Abstract
Background: Previous studies have failed to establish clear advantages for the use of stereoscopic visualization systems in minimal-access surgery. The aim of this study was to objectively assess whether stereoscopic visualization improves performance on bench models using the da Vinci robotic system. Methods: Eleven surgeons carried out a series of four tasks. Positional data streamed from the da Vinci system was analyzed by means of a previously validated custom-designed software-package. An independent blinded observer scored errors. Statistical analysis included the Wilcoxon signed rank test. A p < 0.05 was deemed significant. Results: We found significant improvements in all tasks and for all parameters (p < 0.05). In addition, a significantly lower number of errors was scored using the stereoscopic mode as compared to the standard two-dimensional image (p < 0.001). Conclusion: Robotic-assisted performance on bench models is more efficient and accurate using stereoscopic visualization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
During the last 2 decades, robotic systems have evolved from simple instrument stabilizers, through voice-controlled camera stabilizers (AESOP; Computer Motion, CA, USA), to more comprehensive solutions, such as telemanipulator systems (Zeus and de Vinci [Intuitive Surgical, Mountain view, CA, USA]). At the same time, visualization technologies followed a similar path of evolution, with the ultimate aim of restoring the surgeon’s stereoscopic vision.
The application of stereoscopic visualization systems in laparoscopic surgery has gone through several stages of development since the early 1990s [18, 19]. The first generation of stereoscopic vision systems, which consisted of two separate video cameras attached to a single optical channel (standard endoscope) that alternately transmitted signals to a head-mounted device, produced sufficient stereoscopic visualization. However, these systems did not find much favor with the surgical community because they were ergonomically unsuitable. Moreover, they were associated with neurophysiological side effects that were attributed mainly to the optical shuttering, which caused ocular fatigue, headaches, and nausea [4, 9, 13, 17]. The next generation of stereoscopic vision systems relied on the same basic principle. However, the images were displayed on a monitor, while the surgeon wore simple lightweight polarizing glasses, so that each eye received only a single respective signal. The right and left signals then fused in the visual cortex to produce a three-dimensional (3D) image. Although several studies have demonstrated the advantages of 3D systems [3, 16], others have suggested that the benefit is negligible. This lack of proven efficacy, together with the excessive costs of these systems, has led experienced surgeons to regard them as unnecessary. Also, these studies have shown that with increasing skill and experience, the acquisition of other visual and spatial cues adequately compensates for the lack of stereoscopic vision [3, 5, 6, 10, 11].
It was expected that surgical robotic systems would be able to overcome most of the shortcomings of conventional minimally invasive surgery (MIS). The da Vinci telemanipulator system is a master–slave system where the surgeon is seated at the master console, which is equipped with ergonomically designed controls together with a stereoscopic visualisation system (Fig. 1). The slave unit carries out the operative procedure on the patient using two mechanical arms. The arms are equipped with wrist-like working tips with seven degrees of freedom of motion, almost replicating the human wrists.
The da Vinci stereoscopic visualization system is comprised of four subunits that are interconnected (Fig. 2). The first unit features a custom-designed endoscope with two separate optic channels with a distance of 6 mm between their longitudinal axis; thus, it re-creates the most important aspect of stereopsis, which is binocular disparity. This is connected to a camera head, which holds two three charge-coupled device (CCD) chip cameras. The image is then processed through a noise reduction system, enhanced, scanned, and then displayed through the stereo viewer, which consists of two high-resolution monitors, where the surgeon receives a fused 3D image of the operative field.
A scheme of the stereoscopic visualization system. Two types of scopes are available, 0° and 30°, equipped with two separate optic channels and light sources. The system delivers 752 (horizontal) ×582 (vertical) pixels. Image focusing is managed through a foot switch or buttons located at surgeon console and under the surgeon’s control. Magnification is also surgeon-controlled by adjusting the depth of the endoscope. The assistant can watch the procedure on a standard monitor positioned on top of the vision cart.
From recent studies, it is evident that the learning curve for robotic surgery is shorter than that for laparoscopic surgery [7, 12]. The aim of this study was first to objectively assess whether stereoscopic visualization improves the surgeon’s performance using the da Vinci robotic system on tasks carried out on bench models and then to quantify these results by means of motion analysis.
Materials and Methods
Subjects
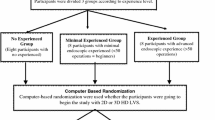
Eleven surgeons with limited experience with the use of the da Vinci system (all of whom participated in a previous study using bench models only) took part in this study. They were randomized to begin the experiment either with two-dimensional (2D) or 3D vision (using the 2D/3D selector on the surgeon’s console) and blinded to mode selection. We decided to use the 2D/3D selector rather than an external monocular imaging system because the 2D mode on the da Vinci is equivalent to a standard monitor image used for conventional laparoscopy.
System
The system used was the da Vinci Surgical System. In this study, we used two regular-size robotic needle holders. The tasks were set up in a standard laparoscopic box trainer. The camera angle and distance with respect to the working surface as well as the angles of the instruments were standardized and fixed for all subjects.
Tasks
There were four tasks designed to replicate the most common maneuvers used in robotic-assisted laparoscopic surgery.
The first task was a transfer task. Two disks, 5 cm in diameter, were positioned 2.5 cm apart from each other. Each disk was divided into four quadrants with different colors and numbers. On each quadrant there was one curved needle. Subjects were instructed to pick a needle from the left-sided disk with the left-hand instrument and transfer it to the right-hand instrument before placing it as gently as possible on the corresponding colored zone/number on the right-sided disk (Fig. 3A). The drill was repeated four times from each side.
Tasks 1–4. A Pick and place—moving curved needles from right to left to corresponding colors/numbers (used in case of color blindness). B Rope passing—“walking along the rope” by grasping it on the marks, from top to bottom, and returning to top, C Intracorporeal knot tying—inserting three stitches in a suturing pad following standardized instructions. Suture material used was Vicryl 3/0. D V-box—similar to the first task but within very limited space, moving delicate foam balls measuring 4 mm in diameter.
The second task was rope passing. The subjects were instructed to pass the rope between right- and left-hand instruments by grasping it at predetermined marks, from one end to the other and back to the starting point. The rope was 2 mm thick and 30 cm long, with marks at every 1 cm (Fig. 3B).
The third task was a suturing task. Subjects were asked to insert three consecutive sutures in a suturing pad using predetermined marks as entry and exit points (Pharmabotics, UK ) (Fig. 3C). Each knot was tied with four throws. The suture material was Vicryl 3/0 (USSC).
The last task was the V-box task. This task was specifically designed to simulate maneuverability within a confined space, with emphasis on the wristed working tips. This novel model is comprised of two standard laboratory well stands (TPPR, Europe/Switzerland). Each stand is divided into six wells measuring 2.4 cm in diameter on the left and 1.2 cm on the right. The stands were positioned and fixed in a 90° angle, thus creating a V-shaped model. Subjects were asked to grasp foam balls measuring 4 mm in diameter from the larger wells using the left-hand instrument and then transfer them to the right-hand instrument before placing them into the smaller wells with the corresponding colors and back again to the larger wells (Fig. 3D).
Assessment
The assessment of performance was carried out using qualitative and quantitative measures.
Qualitative analysis
All task performances were recorded on digitalized videotapes. A blinded observer watched the videos and scored errors using a simple error score. Errors were defined according to tasks (Table 1).
Quantitative analysis
The analysis was performed by recording positional data using the system’s Application Programming Interface (API), which is a protocol for data streaming. It allows authorized users to access positional data such as the x, y, and z coordinates of the master control. The data were analyzed using specifically designed software, Robotic Video Motion Analysis Software (RoViMAS). This package is based on the Imperial College Surgical Assessment device (ICSAD), a previously validated motion analysis system designed by the computing division at Imperial College London [1, 2]. This software analyzes positional data and other kinematics and generates results such as time taken, number of movements made, and total distance traveled by each hand, number of occurrences of distances traveled per unit time, average distance traveled per movement, trajectory calculations, densities of movements per unit surface, etc. (Fig. 4).
RoViMAS output sheet. The analysis includes all positional data processed per task and per subject. The tracing seen in the upper part represents the hand movements, and a zoom-in option is available at any given point on the tracing. There is a 3D reconstruction of hand movements, trajectories, and flight path. It can be synchronized with video in real time.
Statistical analysis
Since the data were nonparametric, the Wilcoxon signed rank test was used and a p < 0.05 was deemed significant.
Results
Eleven surgeons completed the four tasks under both conditions (2D and 3D imaging). Significant differences in performance were found between 2D and 3D in all of the tasks for time taken, total number of movements made, total distance traveled by both hands, and number of errors made (Fig. 5A–D). Percentage improvements were highly significant, as noted in Table 2. Even more interesting were the findings of a significantly higher average movement path length by each hand when the subject was performing with stereoscopic visualization (Fig. 6A,B). This pattern was consistent throughout the study. The surgeons’ hands traveled longer distances, but targeting was better and fewer movements were needed to complete the tasks.
A–D Box plots for time taken, total number of movements, total path length, and number of errors made. In each box plot, all four tasks are represented. The median is the horizontal dark line, the interquartile range is the colored box and the whiskers represent the whole range. Outliers are presented as marked and numbered dots. The p values are inserted in the box plots.
In summary, we found significant improvements in performance for all parameters when stereoscopic visualization was used, as analyzed by both quantitative and qualitative methods of assessments.
Discussion
With the advent of laparoscopic surgery, surgeons have had to adapt to the loss of direct vision, as a 3D image, of the operative site, the lack of tactile feedback and compromised hand–eye coordination. The use of long instruments, with limited degrees of freedom, through fixed ports (the fulcrum effect) further reduced the surgeon’s dexterity. It is well established that in open surgery most of the sensory input is derived from vision, whereas the remainder comes from tactile feedback. However, in laparoscopic surgery, the surgeon is forced to rely even more on the visual input as tactile feedback decreases [8, 14, 15]. Thus, the prolonged learning curve associated with laparoscopic surgery is partly due to the lack of stereoscopic vision.
Although the da Vinci system provides true stereoscopic vision, there is a total lack of tactile feedback; thus, the operator has to rely solely on the visual input. However, this study clearly demonstrates, by means of objective measures, that performance using the 3D mode was significantly better than that in 2D—mainly because visualization mode was the only condition changed throughout the experiment. Time taken for the execution of all tasks was reduced by as much as a third. Dexterity improved by as much as 25%, as reflected by the reduction in the total number of movements made and distance traveled by both hands. Accuracy also improved by nearly 100%, as demonstrated by the reduction in the number of errors made.
The finding of higher average movement path length in the 3D mode, coupled with the reduction in the total number of movements made, as compared to the 2D mode for all the tasks suggests that surgeons have less difficulty in targeting the instruments and do so with higher precision and in less time. The use of motion analysis provides us with quantitative measures of performance, thus enabling us to objectively assess dexterity parameters such as number of movements, path length, and time taken to complete a task, as well as other parameters.
One of the limitations of this study is the use of the error score, which was developed due to the inability of motion analysis to assess quality of performance. One observer carried out the error scoring. This is a visual count of performance errors, anchored by a number of clear descriptors, which could be subject to bias. However, this potential bias was minimized by the fact that the observer was blinded.
Future studies should integrate more performance parameters, such as trajectories of instrument tips (flight path) and densities of instrument location throughout a task (demonstrating the actual working perimeter), as well as concentrating on the development of objective methods for the assessment of the quality of the final product.
In conclusion, this study has demonstrated the advantage of stereoscopic (3D) vision over monocular (2D) vision in a dry lab experiment with the da Vinci system. In view of the complete lack of tactile feedback, the reliance on visual cues necessitates the development of visualization systems of the highest quality to enhance the performance of surgical procedures with telemanipulator systems.
References
V Datta S Mackay M Mandalia A Darzi (2001) ArticleTitleThe use of electromagnetic motion tracking analysis to objectively measure open surgical skill in the laboratory-based model. J Am Coll Surg 193 479–485 Occurrence Handle10.1016/S1072-7515(01)01041-9 Occurrence Handle1:STN:280:DC%2BD3Mnmtlanug%3D%3D Occurrence Handle11708503
V Datta A Chang S Mackay A Darzi (2002) ArticleTitleThe relationship between motion analysis and surgical technical assessment. Am J Surg 184 70–73 Occurrence Handle10.1016/S0002-9610(02)00891-7 Occurrence Handle12135725
A Chan S Chung A Yim J Lau E Ng A Li (1997) ArticleTitleComparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc 11 438–440
WK Cheah JE Lenzi J So F Dong CK Kum P Goh (2001) ArticleTitleEvaluation of a head-mounted display (HMD) in the performance of a simulated laparoscopic task. Surg Endosc 15 990–991 Occurrence Handle1:STN:280:DC%2BD3MrlvVCrtw%3D%3D Occurrence Handle11443461
GB Hanna A Cuschieri (2000) ArticleTitleInfluence of two-dimensional and three-dimensional imaging on endoscopic bowel suturing. World J Surg 24 444–449 Occurrence Handle1:STN:280:DC%2BD3c7ntlOqtw%3D%3D Occurrence Handle10706917
GB Hanna SM Shimi A Cuschieri (1998) ArticleTitleRandomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 351 248–251 Occurrence Handle10.1016/S0140-6736(97)08005-7 Occurrence Handle1:STN:280:DyaK1c7hvVaktQ%3D%3D Occurrence Handle9457094
JD Hernandez SD Bann Y Munz K Moorthy V Datta S Martin A Dosis F Bello A Darzi T Rockall (2002) ArticleTitleThe learning curve of a simulated surgical task using the da Vinci telemanipulator system. Br J Surg 89 IssueIDSuppl 17–18 Occurrence Handle10.1046/j.1365-2168.89.s.1.9_9.x
J Hofmeister TG Frank A Cuschieri NJ Wade (2001) ArticleTitlePerceptual aspects of two-dimensional and stereoscopic display techniques in endoscopic surgery: review and current problems. Semin Laparosc Surg 8 12–24 Occurrence Handle10.1053/slas.2001.20835 Occurrence Handle1:STN:280:DC%2BD3Mzps1agtg%3D%3D Occurrence Handle11337734
DB Johns JD Brewer NJ Soper (1996) ArticleTitleThe influence of three-dimensional video systems on laparoscopic task performance. Surg Laparosc Endosc 6 191–197 Occurrence Handle10.1097/00019509-199606000-00005 Occurrence Handle1:STN:280:BymA3s3pvFM%3D Occurrence Handle8743361
EM McDougall JJ Soble JS Wolf SY Nakada OM Elashry RVAD Clayman (1996) ArticleTitleComparison of three-dimensional and two-dimensional laparoscopic video systems. J Endourol 10 371–374 Occurrence Handle1:STN:280:ByiD38nlsF0%3D Occurrence Handle8872737
MD Mueller C Camartin E Dreher W Hanggi (1999) ArticleTitleThree-dimensional laparoscopy: gadget or progress? A randomized trial on the efficacy of three-dimensional laparoscopy. Surg Endosc 13 469–472 Occurrence Handle1:STN:280:DyaK1M3ksVOnuw%3D%3D
SM Prasad HS Maniar NJ Soper RJ Damiano Jr ME Klingensmith (2002) ArticleTitleThe effects of robotic assistance on learning curves for basic laparoscopic skills. Am J Surg 183 702–707 Occurrence Handle10.1016/S0002-9610(02)00871-1 Occurrence Handle12095605
F Peli (1998) ArticleTitleThe visual effects of head-mounted display (HMD) are not distinguishable from those of desk-top computer display. Vision Res 38 2053–2066 Occurrence Handle10.1016/S0042-6989(97)00397-0 Occurrence Handle1:STN:280:DyaK1M%2FhsVKrtw%3D%3D Occurrence Handle9797951
RM Satava (1993) ArticleTitle3-D vision technology applied to advanced minimally invasive surgery systems. Surg Endosc 7 429–431 Occurrence Handle1:STN:280:ByuD38vkvFU%3D Occurrence Handle8211624
MO Schurr W Kunert A Arezzo G Buess (1999) ArticleTitleThe role and future of endoscopic imaging systems. Endoscopy 31 557–562 Occurrence Handle1:STN:280:DC%2BD3c%2FgtFejuw%3D%3D Occurrence Handle10533742
N Taffinder SGT Smith J Huber RCG Russel A Darzi (1999) ArticleTitleThe effect of a second-generation 3D endoscope on laparoscopic precision of novices and experienced surgeons. Surg Endosc 13 1087–1092 Occurrence Handle1:STN:280:DC%2BD3c%2FivVCjug%3D%3D Occurrence Handle10556444
RA Tyrrell HW Leibowitz (1990) ArticleTitleThe relation of vergence effort to reports of visual fatigue following prolonged near work. Hum Factors 32 341–357 Occurrence Handle1:STN:280:By6D2sfotFU%3D Occurrence Handle2258180
P van Bergen W Kunert GF Buess (2000) ArticleTitleThe effects of high-definition imaging on surgical task efficiency in minimally invasive surgery: an experimental comparison between three-dimensional imaging and direct vision through a stereoscopic TEM rectoscope. Surg Endosc 14 71–74 Occurrence Handle10.1007/s004649900015 Occurrence Handle1:CAS:528:DC%2BD3cXmt1eitQ%3D%3D Occurrence Handle10653241
M Wentink JJ Jakimowicz LM Vos DW Meijer PA Wieringa (2002) ArticleTitleQuantitative evaluation of three advanced laparoscopic technologies: a stereo endoscope, an image projection display and a TFT display. Surg Endosc 16 1237–1241 Occurrence Handle10.1007/s00464-001-9127-1 Occurrence Handle1:STN:280:DC%2BD38vjt1egtA%3D%3D Occurrence Handle11984691
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munz, Y., Moorthy, K., Dosis, A. et al. The benfits of stereoscopic vision in robotic-assisted performance on bench models . Surg Endosc 18, 611–616 (2004). https://doi.org/10.1007/s00464-003-9017-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-003-9017-9