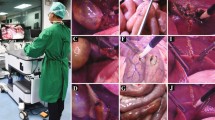
Abstract
Objective
To introduce a novel laparoscopic training system with a continuously perfused ex-vivo porcine liver for hepatobiliary surgery.
Background
Existing models for laparoscopic training, such as box trainers and virtual reality simulators, often fail to provide holistic training and real haptic feedback. We have formulated a new training system that addresses these problems.
Methods
Real-Liver Laptrainer consists of a porcine liver, customized mannequin, ex-vivo machine perfusion system, and monitoring software. We made a detailed comparison of Real-Liver Laptrainer with the LapSim virtual reality simulator and the FLS Trainer Box systems. Five laparoscopic surgeons assessed the new system on multiple features. We assessed the performances of 43 trainees who used the new system to perform laparoscopic cholecystectomy (LC) three times.
Results
Real-Liver Laptrainer offered more functions and better tactile feedback than the FLS or LapSim system. All five surgeons graded the quality of the new system as realistic. The utility of the system for training was scored as 3.6 ± 1.1 on a scale of 1–5. Between the first and third attempts, the number of successfully performed LCs increased (9 vs 14 vs 23; P = .011), while the numbers of liver damage incidents (25 vs. 21 vs. 18, P = .303) and gallbladder perforations decreased (17 vs. 12 vs. 9, P = .163). The mean LC operation time significantly decreased (63 vs. 50 vs. 44, P < .0001).
Conclusion
Real-Liver Laptrainer is a feasible, stable, and practical training model that has potential for improving the laparoscopic skills of surgeons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopy has become the standard approach for many conditions in most surgical specialties, driven by the desire for less surgical trauma, faster postoperative recovery, shorter hospital stay, and better cosmetic results [1, 2]. However, laparoscopy training offers some challenges, including the “fulcrum effect” (i.e., the direction of movement of the hands is opposite to the direction of movement of the instrument [3]), limited tactile feedback or haptics, two-dimensional vision [4], and narrow exposure of the operative site. Learning and building these skills in the operating room is not ideal [5].
Many new techniques for laparoscopic training have been explored. Today, the most basic skills can be acquired in a box trainer, which is simple structure that is easily manipulated [6, 7]. Specific operation models can be trained in virtual reality (VR) simulators [8, 9]. However, these models are inadequate in providing advanced surgical skill training [7, 10] and unable to provide haptic sensations through force feedback [11], which are fundamental in the simulation of surgical realities [12]. Live animal models can also be used to acquire laparoscopic skills. In practical use, however, animal models are costly and inconvenient, as they require specialized personnel and permission from local bioethics committees [11, 13]. In view of the shortage and high costs of simulators, the development of new models for clinical training in laparoscopy has gained considerable importance.
In the present study, we describe our development of a more effective and affordable system, Real-Liver Laptrainer, to help trainees acquire a wide range of skills in performing laparoscopic surgery. Real-Liver Laptrainer combines the use of a porcine liver, customized mannequin, ex-vivo machine perfusion, and computer monitoring to provide a more holistic and realistic environment for trainees to learn skills.
Methods
Procurement and preservation of porcine livers
The porcine livers used in this study were from Zhaoyang Slaughter House in Xi’an, China. As the slaughterhouse forbade us from injecting anticoagulant into the live pig before liver procurement, we faced the issue of thrombogenesis in the porcine liver vessels. Pigs in the slaughterhouse were executed by cardiac bloodletting. The time spent on the procedure from bloodletting to portal vein catheterization and perfusion was controlled to be within 10 min. This time period is sufficiently short to protect most of the blood vessels in the liver from thrombosis. Then, we used a liver acquisition technique that is used in liver transplantation procedures when heparinization is not performed in advance.
Customized mannequin
The customized mannequin was designed to simulate the human anatomical structure, particularly the pneumoperitoneum, during laparoscopic surgery. An artificial abdominal wall was designed and customized to reduce the complexities of training. Real pneumoperitoneum is difficult to achieve, as it would require the use of a CO2 insufflator and related instruments. The customized abdominal wall was a dome-like cushion made from medical silica and wire mash, which was wrapped into silica gel before it was heated and formed. The molded and silica-embedded wire mash can be used to simulate the elasticity and rigidity of the abdominal wall under the state of pneumoperitoneum. Four snap fasteners, though which the cushion could be installed and removed easily from the mannequin, were placed at the two sides of the artificial abdominal wall. These fasteners also provided the fulcrum around which the laparoscopic instruments were operated (Fig. 1).
Ex-vivo machine perfusion system
An ex-vivo machine perfusion system was designed to simulate the virtual blood cycle, blood supply to the liver, and hemorrhaging during the operation. The system consisted of four parts: two peristaltic pumps (Longer Pump Co.), tubes, a vacuum extractor (SMAF Co.), and two reservoirs. Different peristaltic pump models were used to simulate the physiological blood flow dynamics through the human portal vein (T300 pump; flow rate range: 0–1200 ml/min) and the hepatic artery (T-S107 and JY15-12 pumps; flaw rate range: 0–170 ml/min). Tubes communicated between the reservoirs and pumps, as well as between the pumps and discharge points, which were adapted to be associated with the porcine liver. We used customized tubes having similar diameters, thicknesses, and elasticities to the portal vein, postcava, and hepatic artery. The purpose of the vacuum extractor was to collect leaking fluid in the artificial abdominal cavity during training. Collected fluid was treated as hemorrhage during the operation. One of the two reservoirs played the role of the heart, which circulated the fake blood. The other reservoir contained the collected fluid. The mass of this fluid, measured by an electronic scale, was used in the monitoring software to calculate the bleeding volume (Fig. 1).
Monitoring software
The monitoring software was written based on LabVIEW 8.6. The software consisted of four modules for regulating flow, displaying the hemorrhage volume, displaying the portal vein and postcava pressures, and displaying the laparoscopic image. With this software, trainees could easily regulate the “blood flow” to any clinical status they wanted to simulate, while directly monitoring pressure changes and hemorrhaging. Moreover, because the software could display the laparoscopic image, there was no need for a specific monitor for the laparoscope (Fig. 2).
Training procedures on the real-liver Laptrainer
Installing and connecting porcine liver
The trainee removed the liver from the organ cluster and fixed the plastic connectors to the inside of the vessels. The trainee fixed the other point of the connectors to the inside of the corresponding tube. Finally, the trainee installed the liver (Fig. 3).
Using the ex-vivo machine perfusion system
The trainee properly positioned the tubes and clicked the training program to start the pump. The trainee gradually increased the speed of the pumps according to the change in liver shape, until the human blood flow rate was reached.
Locating and placing trocars
The trainee selected the type and size of trocars needed, and placed the trocars in their proper places. The model allowed the trainee to start the procedure from the skin incision and trocar insertion to simulate clinical realities.
Inspecting the abdominal cavity and locating the gallbladder
The trainee practiced simple laparoscopy to inspect the abdominal cavity and locate the gallbladder.
Selecting and utilizing instruments
One goal of the training system was to train students in choosing the right laparoscopic instruments for laparoscopic cholecystectomy (LC) and how to use them properly in operation. Therefore, the trainee used laparoscopic training instruments in this simulator as they would do clinically.
LC training procedures
The trainee could be trained in the major procedures of LC in this simulator, including: identifying, grasping, and retracting the gallbladder fundus, dissecting Calot’s Triangle, clipping the cystic duct and cystic artery, and removing the gallbladder (Fig. 4).
Comparison of functions on the real-liver Laptrainer, FLS trainer box, and LapSim
Training functions of the Real-Liver Laptrainer, the FLS Trainer Box (Limbs & Things Ltd.), and the LapSim (Surgical Science Ltd.) VR simulators were compared in terms of basic and advanced skills, team training, surgery type, and real tactile feedback. Parameters and data were obtained from the simulator devices and the product brochures.
Evaluation of real-liver Laptrainer by expert surgeons
Five attending surgeons were asked to evaluate the developed simulator. Each surgeon had at least 5 years’ experience in performing LCs or had performed at least 500 LC procedures. After performing LC in the Real-Liver Laptrainer, each surgeon graded the quality of the mannequin with porcine liver and the perfusion system from the perspectives of the anatomical simulation, the operative action, and the reaction of tissues to actions (as realistic or nonrealistic), the haptics during operation (as poor, fair, good, or excellent), and the laparoscopic performance (as successful, difficult, or impossible). On a scale of 1–5, they were asked to compare the procedure in the simulator with LC in the operating room, and to quantify their impression of the value of the simulator for training. Criteria of these evaluations are given in Table 1.
Application of real-liver Laptrainer in laparoscopic training and outcomes
We enrolled 43 first-year surgery students without any experience of laparoscopic surgery from Xi’an Jiaotong University Medical College. Each participant performed LC thrice in the Real-Liver Laptrainer system. Their performances were evaluated by the following measures: time, successful installation of porcine liver, stability of the perfusion state, successful removal of the gallbladder, and damage to the model (e.g., rupture, laceration, or disruption of any part).
Statistical analysis
Data of training outcomes were expressed as the mean and range. Data of experts’ assessment scores were expressed as the mean ± standard deviation (SD). Statistical significance was determined by using SPSS 12.0 software (SPSS, San Rafael, CA, USA). Analysis of variance followed by a χ 2 test was used to determine any significant difference among three attempts. A P value of less than .05 was considered significant.
Results
Comparison of different simulators
Real-Liver Laptrainer had prominent advantages compared to the FLS Trainer Box and LapSim VR simulator (Table 2). Real-Liver Laptrainer can teach basic skills (touching, grasping, traction, and translocation) similarly to the FLS Trainer Box and advanced skills similarly to the LapSim VR simulator. Trainees can acquire real tactile feedback on Real-Liver Laptrainer in contrast to the haptic feeling produced by resistance on LapSim. In addition to surgical training, Real-Liver Laptrainer can be used to teach camera operation during surgery. Among the three training models, only Real-Liver Laptrainer allows customer-made models for individualized training. Currently, Real-Liver Laptrainer can only train on LC procedures, whereas LapSim provides training models of appendectomy, cholecystectomy, hysterectomy, and other bariatric and gynecologic surgeries.
Expert evaluation of real-liver Laptrainer
All five experts graded the quality of the mannequin with porcine liver and the perfusion system as realistic. Experts graded the haptics of the system as good (2 experts), excellent (2 experts), or fair (1 expert). All experts considered their laparoscopic performance in Real-Liver Laptrainer to be successful. They considered the simulation to be easier than the actual procedure, with a rating of 2.6 ± 0.6. Three experts considered the simulation to have medium difficulty, and two experts considered to simulation to be easy. In terms of training utility, experts gave the system a mean score of 3.6 ± 1.1.
Application of real-liver Laptrainer in laparoscopic training and outcomes
Forty-three participants completed three LCs in the new model (total 129 LC simulations). All installations of the porcine liver were successful. The perfusion system worked well 98 times. In seven LC procedures, the software failed to start the perfusion pumps. In these cases, the pumps were enabled manually and fixed later. In 12 LCs, the portal vein pressure did not reach the desired level, but the simulation could still continue. In these cases, the problem was due to leaking at a connection joint of a tube inside the mannequin. In 12 LCs, the pressure in the liver was so high that the tube system broke the joints. In these cases, we stopped the perfusion system and changed to another well-perfused porcine liver. Good stability of the perfusion state was achieved for the other 117 simulated LCs.
Outcomes of the three LC attempts are summarized in Table 3. From the first to the third attempt, the number of successfully completed operations increased (9 vs. 14 vs. 23; P = .011), the number of episodes of liver damage decreased (25 vs. 21 vs. 18, P = .303), and the number of perforations of the gallbladder decreased (17 vs. 12 vs. 9, P = .163). The mean LC time of the last attempt was 12 and 30% shorter than that of the second and first attempt, respectively (63 vs. 50 vs. 44 min, P < .0001). In some cases, trainees touched the connecting tubes with their sharp instruments but did not cause damage because the tubes were thick and hard, which prevented laceration. In such cases, there was little damage to other parts of the model (e.g., rupture, laceration, or other disruption).
Cost
This model incorporates reusable and disposable components, which are readily available and inexpensive. Reusable parts, including the laparoscope and training instrument, cost only ¥20,000 in total (about $3300). Disposable parts cost ¥30 (about $5).
Discussion
Here, we describe an effective and affordable system to help trainees acquire a wide range of skills in performing laparoscopic surgery. Compared to conventional training simulators, a prominent advantage of Real-Liver Laptrainer is that it uses several sources to create a more holistic and realistic clinical scenario. Real-Liver Laptrainer overcomes the shortcomings of LapSim in producing force feedback. Different tactile responses associated with different tissues could be captured and felt directly by the trainees, thus facilitating the acquisition of fundamental skills. Real-Liver Laptrainer permits training in advanced laparoscopic techniques that are unattainable using FLS Trainer Box. Trainees would be able to transfer their acquired skills to the clinical setting.
Haptic feedback is an indispensable part of laparoscopic training. Vapenstad [14] studied the validation of a proficiency-based training setup with Xitact IHP haptic feedback instrument ports on the LapSim VR simulator. They concluded that the simulation of haptic feedback influences the training performance on laparoscopic simulators and is an important part of validating a training setup. After training with and without haptic feedback, 90% of surgeons preferred the system without haptic feedback [15]. They perceived the system with haptic feedback to add additional friction, making it unrealistic and not mechanically transparent. In our study, five laparoscopic surgeons performed LCs on the Real-Liver Laptrainer. Most of them spoke highly of the system, regarding it as a new, effective training model and considering the real tactile feedback to be the most attractive feature. However, one expert considered the system to be lacking industrial design and recommended further modifications.
Students were trained on the system 129 times for LC training. Our findings revealed the good internal consistency and stability of the system. The mean operating time of trainees decreased significantly from 63 to 44 min across three attempts. Operating time was defined as the time from when the trainee first grasped the gallbladder until they pulled the gallbladder out of the abdominal cavity. Time spent assembling the simulator was recorded separately. The success rate significantly increased from 21 to 53% across the three attempts. Rates of damage to the liver and gallbladder decreased, but not significantly. The lack of significance in this result may have been due to poor accuracy or refinement of the definition and classification of damage.
Real-Liver Laptrainer is a problem-based learning model that focuses on the main procedures of laparoscopic surgeries for the hepatobiliary system. In this study, trainees completed the major procedures of LC, during which they learned grasping, dissecting, suturing, and knotting. They learned these skills in a reasonable order and simultaneously, rather than learning one skill at a time. Another important advantage of the new system is that the real liver offers a regional blood supply and provides training in hemorrhage control. Real-Liver Laptrainer shows blood loss immediately, thereby indicating the level of performance. Before training, some students participated in the procurement and preservation of porcine livers at the slaughterhouse. These students learned and performed some techniques relevant to liver transplantation. This organ transplantation training can benefit surgical students and young doctors because, at present, no medical center in China provides such training. For this study, we chose LC as the procedure of interest because its operation is relatively simple and easy to quantify. We have also used this system for training in laparoscopic liver resection because the Real-Liver Laptrainer can mimic the portal pressure and blood flow parameters through the liver. Several experts evaluated the system and determined that it can be used to reproduce the liver resection process. However, we have not yet acquired enough data on liver resection training for those results to be reported in this article.
Our system can be reproducibly used in laparoscopic training. After LC, the remaining liver can be used for laparoscopic hepatectomy and hepatic cyst models, which can be applied to the training of laparoscopic fenestration and drainage. Nevertheless, the addition of other animal organs (e.g., lung, stomach, and uterus) to this simulator would expand the training range to thoracic, gynecologic, and urologic laparoscopic surgeries. The open design of the system allows users to build models autonomously to train different surgeries or even to innovate operations.
Our study and system have some limitations. Major problems in the use of Real-Liver Laptrainer are regarding the lack of techniques to preserve the porcine hepatic artery, which may affect the perfusion results. We will cooperate more closely with the slaughterhouse in the future to guarantee double perfusion (via the portal vein and hepatic artery). Moreover, the stability of the perfusion system cannot be guaranteed, and the industrial design of the simulator is not yet satisfactory. We are currently working with professional experts to improve our novel system.
In summary, the ex-vivo biological training system developed in the present study could provide better opportunities for trainees to acquire and practice laparoscopic skills in a more realistic environment, which is unattainable using conventional VR simulators and box trainers. Real-Liver Laptrainer is a feasible and effective training system that is economically accessible and could be used efficiently to improve skill teaching and learning in many areas and for many purposes. Development of the corresponding assessment system is in progress. At this time, the first laparoscopic student tests have been performed. In future studies, we plan to apply Real-Liver Laptrainer in more hospitals and to train more surgeons to test its efficacy and feasibility. Furthermore, more studies using this model are warranted, specifically evaluating trainee acquisition of skills and qualitative comparisons with training on box trainers and VR simulators.
References
Hendolin HI, Paakonen ME, Alhava EM, Tarvainen R, Kemppinen T, Lahtinen P (2000) Laparoscopic or open cholecystectomy: a prospective randomised trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg 166:394–399
Lee JH, Yom CK, Han HS (2009) Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 23:1759–1763
Crothers IR, Gallagher AG, McClure N, James DT, McGuigan J (1999) Experienced laparoscopic surgeons are automated to the “fulcrum effect”: an ergonomic demonstration. Endoscopy 31:365–369
Perkins N, Starkes JL, Lee TD, Hutchison C (2002) Learning to use minimal access surgical instruments and 2-dimensional remote visual feedback: how difficult is the task for novices? Adv Health Sci Educ Theory Pract 7:117–131
Stefanidis D, Scerbo MW, Korndorffer JR Jr, Scott DJ (2007) Redefining simulator proficiency using automaticity theory. Am J Surg 193:502–506
Munz Y, Kumar BD, Moorthy K, Bann S, Darzi A (2004) Laparoscopic virtual reality and box trainers: is one superior to the other? Surg Endosc 18:485–494
Dunkin B, Adrales GL, Apelgren K, Mellinger JD (2007) Surgical simulation: a current review. Surg Endosc 21:357–366
Gallagher AG, Ritter EM, Champion H, Higgins G, Fried MP, Moses G, Smith CD, Satava RM (2005) Virtual reality simulation for the operating room: proficiency-based training as a paradigm shift in surgical skills training. Ann Surg 241:364–372
Seymour NE (2008) VR to OR: a review of the evidence that virtual reality simulation improves operating room performance. World J Surg 32:182–188
Rassweiler J, Klein J, Teber D, Schulze M, Frede T (2007) Mechanical simulators for training for laparoscopic surgery in urology. J Endourol Soci 21:252–262
Schijven M, Jakimowicz J (2003) Virtual reality surgical laparoscopic simulators. Surg Endosc 17:1943–1950
Dankelman J (2008) Surgical simulator design and development. World J Surg 32:149–155
Velazquez-Avina J, Sobrino-Cossio S, Chavez-Vargas C, Sulbaran M, Monkemuller K (2014) Development of a novel and simple ex vivo biologic ERCP training model. Gastrointest Endosc 80:1161–1167
Vapenstad C, Hofstad EF, Bo LE, Chmarra MK, Kuhry E, Johnsen G, Marvik R, Lango T (2013) Limitations of haptic feedback devices on construct validity of the LapSim(R) virtual reality simulator. Surg Endosc 27:1386–1396
Vapenstad C, Hofstad EF, Lango T, Marvik R, Chmarra MK (2013) Perceiving haptic feedback in virtual reality simulators. Surg Endosc 27:2391–2397
Acknowledgements
The authors want to thank all medical students and surgeons for their participation in all trials and the staff at Institute of Advanced Surgical Technology and Engineering of the First Affiliated Hospital of Xi’an Jiaotong University for the help and facilities to carry out this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
This study was supported by a grant from the National Natural Science Foundation of Equipment awarded to Yi Lv (Grant No. 81127005). Wenyan Liu, Xinglong Zheng, Rongqian Wu, Yinbin Jin, Shu Kong, Jianpeng Li, Jianwen Lu, Huan Yang, Xianghua Xu, Yi Lv, and Xiaogang Zhang have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Liu, W., Zheng, X., Wu, R. et al. Novel laparoscopic training system with continuously perfused ex-vivo porcine liver for hepatobiliary surgery. Surg Endosc 32, 743–750 (2018). https://doi.org/10.1007/s00464-017-5731-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5731-6