric surgery patients leads to a limited sample size. This is a retrospective study, which hinders the evaluation of different treatment modalities as well as the order of operative interventions for the concurrent conditions. For several patients it is not clear if postoperative symptoms are reflective of inadequate response to achalasia treatment or instead is secondary to their bariatric procedure. Symptoms of dysphagia and reflux are known potential complications of bariatric surgery that confound our ability to monitor response to treatment for achalasia. As not all initial evaluations or even procedures were performed at our institution, there is of course added potential variability. For those patients treated in our institution, practice patterns favored LHM over other common interventions such as pneumatic dilation or POEM, so evaluation of these other therapeutic modalities is limited.
Another difficulty in this patient population was the duration of time between initial bariatric surgery and diagnosis for achalasia. The average interval between bariatric surgery and achalasia diagnosis was 8.3 years postoperatively. We are unable to ascertain whether the majority of patients in Group 2 had preoperative symptoms for dysphagia. Two patients (Patients 10, 12), had reported symptoms concerning for achalasia preoperatively but further work-up was incomplete and decision-making on management is unclear in the medical record. Similarly, comparison studies underreported the onset of symptoms relative to bariatric surgery. Ideally, patients would have been assessed for possible achalasia symptoms prior to their initial bariatric surgery to better determine if the condition was preexisting or if it developed postoperatively de novo.
Follow up reporting has also inevitably affected data collection. Although the average duration was 20.5 months, for 4 of the 12 patients, follow up has been less than a year to date. While follow up in this study was longer than many comparable publications, it remains suboptimal given the chronicity of achalasia. Limited follow up makes drawing conclusions difficult in our series.
Weight loss was not always feasible to assess when bariatric surgery had been performed many years prior. The effect of achalasia treatment coupled with bariatric surgery on weight loss is an important question that should be addressed in future prospective studies.
The comparison studies are similarly limited by their retrospective nature and short follow up duration. Moreover, there exists a possibility of a selection bias for positive studies. Of the 22 comparison articles, only 1 mentions a patient needing multiple surgeries for incisional herniae following their prior bariatric procedure [30]. Another specifically describes a patient with no complications at 3 month follow up [22]. Aside from these notable exceptions, the comparison studies focus exclusively on the symptomatic relief of achalasia without commentary on any postoperative complications related to bariatric surgery. One potential reason for this is the difficulty capturing older data, as the studies often include 2 separate surgical procedures. This is particularly true in the groups with bariatric surgery prior to achalasia diagnosis.
Conclusions
Our study details the clinical course of 13 bariatric surgery patients who required procedural treatment for achalasia and demonstrates the relative lack of efficacy of prolonged symptomatic relief in this population. Complications and the need for additional procedures were common. Despite numerous treatment modalities, a clear optimal intervention for these patients remains unclear. Patients who underwent treatment for achalasia before having bariatric surgery tended to have a more favorable resolution of their symptoms, though no statistical analysis was performed. These conclusions underscore the tremendous need for further prospective study of this topic. Additionally, thorough and systematic symptom screening for esophageal dysmotility at the time of bariatric surgery evaluation should be incorporated into practice, prompting additional work-up as indicated.
References
Fisichella PM et al (2015) The surgical management of achalasia in the morbid obese patient. J Gastrointest Surg 19(6):1139–1143
Hong D et al (2004) Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg 14(6):744–749
O'Rourke RW et al (2007) Simultaneous surgical management of achalasia and morbid obesity. Obes Surg 17(4):547–549
Aiolfi A et al (2019) Management of esophageal achalasia after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg 29(5):1632–1637
Sadowski DC et al (2010) Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 22(9):e256–e261
Almogy G, Anthone GJ, Crookes PF (2003) Achalasia in the context of morbid obesity: a rare but important association. Obes Surg 13(6):896–900
Koppman JS et al (2007) Esophageal motility disorders in the morbidly obese population. Surg Endosc 21(5):761–764
Jaffin BW, Knoepflmacher P, Greenstein R (1999) High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg 9(4):390–395
Werner YB et al (2019) Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med 381(23):2219–2229
Kumbhari V et al (2013) Efficacy and safety of pneumatic dilatation for achalasia in the treatment of post-myotomy symptom relapse. Am J Gastroenterol 108(7):1076–1081
van Hoeij FB et al (2018) Management of recurrent symptoms after per-oral endoscopic myotomy in achalasia. Gastrointest Endosc 87(1):95–101
Vigneswaran Y et al (2014) Peroral endoscopic myotomy (POEM): feasible as reoperation following Heller myotomy. J Gastrointest Surg 18(6):1071–1076
Hagen ME et al (2010) Morbid obesity with achalasia: a surgical challenge. Obes Surg 20(10):1456–1458
Kaufman JA, Pellegrini CA, Oelschlager BK (2005) Laparoscopic Heller myotomy and Roux-en-Y gastric bypass: a novel operation for the obese patient with achalasia. J Laparoendosc Adv Surg Tech A 15(4):391–395
Pandolfino JE, Gawron AJ (2015) Achalasia: a systematic review. JAMA 313(18):1841–1852
Schlottmann F, Patti MG (2018) Esophageal achalasia: current diagnosis and treatment. Expert Rev Gastroenterol Hepatol 12(7):711–721
Coleman HG, Xie SH, Lagergren J (2018) The epidemiology of esophageal adenocarcinoma. Gastroenterology 154(2):390–405
Bashir U et al (2019) Peroral endoscopic myotomy is feasible and safe in a gastric bypass population. Obes Surg 29(11):3523–3526
Rohof WO et al (2013) Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 144(4):718–725; quiz e13–4
Qiu J et al (2018) Revisional bariatric surgery for weight regain and refractory complications in a single MBSAQIP Accredited Center: what are we dealing with? Obes Surg 28(9):2789–2795
Spann MD et al (2020) Efficacy and safety of recurrent paraesophageal hernia repair with Roux-en-Y gastric bypass. Am Surg 86(3):250–255
Herbella FA et al (2005) Obesity and symptomatic achalasia. Obes Surg 15(5):713–715
Boules M et al (2016) Achalasia after bariatric surgery. J Laparoendosc Adv Surg Tech A 26(6):428–432
Sanaei O et al (2019) Peroral endoscopic myotomy for the treatment for achalasia patients with Roux-en-Y gastric bypass anatomy. Endoscopy 51(4):342–345
Benavente-Chenhalls LA, Sherman V, Reardon PR (2011) Laparoscopic Heller myotomy and gastric bypass for achalasia after vertical banded gastroplasty. Surg Obes Relat Dis 7(5):664–665
Birriel TJ, Claros L, Chaar ME (2017) Laparoscopic Heller myotomy after previous Roux-en-Y gastric bypass. Surg Obes Relat Dis 13(11):1927–1928
Chapman R et al (2013) Laparoscopic Heller's myotomy for achalasia after gastric bypass: a case report. Int J Surg Case Rep 4(4):396–398
Johnson WD, Marshall MB (2016) Surgical management of achalasia in a patient with previous gastric bypass. Innovations (Phila) 11(3):214–216
Kim D, Pullat R, Crowley N (2019) Robotic redo Heller myotomy after laparoscopic Heller myotomy in a patient with recurrent achalasia after a Roux-en-Y gastric bypass. Am Surg 85(3):e162–e163
Luo RB, Montalvo D, Horgan S (2017) Peroral endoscopic myotomy after gastric bypass: an effective solution for de novo achalasia. Surg Obes Relat Dis 13(2):e1–e3
Masrur M, Gonzalez-Ciccarelli LF, Giulianotti PC (2016) Robotic Heller myotomy for achalasia after laparoscopic Roux-en-Y gastric bypass: a case report and literature review. Surg Obes Relat Dis 12(9):1755–1757
Nguyen D et al (2016) Heller oesophagomyotomy as treatment for achalasia after gastric bypass for morbid obesity. Ann R Coll Surg Engl 98(1):e3–5
Oh HB, Tang SW, Shabbir A (2014) Laparoscopic Heller's cardiomyotomy and Roux-En-Y gastric bypass for missed achalasia diagnosed after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 10(5):1002–1004
Ramos AC et al (2009) Achalasia and laparoscopic gastric bypass. Surg Obes Relat Dis 5(1):132–134
Torghabeh MH et al (2015) Achalasia 5 years following Roux-en-y gastric bypass. J Minim Access Surg 11(3):203–204
Yang D, Draganov PV (2014) Peroral endoscopic myotomy (POEM) for achalasia after Roux-en-Y gastric bypass. Endoscopy 46(Suppl 1):E11–E12
Aiolfi A et al (2018) Esophagectomy for end-stage achalasia: systematic review and meta-analysis. World J Surg 42(5):1469–1476
Losh JM, Sanchez B, Waxman K (2017) Refractory pseudoachalasia secondary to laparoscopically placed adjustable gastric band successfully treated with Heller myotomy. Surg Obes Relat Dis 13(2):e4–e8
Khan MA et al (2017) Is POEM the answer for management of spastic esophageal disorders? A systematic review and meta-analysis. Dig Dis Sci 62(1):35–44
Acknowledgements
The authors of this paper would like to thank the OHSU Department of Surgery for facilitating the data collection required for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Trevor Crafts, Victoria Lyo, Priya Rajdev, and Stephanie Wood have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crafts, T.D., Lyo, V., Rajdev, P. et al. Treatment of achalasia in the bariatric surgery population: a systematic review and single-institution experience. Surg Endosc 35, 5203–5216 (2021). https://doi.org/10.1007/s00464-020-08015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08015-3