Abstract
Background
Proximal gastrectomy is not widely performed because the procedure is complicated, particularly under laparoscopy. We developed a simple laparoscopic technique of hand-sewn esophagogastrostomy with an anti-reflux mechanism. This study aimed to evaluate and compare the postoperative body weight loss (BWL) and quality of life (QOL) following laparoscopic proximal gastrectomy (LPG) and laparoscopic total gastrectomy (LTG) in patients with upper gastric cancer.
Methods
We retrospectively analyzed patients with stage I upper gastric cancer undergoing LPG or LTG at Kyoto University Hospital between March 2006 and June 2014. The main outcome measures were the % BWL 1 year after gastrectomy, postoperative anastomotic stricture, and reflux esophagitis. Additionally, patient-reported outcomes were evaluated using the Post-Gastrectomy Syndrome Assessment Scale (PGSAS)-45 in patients presenting at the outpatient clinic and exhibiting no recurrence.
Results
A total of 62 patients were included in this study (LTG, n = 42 vs. LPG, n = 20). The % BWL at 12 months in the LPG group was less than that in the LTG group (−16.3 vs. −10.7%). Multivariate analysis revealed that LPG was associated with less BWL (P = 0.003). Anastomotic stricture occurred more frequently in the LPG group than in the LTG group (0 vs. 25%). One patient in each group exhibited grade B severity of reflux esophagitis (based on the Los Angeles classification). In the questionnaire survey, LPG was better than LTG in terms of diarrhea and dissatisfaction with symptoms. In terms of reflux symptoms, patients in the LPG group experienced less acid and bile regurgitation symptoms compared with those in the LTG group.
Conclusions
LPG with hand-sewn esophagogastrostomy results in less postoperative BWL and better QOL than LTG despite higher rates of anastomotic stricture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The incidence of gastric cancer in the upper third of the stomach has been increasing [1–3], and the current standard therapy for it is a total gastrectomy. However, patients who have undergone this procedure experience diminished appetite, severe body weight loss (BWL), and symptoms such as heart burn, nausea, and vomiting (known as post-gastrectomy syndrome) [4–6]. Proximal gastrectomy has been suggested as a function-preserving surgical option for early-stage upper gastric cancer to improve the patients’ postoperative condition. However, the major concern following this procedure is postoperative gastroesophageal reflux following esophagogastric anastomosis [7–11]. Several types of procedures have been suggested to prevent postoperative reflux, including esophagogastric anastomosis with anti-reflux mechanism, jejunal interposition, and double-tract reconstruction. However, proximal gastrectomy is not widely performed as the procedure is complicated, particularly under laparoscopy, which has become a standard approach for patients with early gastric cancer [12].
We previously developed a simple laparoscopic technique for esophagogastrostomy with an anti-reflux mechanism [13]. In this method, fixation of the esophagus to the anterior wall of the stomach using a knifeless linear stapler allows easier creation of a hand-sewn esophagogastric anastomosis with posterior fundoplication. Although the technique and short-term outcomes have been reported elsewhere [13], the clinical benefits of laparoscopic proximal gastrectomy (LPG) using our technique have yet to be comprehensively evaluated. This study aimed to retrospectively evaluate and compare the short-term surgical outcomes, postoperative BWL, and quality of life (QOL) following LPG with hand-sewn esophagogastrostomy and laparoscopic total gastrectomy (LTG) for clinical stage I upper gastric cancer.
Materials and methods
Setting
This cohort study was conducted at the Department of Surgery, Kyoto University Hospital, in accordance with the Declaration of Helsinki and was approved by the ethics committee of Kyoto University.
Eligible patients
All consecutive patients with histologically diagnosed gastric adenocarcinoma undergoing gastrectomy between March 2006 and June 2014 were identified using a prospectively maintained database. Patients underwent gastrointestinal endoscopy, upper gastrointestinal X-rays, and multi-detector-row computed tomography of the chest and abdomen to determine the clinical stage. Patients who were diagnosed with clinical stage I cancer limited to the upper third of the stomach with no lymph node involvement (T1N0 or T2N0) and did not receive endoscopic treatment were included. Indication for endoscopic resection was determined according to the Japanese gastric cancer treatment guidelines [14, 15]. Proximal gastrectomy with hand-sewn esophagogastrostomy was indicated for patients with tumors that did not invade the esophagus, allowing preservation of two-thirds of the stomach. Those with tumors invading the esophagus, those with multiple primary cancers, or those undergoing laparotomy or LPG with different reconstructions were excluded from this study.
Outcomes
The primary outcome was the % BWL 1 year after gastrectomy. Changes from preoperative baseline were compared on the basis of the type of resection (LTG or LPG). The secondary outcomes were as follows: (1) operative time, (2) intraoperative estimated blood loss, (3) early complications, (4) late complications (i.e., anastomotic stenosis and reflux esophagitis), (5) overall survival, and (6) postoperative patient-reported outcomes including QOL.
Data collection
Detailed information on the patients, including preoperative examinations, surgical procedures, and early complications, was extracted from the database. Tumors were staged in accordance with the third English Edition of the Japanese Classification of Gastric Carcinoma [16]. Early complications were diagnosed and graded according to the Clavien–Dindo classification [17]. Patients who developed grade II or higher complications within 30 days or during the postoperative hospital stay were identified as having early complications.
Postoperative body weight, date of death or the last follow-up, and endoscopic findings were obtained from the original medical records. Anastomotic stricture and reflux esophagitis were diagnosed on the basis of the endoscopic findings. Patients requiring endoscopic balloon dilations were identified as having anastomotic stenosis. The number of dilations was also recorded. Reflux esophagitis was diagnosed according to the Los Angeles classification [18]. The necessity for medications for esophageal reflux was also evaluated.
Assessment of patient-reported outcomes
In cooperation with an expert in QOL measurement (K. M.), postoperative patient-reported outcomes were assessed using a questionnaire, the Post-Gastrectomy Syndrome Assessment Scale (PGSAS)-45 [19], which comprises the PGSAS-37 and the eight-item Short-Form Health Survey (SF-8) [20]. Permission to use the PGSAS-37 and SF-8 was obtained from the Japan Post-Gastrectomy Syndrome Working Party and iHope International Inc., respectively.
Patients who presented at the outpatient clinic at least 1 year after the operation were approached consecutively between November 2014 and July 2015. The following patients were excluded: patients currently receiving or received chemotherapy or radiotherapy within 1 year and patients with recurrence or other malignant diseases. Written informed consent was obtained from all participants.
The PGSAS-45 comprises 45 questions classified into the symptom, QOL, and living status domains. The symptom domains comprise seven symptom subscales, including esophageal reflux, abdominal pain, meal-related distress, indigestion, diarrhea, constipation, and dumping symptoms. Total symptom score was calculated as the mean of the seven symptom subscales. The QOL domain consisted of general QOL from the SF-8 and dissatisfaction items. Physical and mental component summary measures were calculated by weighting each SF-8 item using a norm-based scoring method given in the instrument guidelines [20]. The dissatisfaction items consisted of three measures, i.e., dissatisfaction with symptoms, dissatisfaction at the meal, and dissatisfaction at working. Additionally, dissatisfaction with daily life was calculated as the mean of the three dissatisfaction scores. The living status domain consisted of changes in body weight, quality of ingestion, amount of food ingested per meal, necessity for additional meals, and ability to work.
Surgical procedures and follow-up
Surgical procedures for LTG and LPG have been reported elsewhere in detail [13, 21–24]. We selected the extent of lymphadenectomy according to the Japanese guidelines: D1+ for cT1 tumors and D2 for cT2 tumors [15]. However, for T2 tumors that did not invade the greater curvature, the dissection of splenic hilar lymph nodes was omitted; thus, lymphadenectomy was recorded as D1+. After January 2012, the da Vinci Surgical System was used for lymph node dissection in patients who met the requirements of the protocol by evaluating feasibility. In LPG, the pyloric and celiac branches of the vagal trunk were preserved.
After total gastrectomy, a Roux-en-Y reconstruction was performed with functional end-to-end esophagojejunal anastomosis using a 45-mm endoscopic linear stapler. The entry hole was also closed using the stapler [24]. After proximal gastrectomy, following fixation of the esophagus to the anterior wall of the remaining stomach using a knifeless stapler, a hand-sewn esophagogastrostomy with posterior fundoplication was created [13].
All patients received outpatient follow-up at least once every 6 months, and their body weights were recorded longitudinally. Patients who underwent LPG had a routine endoscopic examination 6 months after surgery and then once a year. Those who underwent LTG had an endoscopy only when they exhibited symptoms or requested the examination.
Sample size calculation
The sample size calculation was based on the primary outcome of the % BWL at 12 months. The following parameters were used: power of 80%, alpha error of 5%, a standard deviation of 6%, and LTG/LPG ratio of 2:1. To detect a difference of 5% in the % BWL between the two groups, 34 patients undergoing LTG and 17 patients undergoing LPG were required for this study.
Statistical analysis
Continuous variables have been expressed either as means and standard deviations and compared using the t test, or as medians and ranges or interquartile ranges and compared using the Mann–Whitney U test, as appropriate. Categorical data have been expressed as numbers and proportions and were compared using Fisher’s exact test. Survival curves were estimated for each group using the Kaplan–Meier method and compared statistically using the log-rank test.
To adjust for confounding factors, multiple regression analyses were performed. Preoperative factors [age, gender, body mass index, American Society of Anesthesiologists (ASA) physical status, history of smoking, serum albumin, serum creatinine, forced expiratory volume in one second (FEV1.0%), and clinical T factor] with P values <0.2 in the univariate analysis were included in the multivariate analyses. To assess patient-reported outcomes, the period from surgery to the date of the questionnaire survey was also considered as an explanatory variable. Results have been presented as regression coefficient (β) and 95% confidence interval (CI). Two-tailed P values <0.05 were considered statistically significant. All statistical analyses were performed using STATA statistical software, version 12.1 (Stata Corp., College Station, TX, USA).
Results
Clinical characteristics
Figure 1 shows a flowchart depicting the patient selection process. A total of 62 patients met all the protocol requirements and were included in this study. The patients were classified into an LTG group (n = 42) and an LPG group (n = 20). Table 1 presents the clinical characteristics of the patients in the two groups. The LPG group had a greater proportion of patients with high ASA physical status than the LTG group. After obtaining informed consent, four patients with small T2 tumors located near the esophagogastric junction underwent LPG.
Flow diagram for selecting patients. A total of 62 patients were enrolled in this study, and the operative outcomes were compared between the laparoscopic total gastrectomy (LTG) and laparoscopic proximal gastrectomy (LPG) groups. With regard to assessment of body weight loss, body weight data were unavailable in four patients, leaving 50 patients to be analyzed. With regard to postoperative patient-reported outcomes, 28 patients were analyzed
Operative features and outcomes
The operative features and outcomes are listed in Table 2. As no patients had T2 tumors that invaded the greater curvature, splenic hilar lymph node dissection was omitted, except in one patient. Laparoscopic gastrectomy was completed in all patients undergoing LPG, but open conversion was required in one patient undergoing LTG. A positive surgical margin was seen on pathological examination (R1 resection) in one patient in the LPG group, and laparoscopic completion gastrectomy was performed 21 days after the initial surgery. The estimated blood loss in the LPG group was significantly lower than that in the LTG group. The operative time and the incidence of morbidity were also less in the LPG group, but this was not statistically significant. Surgical complications such as pancreatic fistula, anastomotic leakage, or intra-abdominal abscess occurred in seven patients (16%) in the LTG group and 1 patient (5%) in the LPG group.
The median follow-up period was 50 months (range 2–98). There was no difference in overall survival between the groups (P = 0.90). The 3-year survival rates were similar between the two groups (92%) (Supplemental figure). During follow-up, 28 patients (67%) in the LTG group and 19 patients (95%) in the LPG group underwent at least one endoscopic examination. Five patients (25%) in the LPG group and zero patients in the LTG group exhibited anastomotic stenosis. Of the five patients in the LPG group, three experienced occasional vomiting and one required in-hospital treatment. All patients were relieved of their symptoms after serial endoscopic dilation, and none of the patients required any long-term interventions or surgery. The median number of dilations required was 3 (range 1–5).
During the follow-up period, endoscopic examination revealed reflux esophagitis in three patients (7%) in the LTG group and five patients (25%) in the LPG group (P = 0.10). The severity of the reflux esophagitis was mostly grade A, except for one patient in each group with grade B esophagitis. In the LPG group, 18 patients (90%) received proton pump inhibitor therapy after surgery for a median period of 9 months (range 1–50). All five incidences of reflux esophagitis in the LPG group were observed in patients who had stopped proton pump inhibitor therapy. However, as they did not complain of any reflux-related symptoms, proton pump inhibitors were not readministered. Grade B esophagitis in the LPG group healed spontaneously, and grade C or D esophagitis was not observed in either group.
Postoperative BWL
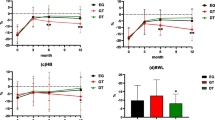
As shown in Fig. 1, 50 patients were analyzed for postoperative body weight. Changes in the % BWL over time after surgery are shown in Fig. 2. The % BWL at 12 months in the LPG group was significantly lower than that in the LTG group (LTG −16.3% vs. LPG −10.7%; P = 0.034). To adjust for confounding factors, multivariate regression analysis was performed (Table 3). In the univariate analysis, the P values of body mass index and a history of smoking were <0.2. In the multivariate analysis, after including these factors, LPG was seen to be associated with lower BWL at 12 months (β = 7.23; 95% CI 2.61–11.83; P = 0.003).
Assessment score with the PGSAS-45
As shown in Fig. 1, 28 patients were analyzed for patient-reported outcomes. The median period from operation to the survey was 23 months (range 13–66) in the LPG group and 37 months (range 13–64) in the LTG group (P = 0.07). All results of the outcome measures are given in Table 4. The scores for diarrhea and dissatisfaction with symptoms were significantly lower (i.e., better condition) in the LPG group than in the LTG group. Except for these measures, no differences were observed in the assessment scores of the PGSAS-45 between the groups.
To adjust for confounding factors, multiple regression analyses were performed (Table 5). In the univariate analysis of the diarrhea subscale, serum creatinine exhibited P < 0.2. However, even after adjustment for this factor, LPG was significantly associated with lower score for diarrhea (β = −0.90; 95% CI −1.68 to −0.13; P = 0.024, i.e., better condition). In the univariate analysis of dissatisfaction with symptoms, gender, serum albumin, and a history of smoking presented P value <0.2. In the multivariate analysis including these factors, LPG was the only significant factor associated with lower score for dissatisfaction with symptoms (β = −1.02; 95% CI −1.88 to −0.17; P = 0.021; i.e., better condition).
Acid or bile regurgitation score, which is a part of the esophageal reflux subscale, indicates how troublesome the symptom is to a patient. In the LTG group, nine (53%) and eight (47%) patients experienced at least mildly (a score of greater than or equal to 3) troublesome acid and bile regurgitation, respectively. On the other hand, zero and two (18%) patients in the LPG group experienced these troublesome symptoms, respectively (P = 0.002 and 0.09).
Discussion
The incidence of upper gastric cancer and the percentage of the aged population are increasing in Japan. They have resulted in the need for a less invasive and function-preserving surgical treatment option. For patients with early-stage upper gastric cancer, LPG is theoretically the ideal treatment option [25]. However, the application of this procedure is still limited due to technical difficulties, anastomosis-related complications, and uncertainty of long-term QOLs. To solve these problems, we previously developed a simple laparoscopic technique of hand-sewn esophagogastrostomy with a rigorous anti-reflux mechanism [13]. This study clearly demonstrated that LPG using our method was independently associated with less BWL compared with LTG 1 year after surgery. Further post hoc analysis revealed that the difference in the % BWL persisted at 2 years after gastrectomy (LTG −15.6% vs. LPG −9.2%; P = 0.032). Since an underweight individual is associated with a higher risk of mortality and poorer QOL [26, 27], the significant difference in BWL between LTG and LPG is not negligible for patients with early-stage gastric cancer.
With regard to reflux esophagitis, which is the major concern associated with LPG with esophagogastrostomy, endoscopic examination revealed no severe esophagitis (grade C/D) after LPG, although mild endoscopic findings were observed in 25% of the patients (grade A in 20% and grade B in 5%). Importantly, no patient in the LPG group complained of any clinical symptoms of regurgitation. In the postoperative assessment with PGSAS-45, LPG caused less troublesome symptoms of regurgitation than LTG. These results suggest that LPG with esophagogastrostomy may be clinically superior to LTG with regard to reflux symptoms.
Moreover, LPG was also seen to be associated with fewer diarrhea symptoms. A previous Japanese nationwide study using PGSAS-45 comparing proximal gastrectomy with total gastrectomy confirmed the same results [28]. The remnant stomach and pyloric sphincter may play an important role in the control of ingested food. The preservation of the celiac branch of the vagus nerve, which was performed only in the LPG group, may also be beneficial for preventing diarrhea [29, 30], although the effect of nerve preservation remains controversial. In addition, the dissatisfaction with symptoms score in patients undergoing LPG was less than in those undergoing LTG. LTG did not have any advantages over LPG with regard to the assessment score of PGSAS-45. Better QOL after LPG observed in this study may have resulted from proper selection of patients. We did not perform LPG when more than two-thirds of the stomach could not be preserved.
The only potential disadvantage of LPG with hand-sewn esophagogastrostomy was the frequent occurrence of postoperative anastomotic stricture. The hand-sewn method was chosen, as we believe that it creates a softer anastomosis that can be completely shut by the pressure of the pseudofornix, thus ensuring an anti-reflux mechanism. However, there are some technical considerations with regard to creation of a sufficiently sized stoma using this method. Since the length of the incision on the remnant stomach determines the size of the stoma, an incision longer than 3 cm should be made and over-tightening of the running sutures should be avoided. We believe that careful adherence to these technical tips can decrease the risk of anastomotic stricture.
Recently, Kuroda et al. [31] reported favorable results with regard to endoscopic reflux esophagitis in 13 patients undergoing LPG and valvuloplastic esophagogastrostomy using the double-flap technique. However, this procedure appears to be technically more complex than the method of reconstruction proposed by us. The operation time is likely to be longer, and similar to this study, anastomotic stenosis was identified as a potential complication that requires careful attention. However, the use of a validated questionnaire and adjustment for patient background are required to compare these reconstruction techniques accurately.
This study has several limitations. First, the study design made it difficult to avoid selection and information biases completely. However, laparoscopic gastrectomy has been our standard treatment option for early-stage gastric cancer since 2006. As shown in Fig. 1, this allows us to enroll consecutive patients diagnosed with clinical stage I upper gastric cancer using the prospective database, which also includes perioperative information. We believe that this allows us to minimize these biases to a certain extent. Second, the questionnaire survey was performed at a single point in time. As baseline assessment with PGSAS-45 was lacking, we could not compare changes from baseline status between the two procedures. Third, the sample size of this study was limited, making it difficult to detect small differences between LTG and LPG. A sample size was not calculated for the secondary outcomes, and confidence intervals for the significant effects were also wide. However, we believe that the results of this study revealed important differences in outcomes that are relevant to the patient’s daily life.
In conclusion, LPG with a hand-sewn esophagogastric anastomosis using a knifeless endoscopic linear stapler has several advantages over LTG in terms of less postoperative loss of body weight, fewer diarrhea symptom, and better QOL despite higher rates of anastomotic stenosis. From a patient-centered viewpoint, LPG using our method of reconstruction can be considered as a suitable treatment option for early upper gastric cancer.
References
Carr JS, Zafar SF, Saba N, Khuri FR, El-Rayes BF (2013) Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer 44(2):143–151
Blaser MJ, Saito D (2002) Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol 14(2):107–113
Deans C, Yeo MSW, Soe MY, Shabbir A, Ti TK, So JBY (2011) Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg 35(3):617–624
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A (2008) Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg 247(5):759–765
Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, Barbul A (2005) Postoperative changes in body composition after gastrectomy. J Gastrointest Surg 9(3):313–319
O’Gorman P, McMillan DC, McArdle CS (1998) Impact of weight loss, appetite, and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr Cancer 32(2):76–80
Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, Suh YS, Kong SH, Lee KU, Yang HK (2015) Clinical outcome of modified laparoscopy-assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper-third early gastric cancer with special references to postoperative reflux esophagitis. J Gastric Cancer 15(3):191–200
Yoo CH, Sohn BH, Han WK, Pae WK (2004) Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat 36(1):50–55
An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S (2008) The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 196(4):587–591
Karanicolas PJ, Graham D, Gonen M, Strong VE, Brennan MF, Coit DG (2013) Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg 257(6):1039–1046
Ahn SH, Lee JH, do Park J, Kim HH (2013) Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 16(3):282–289
Uyama I, Okabe H, Kojima K, Satoh S, Shiraishi N, Suda K, Takiguchi S, Nagai E, Fukunaga T (2015) Gastroenterological surgery: stomach. Asian J Endosc Surg 8(3):227–238
Okabe H, Obama K, Tanaka E, Tsunoda S, Akagami M, Sakai Y (2013) Laparoscopic proximal gastrectomy with a hand-sewn esophago-gastric anastomosis using a knifeless endoscopic linear stapler. Gastric Cancer 16(2):268–274
Japanese Gastric Cancer Society (2004) Guidelines for diagnosis and treatment of carcinoma of the stomach April 2004 edition. http://www.jgca.jp/pdf/Guidelines2004_eng.pdf. Accessed 1 Dec 2016
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L (1999) Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 45(2):172–180
Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Oshio A, Suzukamo Y, Terashima M, Kodera Y (2015) Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer 18(1):147–158
Fukuhara S, Suzukamo Y (eds) (2004) Manual of the SF-8 Japanese version. Institute for Health Outcomes and Process Evaluation research, Kyoto
Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y (2010) Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg 211(1):e1–e6
Satoh S, Okabe H, Kondo K, Tanaka E, Itami A, Kawamura J, Nomura A, Nagayama S, Watanabe G, Sakai Y (2009) Video. A novel laparoscopic approach for safe and simplified suprapancreatic lymph node dissection of gastric cancer. Surg Endosc 23(2):436–437
Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y (2014) Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg 259(1):109–116
Okabe H, Obama K, Tanaka E, Nomura A, J-i Kawamura, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y (2009) Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc 23(9):2167–2171
Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M (2003) Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 90(7):850–853
Sasazuki S, Inoue M, Tsuji I, Sugawara Y, Tamakoshi A, Matsuo K, Wakai K, Nagata C, Tanaka K, Mizoue T, Tsugane S, Research Group for the Development and Evaluation of Cancer Prevention (2011) Body mass index and mortality from all causes and major causes in Japanese: results of a pooled analysis of 7 large-scale cohort studies. Int J Epidemiol 21(6):417–430
Zhu Y, Wang Q, Pang G, Lin L, Origasa H, Wang Y, Di J, Shi M, Fan C, Shi H (2015) Association between body mass index and health-related quality of Life: the “obesity paradox” in 21,218 adults of the Chinese general population. PLoS ONE 10(6):e0130613
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, Nakada K (2015) Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by Postgastrectomy Syndrome Assessment Scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer 18(2):407–416
Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Nakada K (2016) Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer 19(1):302–311
Kim SM, Cho J, Kang D, Oh SJ, Kim AR, Sohn TS, Noh JH, Kim S (2016) A randomized controlled trial of vagus nerve-preserving distal gastrectomy versus conventional distal gastrectomy for postoperative quality of life in early stage gastric cancer patients. Ann Surg 263(6):1079–1084
Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, Shirakawa Y, Fujiwara T (2016) Double-flap technique as an antireflux procedure in esophagogastrostomy after proximal gastrectomy. J Am Coll Surg 223(2):e7–e13
Acknowledgements
The authors would like to thank Dr. Kenjiro Hirai, Dr. Tomoaki Okada, and Dr. Riki Ganeko for their support in conducting the questionnaire survey. The authors would also like to thank Dr. Koya Hida and Dr. Ryo Takahashi for their contribution to the design of the study.
Funding
This work was supported by Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Tatsuto Nishigori, Hiroshi Okabe, Shigeru Tsunoda, Kazutaka Obama, Hisahiro Hosogi, Shigeo Hisamori, Ms. Kikuko Miyazaki, Profs. Hisashi Shinohara, Takeo Nakayama, and Yoshiharu Sakai have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
464_2016_5403_MOESM1_ESM.tif
Supplemental Figure. Overall survival of patients in both groups. There were no significant differences in the overall survival rates between the groups (P = 0.90). LTG, laparoscopic total gastrectomy; LPG, laparoscopic proximal gastrectomy. (TIFF 26 kb)
Rights and permissions
About this article
Cite this article
Nishigori, T., Okabe, H., Tsunoda, S. et al. Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc 31, 3664–3672 (2017). https://doi.org/10.1007/s00464-016-5403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5403-y