Abstract
Background
Studies comparing the incidence of reflux esophagitis (RE) and patients’ quality of life (QoL) when using circular stapler (CS) and linear stapler (LS) in esophagojejunostomy (EJS) after laparoscopic total gastrectomy (LTG) are rare, and certainly there are not enough to make a definitive decision on best practice. Presented herein is a study on the comparison of the short-term outcomes, QoL of the patients with the focus on the incidence of RE after both linear and circular stapling in LTG.
Methods
From January 2014 to October 2018, 120 patients were analyzed; of these, 42 patients underwent laparoscopy-assisted total gastrectomy (LATG) with CS (CS group) and 78 patients who underwent totally laparoscopic total gastrectomy (TLTG) with LS (LS group). We examined the results obtained in terms of perioperative outcomes, reflux-related assessments (GerdQ questionnaire and endoscopy findings with all cases; 24-h pH monitoring with limited cases), and EORTC QLQ-C30 and QLQ-STO22. In addition, questionnaires were also supplied to patients and the results were recorded.
Results
The incidence of anastomotic stenosis (7.1% vs. 0; P < 0.05) and the median intraoperative blood loss (180.0 vs. 100.0 mL; P < 0.05) of the CS group were higher than the LS group. The factor aside, no significant differences were observed between the two groups with regard to the incidence of RE assessed by the QLQ-STO22 reflux scale, the GerdQ scores, endoscopy (in all cases), or the percent time of pH > 7 (in limited cases) (P > 0.05). In the EORTC QLQ-C30 and QLQ-STO22, it was noted that the score of constipation [0 (0, 0) vs. 0 (0, 33.3); P = 0.028] and postoperative dysphagia [0 (0, 0) vs. 0 (0, 22.2); P = 0.046] of the LS group in a 1-year follow-up were lower than the CS group.
Conclusions
TLTG with LS generated better results than LATG with CS in terms of the incidence of anastomotic stenosis, intraoperative blood loss, and postoperative constipation and dysphagia. Furthermore, when compared with circular stapling, linear stapling in EJS did not increase the incidence of RE assessed by the QLQ-STO22 reflux scale, the GerdQ scores, endoscopy (in all cases), or the percent time of pH > 7 (in limited cases).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Kitano et al. [1] reported on laparoscopy-assisted distal gastrectomy (LADG) in 1994, laparoscopic gastrectomy became the approach most commonly adopted by medical practitioners to treat this condition [2,3,4]. In 1999, Azagra JS et al. began laparoscopy-assisted total gastrectomy (LATG) with a circular stapler (CS) for the esophagojejunostomy (EJS) via an 8-cm subxiphoid incision [5]. However, LATG has some drawbacks that must also be considered. For example, performing the EJS in LATG is often challenging because of the restriction of working space resulting from the mini-laparotomy; this is especially so for overweight patients. The next development occurred also in 1999, when Uyama et al. began successfully performing totally laparoscopic total gastrectomy (TLTG) with a linear stapler (LS) [6]. TLTG has been shown to have many merits, such as smaller wounds, a larger workspace, and no limitation due to the patient’s body size. More and more surgeons now prefer TLTG as an appropriate method for upper body gastric cancer, or even adenocarcinoma of the esophagogastric junction (AEG), despite the high demand the procedure places on a surgeon’s operating skills [7,8,9]. Nowadays, LATG with CS and TLTG with LS (overlap anastomosis) are two of the most widely used laparoscopic total gastrectomy (LTG) procedures.
Physiologically, the lower esophageal sphincter pressure (LESP) is an essential pressure barrier for preventing the reflux of gastric contents into the esophagus. Tomita et al. reported that the preservation of the lower esophageal sphincter (LES) might be necessary to prevent RE after total gastrectomy reconstructed by Roux-en-Y [10]. Based on it, significant numbers of surgeons adhere to the view that the use of LS in EJS may increase the incidence of RE when compared with CS. Although several studies have now considered the anastomotic complications, such as leakage and stenosis, between CS and LS [11, 12], the number of studies comparing the incidence of RE and patients’ quality of life (QoL) after LTG between these two staplers remains insufficient for definitive conclusions to be drawn. Therefore, we have conducted our research and compared the short-term outcomes and QoL of the patients between linear and circular stapling in EJS after LTG in order to clarify whether linear stapling would increase the incidence of RE.
Methods
Patients
The medical records of the 169 patients who underwent LATG with CS or TLTG with LS (esophagus-incomplete-transecting self-pulling overlap anastomosis, a modified overlap method we developed) in our department from January 2014 to October 2018 were retrospectively assessed. Among these participants, those who were (1) under 75 years of age and (2) diagnosed with gastric carcinoma located in the fundus, upper body, or entire stomach were included. Conversely, patients exhibiting the following were excluded: those with (1) previous history of upper abdominal surgery (except laparoscopic cholecystectomy), (2) adenocarcinoma of the esophagogastric junction, (3) neoadjuvant therapy, (4) combined resection during the gastrectomy, (5) comorbidities that could influence the quality of life (QoL) (e.g., previous or combined malignancies, cardiovascular disease, cerebrovascular disease, neurologic conditions such as dementia and seizure, and severe chronic obstructive pulmonary disease, requiring persistent medical aid), and (6) recurrent gastric cancer within 1 year of surgery and those who died within 1 year after their surgery. EORTC QLQ-C30 and QLQ-STO22 questionnaire responses, along with clinical data, of the participants were collected and collated during the preoperative period and at three monthly intervals after the surgery. The clinical characteristics of the 120 patients included in our research are outlined in Table 1 for the analysis. The choice of the procedures was primarily down to the operating surgeons’ preference and the patients’ characteristics. In addition, the tendencies toward each type of procedure evolved in our department. Since 2015, our team has gradually moved from performing LATG with CS to TLTG with LS. Indeed, most patients treated before 2015 underwent LATG with CS, whereas most patients treated subsequently underwent TLTG with LS.
Surgical Techniques
Under general anesthesia, all patients were laid supine and subjected to a 15–20° reverse Trendelenburg position. The surgeon was positioned on the left side of the patient. An initial 12-mm trocar for a 30° rigid electrolaparoscope was inserted via surgeon incision through the infraumbilical area. Pneumoperitoneum was established with carbon dioxide and maintained at 12–14 mmHg throughout the operation. Thereafter, four additional ports (two ports with 12-mm diameter and two with 5-mm diameter) were inserted in the upper abdomen [13]. Partial omentectomy with D1 + β lymphadenectomy for early gastric cancer and total omentectomy with D2 lymphadenectomy for advanced gastric cancer were performed. The duodenum was transected with a 45-mm-long LS before removing lymph node groups 7, 8, 9, and 12. Following these procedures, EJS was performed by using a circular or linear stapler, as described below.
EJS Reconstruction in LATG with CS (CS Group)
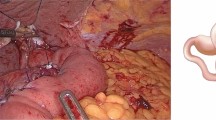
After ensuring adequate mobility of the esophagus, a mini-incision was created in the upper midline or the left subcostal (approximately 8 cm), and a double-ring wound retractor was inserted through the incision to protect the wound from cancer spillage. Essentially, the method of the reconstruction of EJS was the same as the conventional open surgery with a 25-mm CS (Fig. 1a, b). A side-to-side jejunojejunostomy was then performed extracorporeally via a hand-sewn anastomosis [14].
Intraoperative image and sketch of the esophagojejunostomy with a circular stapler in laparoscopy-assisted total gastrectomy and linear staplers in totally laparoscopic total gastrectomy. a Intraoperative image of the esophagojejunostomy with a circular stapler in laparoscopy-assisted total gastrectomy. b Sketch of the esophagojejunostomy with a circular stapler in laparoscopy-assisted total gastrectomy. c Intraoperative image of the esophagojejunostomy with linear staplers in totally laparoscopic total gastrectomy. d Sketch of the esophagojejunostomy with linear staplers in totally laparoscopic total gastrectomy
EJS Reconstruction in TLTG with LS (LS Group)
After carefully mobilizing the esophagus, the esophagus was partially transected 2 cm above the esophagogastric junction (EGJ) with a 45-mm LS (ETS45; Ethicon Endo Surgery, blue cartridge, and with 5–8-mm-width esophagus continuation). The specimen was then placed in a plastic bag which was tied up at the mouth using strings with a part of esophageal wall poking through. After this, the plastic bag containing the specimen was transferred to the right upper peritoneal cavity, and the sufficient traction of the esophagus stump was readily obtained to prevent the esophagus stump being withdrawn into the mediastinum (so-called self-pulling). A small hole was made on the left wall of the esophageal stump, with the nasogastric tube stretch protruding from within. The jejunum was intracorporeally transected 20 cm distal to the ligament of Treitz with a 45-mm LS (white cartridge) after the clipping of the mesentery. A small enterotomy was made 5 cm distal to the stapler line on the antimesenteric side of the jejunal limb. After one fork of the 45-mm LS (blue cartridge) was inserted into the opening, the jejunal limb was drawn up and positioned on the left side of the esophagus. Another fork was carefully inserted into the hole of the esophagus with the guidance of a nasogastric tube. The firing of the stapler converted the two openings into a common entry hole to create a side-to-side esophagojejunostomy (Fig. 1c, d). A V-shaped anastomotic staple line between the esophagus and the jejunal limb was created in an ante colic fashion, and intraluminal hemostasis was confirmed. The 3-0 V-Loc suture (Covidien, Mansfield, MA, USA) was used in the closure of the common entry hole of the stapler by an intracorporeal continuous hand-sewn technique, with the stitch going from inside to inside at the dorsal 1/2–2/3, and from outside to outside at the ventral 1/3–1/2 of the common entry hole (vertical mattress suture method). After the closure of the common entry hole, the occlusion clipping of the joint of the esophagus using a vascular clamp was performed. Afterwards, a side-to-side jejunojejunostomy was performed intracorporeally 50 cm distal to the EJS with a 45-mm LS (white cartridge). Finally, after the extension of the skin incision at the umbilical port site (approximately 4 cm long), the specimen bag was extracted from the abdominal cavity.
Reflux-Related Assessments
Reflux esophagitis (RE) was diagnosed mainly based on the combination of symptoms present, objective testing with endoscopy and ambulatory 24-h esophageal pH monitoring. The esophagitis lesions of RE were classified according to the Los Angeles (LA) classification. Postoperative symptoms related to esophageal reflux were assessed by using the Chinese version GerdQ questionnaire [15] and the EORTC QLQ-STO22 reflux scale. Ambulatory 24-h esophageal pH monitoring was only used as an adjunct in the diagnosis of RE. Based on the patients’ voluntary status, it was decided whether to conduct monitoring of the pH at the end of the esophagus at 1-year follow-up. The pH at the end of the esophagus was monitored over 24 h by a nasogastric pH monitor (Digitrapper MK-III; Synectics Medical, Sweden) placed 5 cm above the EJS with the help of gastroscope, and the percent time pH > 7 were calculated by a computer program.
Perioperative Outcomes
Perioperative outcomes were composed of operation time, anastomosis time, intraoperative blood loss, time to first flatus, time to start a soft diet, hospital stay, and severe postoperative complications (except reflux esophagitis). Anastomosis time ranges from the transection of the esophagus to the closure of the entry hole in the LS group or the firing of the stapler in the CS group. Intraoperative blood loss was calculated by aspirator measurement as well as the gauze weighing method. Postoperative complications were graded according to the Clavien–Dindo classification [16]. Severe postoperative complications were defined as those with grades Clavien–Dindo Classification >I.
Postoperative Nutritional Status
In the case of evaluating the postoperative nutritional status, weight changes, serum levels of hemoglobin, total protein, and albumin were measured 1 year after the operation. Weight changes referred to the change of weight at both 1-year follow-up and at the time of discharge after surgery.
EORTC QLQ-C30 and QLQ-STO22
Since the QoL and clinical outcomes obtained after the primary 3-month period follow-up are often influenced by the chemotherapy, we only compare the clinical outcomes and changes of QoL assessed by EORTC QLQ-C30 and QLQ-STO22 at 6 months (or at 1 month after the last cycle of chemotherapy) and 1 year for the two groups. In the CS group, no person was lost at 6-month follow-up, and one person was lost at 1-year follow-up. In the LS group, two people were lost at 6-month follow-up, and four more people were lost at 1-year follow-up. The rate of missing values was 5.8%.
The Chinese versions of the EORTC QLQ-C30 and QLQ-STO22 were used to assess the QoL [17, 18]. The QoL assessment was based on the responses to all items in questionnaires as completed by the responders themselves in the case of visiting the outpatient department or being contacted via telephone, e-mail, or Wechat. Missing data were processed according to the EORTC scoring manuals [19].
Statistical Analysis
The EORTC QLQ-C30 symptom subscale and the EORTC QLQ-STO22 subscale scores are reported using a scale from 0 to 100. Statistical analyses of the QoL outcomes evaluated the differences between the two groups concerning the overall changes from the preoperative scores (baseline) to those obtained at follow-ups previously detailed.
For the qualitative variables, the statistical data studied were the absolute frequencies (n) and the relative frequencies (%). For the quantitative variables, the average and the median were used as summary measures, and the SD and interquartile range were used to represent variability. For the qualitative variables, personal tests of χ2 and Fisher’s exact test were used. For the quantitative variables, Student’s t-distribution and the Mann–Whitney U tests were used. All statistical analysis was conducted by using SPSS software version 18 (IBM Corp., Armonk, NY).
Results
Clinical Characteristics
In the comparison of the CS group with the LS group, no significant difference was found regarding age, gender, BMI, ECOG performance status, as well as serum levels of hemoglobin, total protein, and albumin. In the analysis of the anatomical and pathological findings, no significant difference was observed in comparing patients undergoing circular stapling and linear stapling as to location, pathological type, depth of infiltration, nodal status, and UICC staging of the tumor (Table 1).
Reflux-Related Outcomes
When the reflux-related outcomes were assessed at 1-year follow-up, no significant difference was evidenced regarding the incidence of RE (4.9% vs. 5.6%; P > 0.05) and the grades according to Los Angeles Classification, and there was also no significant difference in terms of the chronological change of the score in the QLQ-STO22 reflux scale (the change of the score in the QLQ-STO22 reflux scale at 1-year follow-up compared with that before surgery) [0 (0, 11.1) vs. 0 (0, 11.1); P > 0.05], the GerdQ scores (5.4 ± 1.7 vs. 5.3 ± 1.9; P > 0.05), and the percentage of patients with GerdQ score ≥ 8 (9.8% vs. 11.1%; P > 0.05) between the two groups (Table 2). Besides, 26 patients (CS group, 11 patients; LS group, 15 patients) volunteered to receive monitoring of the pH at the end of the esophagus at 1-year follow-up, and there was no significant difference in the median percent time pH > 7 (63.2% vs. 68.9%; P > 0.05) between the two groups (Supplementary Table 1).
Perioperative Outcomes
Significant differences were found in intraoperative blood loss with a median of 180.0 mL in the CS group and 100.0 mL in the LS group (P = 0.001). The incidence of anastomotic stenosis was significantly higher in the CS group as compared with the LS group (7.1% vs. 0; P = 0.041). A comparison of the short-term surgical outcomes for each analyzed group showed no significant differences in terms of the operation time, anastomosis time, time to first flatus, time to start a soft diet, length of hospital stay, and other early complications (Table 3, Supplementary Figs. 1 and 2).
Postoperative Nutritional Status
We routinely provide dietary guidance for patients and recommend vitamin B12, folic acid, and iron supplements. During the 1-year follow-up, the nutritional status of the patients in both groups was seen to be good. No significant differences were found in the postoperative nutritional status at 1-year follow-up between the two groups regarding weight changes, hemoglobin, the serum levels of hemoglobin, total protein, and albumin (Table 4).
EORTC QLQ-C30 and QLQ-STO22
There were no significant differences between the CS group and the LS group in any of functioning parameters at 1-year follow-up, but the emotional functioning scores in the LS group tended to exhibit better than those in the CS group, although the difference did not reach statistical significance [0 (0, 8.3) vs. 4 .2 (0, 25.0); P = 0.133].
As for the QLQ-C30 symptom scales, the constipation score showed significant aggravation in the CS group compared with that in the LS group at 1-year follow-up [0 (0, 0) vs. 0 (0, 33.3); P = 0.028]. The scores for other symptom scales showed no significant differences at 6-month and 1-year follow-up between the two groups (Table 5).
The chronological changes in scores of the EORTC QLQ-STO22 symptom scales are shown in Table 6. The CS group had a poorer score for dysphagia than the LS group at 1-year follow-up [0 (0, 22.2) vs. 0 (0, 0); P = 0.046]. There were no significant differences in other symptom scales of QLQ-STO22 between the two groups.
Discussion
The linear stapling technique in EJS after LTG was first reported by Uyama et al. in 1999 [6], but the disputation on whether it would increase the incidence of RE after LTG or not has been perpetuated ever since. Some surgeons doubted that the LS would increase esophageal refluxing as compared with the CS because the LS destroyed the LES for cutting the end of the esophagus longitudinally, while some other surgeons did not believe so.
For non-operative patients, the diagnosis of RE was based on a combination of symptoms present, objective testing with endoscopy, ambulatory reflux monitoring, and response to a proton pump inhibitor (PPI) therapy [20]. For patients after gastric surgery, a study from Tomita et al. mentioned that severe endoscopic alkaline reflux esophagitis (ARE) was found when the 24-h % time is pH > 8, while symptomatic AREs were present in patients with 24-h % time pH > 7 [10]. Hence, the diagnosis of RE in the present study was primarily based on the combination of presenting symptoms and objective testing with endoscopy, and the ambulatory 24-h esophageal pH monitoring. Unfortunately, the ambulatory 24-h esophageal pH monitoring was not a routine examination after total gastrectomy in our clinical practice, so it was only performed in a limited number of cases at the 1-year follow-up. During the study period, 32 patients in total underwent this examination, and 26 of them were included in the present study.
In the present study, the RE incidence in the LS group assessed by both GerdQ questionnaire and QLQ-STO22 reflux scale showed no significant increase in comparison with that in the CS group. And no significant difference in the incidence of RE between these two kinds of staplers was found via gastroscope examination either. Besides, the 26 patients who volunteered to receive the ambulatory 24-h esophageal pH monitoring also confirmed no significant difference in the RE incidence between these two staples.
As reported, the length of the jejunal loop is the crucial factor in preventing reflux [10, 21,22,23,24], In the present study, we prepared 50 cm of the length of the jejunal loop when performing operations (10 cm longer than the length of the loop in the other reports [10, 21,22,23,24]) and found a relatively low incidence of RE (6/113, 5.3%) compared with the studies mentioned above [10, 21,22,23,24]. Therefore, we speculated that the length of the jejunal loop plays a more critical role in preventing RE than the LES.
In our results, the TLTG with LS was better than the LATG with CS in terms of incidence of anastomotic stenosis and intraoperative blood loss, which is consistent with the results in several other studies [7,8,9, 11, 12, 25,26,27,28,29]. We speculated that this partly lies in the anastomotic stoma of LS larger than that of CS, accordingly reduced the incidence of anastomotic stenosis in the LS group. In addition, we routinely provided dietary guidance for the patients in the study and recommended vitamin B12, folic acid, and iron supplements. One year after surgery, all the patients in both groups were in good nutritional status, which indicated that there was no difference in nutrient intake between the use of LS and CS, especially with correct nutrition guidance.
Oncologic outcomes and safety issues are the foci of gastric cancer surgery, but QoL after surgery has not been taken much concern. To our knowledge, there was no study on the comparison of the QoL between circular stapling and linear stapling. Understanding the possible differences in QoL after LTG with CS and LS will also influence the choice of procedures to some extent. Through the measurement of the EORTC QLQ-C30 and QLQ-STO22, we found that there were no significant differences in most of the parameters between these two groups, excepting the scores of dysphagia and constipation.
In the present study, we observed that the aggravation of postoperative dysphagia was more significant in the CS group than the LS group through the measurement of the QLQ-STO22 symptom scales [0 (0, 22.2) vs. 0 (0, 0); P = 0.046]. Among the possible reasons for this, excepting the stenosis of the anastomotic stoma, vertically cutting the LES might also lead to the lower incidence of dysphagia in the LS group. In addition, due to the changes in anatomical structure and physiological function after total gastrectomy, diarrhea has always been a common concern for surgeons and patients. In the present study, we could also see that the diarrhea symptoms of both of the two groups had aggravated to a certain extent at 1-year follow-up, but there was no significant difference in the degree of aggravation between the two groups. Interestingly, we observed that the symptom of constipation at 1-year follow-up aggravated in the CS group when compared with the LS group through the measurement of the QLQ-C30. The mechanisms of postoperative chronic constipation are complex. Some previous studies [30,31,32,33] considered that the symptom of constipation might be attributed to the emotion changes (depression), the imbalance of gut microbiota, and varying degrees of irregular bowel movement after surgery. However, it is still unknown whether there is any difference in the gut microbiota in patients between these two anastomotic methods, and we believe that this would be well worth exploration in any future research. It was observed that constipation mostly occurred in patients with dysphagia or lower scores for the emotional functioning in the QLQ-C30 in the current study. For example, there were ten patients in the CS group and four patients in the LS group at 1 year after the operations reporting the symptom of constipation. Of these patients, six in the CS group had varying degrees of dysphagia, while none in the LS group experienced dysphagia. The QLQ-C30 also showed that the score for the emotional functioning of the LS group tended to exhibit better scores than the CS group, even though the differences did not reach statistical significance. Hence, it might be assumed at present that the symptom of constipation might have some internal relationship with the emotional changes potentially caused by dysphagia in the CS group.
We recognize that our study has several limitations. First of all, although the diagnosis of RE was made based on the combination of the presenting symptoms, objective testing with endoscopy, and pH monitoring in the present study, and the symptoms and endoscopy findings are both persuasive evidence in diagnosing RE, the study findings would be prone to Type II error because of the small number of patients who had pH monitoring after surgery. Second, there was a temporal difference between these two types of methods in the present study. Third, this is a retrospective study from a single institution with small sample size, and the choice of the procedures was according to the operating surgeons’ preference and the patients’ characteristics, which could lead to selection biases, so a randomized clinical trial or a well-designed larger sample size case-matched study would be needed. Consequently, we will continue to investigate the reasons for the difference in postoperative constipation between the circular stapling and the linear stapling in the future study.
In brief, the present study is the first to focus on the differences in the incidence of RE and postoperative QoL between LATG with CS and TLTG with LS. We believe that the present study could serve as useful background research for future randomized clinical trials investigating the differences of long-term outcomes and postoperative QoL between the circular stapling and the linear stapling in LTG.
Conclusions
Taken collectively, TLTG with LS is better than LATG with CS in terms of incidence of anastomotic stenosis, intraoperative blood loss, and postoperative constipation and dysphagia. Furthermore, compared with circular stapling, linear stapling in EJS seems not to increase the incidence of RE assessed by either the QLQ-STO22 reflux scale, the GerdQ scores, endoscopy (in all cases), or the percent time of pH > 7 (in limited cases). Hence, totally laparoscopic total gastrectomy with linear stapler may be a better choice for laparoscopic total gastrectomy.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-148.
Choi YY, Bae JM, An JY, Hyung WJ, Noh SH. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J Surg Oncol 2013;108:550-556.
Wang JF, Zhang SZ, Zhang NY, Wu ZY, Feng JY, Ying LP, Zhang JJ. Laparoscopic gastrectomy versus open gastrectomy for elderly patients with gastric cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:90.
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350-1357.
Azagra JS, Goergen M, De Simone P, Ibanez-Aguirre J. Minimally invasive surgery for gastric cancer. Surg Endosc 1999;13:351-357.
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer 1999;2:230-234.
Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy methods for the surgical treatment of early gastric cancer near the gastroesophageal junction. J Laparoendosc Adv Surg Tech A 2013;23:204-210.
Chen K, He Y, Cai JQ, Pan Y, Wu D, Chen DW, Yan JF, Maher H, Mou YP. Comparing the short-term outcomes of intracorporeal esophagojejunostomy with extracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. BMC Surg 2016;16:13.
Gong CS, Kim BS, Kim HS. Comparison of totally laparoscopic total gastrectomy using an endoscopic linear stapler with laparoscopic-assisted total gastrectomy using a circular stapler in patients with gastric cancer: a single-center experience. World J Gastroenterol 2017;23:8553-8561.
Tomita R, Sakurai K, Fujisaki S. Significance of the lower esophageal sphincter preservation in preventing alkaline reflux esophagitis in patients after total gastrectomy reconstructed by Roux-en-Y for gastric cancer. Int Surg 2014;99:174-181.
Kawamura H, Ohno Y, Ichikawa N, Yoshida T, Homma S, Takahashi M, Taketomi A. Anastomotic complications after laparoscopic total gastrectomy with esophagojejunostomy constructed by circular stapler (OrVilTM) versus linear stapler (overlap method). Surg Endosc 2017;31:5175-5182.
Kyogoku N, Ebihara Y, Shichinohe T, Nakamura F, Murakawa K, Morita T, Okushiba S, Hirano S. Circular versus linear stapling in esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer: a propensity score-matched study. Langenbecks Arch Surg 2018.
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 2010;211:e25-29.
Kim KH, Kim YM, Kim MC, Jung GJ. Is laparoscopy-assisted total gastrectomy feasible for the treatment of gastric cancer? A case-matched study. Dig Surg 2013;30:348-354.
Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009;30:1030-1038.
Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-526.
Wang JP, Chen ZG, Lin WJ, Cui JN. Assessment of quality of life in cancer patients: EORTC QLQ-C30 for use in China. Acta Psychologica Sinca 2000;32:438-442.
Jiang BF, Xu T, Liu CX, Xu M, Cui YC, Wang JL, Du J, Blazyby J. Development of Chinese version of QLQ-STO22 Chinese Mental Health Journal 2005;19:310-312.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 scoring manual (3rd Edition). Brussels: European Organisation for Research and Treatment of Cancer. 2001.
Azer SA, Reddivari AKR. Reflux esophagitis. Treasure Island (FL): StatPearls Publishing LLC., 2020.
Tonelli F, Corazziari E, Spinelli F. Evaluation of “alkaline” reflux esophagitis after total gastrectomy in Henley and Roux-en-Y reconstructive procedures. World J Surg 1978;2:233-237.
Yoo HY, Venbrux A, Heitmiller R, Ravich WJ, Lee LA. Control of alkaline reflux esophagitis after total gastrectomy by a percutaneous jejunostomy tube. J Clin Gastroenterol 2002;35:46-49.
Morrow D, Passaro ER. Alkaline reflux esophagitis after total gastrectomy. Am J Surg 1976;132:287-291.
Matei D, Dadu R, Prundus R, Danci I, Ciobanu L, Mocan T, Bocsan C, Zaharie R, Serban A, Tantau M, Iancu C, Alexandru I, Al-Hajjar N, Andreica V. Alkaline reflux esophagitis in patients with total gastrectomy and Roux en Y esojejunostomy. J Gastrointestin Liver Dis 2010;19:247-252.
Chen K, Pan Y, Cai JQ, Wu D, Yan JF, Chen DW, Yu HM, Wang XF. Totally laparoscopic versus laparoscopic-assisted total gastrectomy for upper and middle gastric cancer: a single-unit experience of 253 cases with meta-analysis. World J Surg Oncol 2016;14:96.
Kim EY, Choi HJ, Cho JB, Lee J. Totally laparoscopic total gastrectomy versus laparoscopically assisted total gastrectomy for gastric cancer. Anticancer Res 2016;36:1999-2003.
Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol 2015;21:9656-9665.
Liao GQ, Ou XW, Liu SQ, Zhang SR, Huang W. Laparoscopy-assisted total gastrectomy with trans-orally inserted anvil (OrVil): a single institution experience. World J Gastroenterol 2013;19:755-760.
Zuiki T, Hosoya Y, Kaneda Y, Kurashina K, Saito S, Ui T, Haruta H, Hyodo M, Sata N, Lefor AT, Yasuda Y. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surg Endosc 2013;27:3683-3689.
Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 2020.
Staller K, Barshop K, Kuo B, Ananthakrishnan AN. Depression but not symptom severity is associated with work and school absenteeism in refractory chronic constipation. J Clin Gastroenterol 2018;52:407-412.
Liang YX, Wen P, Wang Y, OuYang DM, Wang D, Chen YZ, Song Y, Deng J, Sun YM, Wang H. The constipation-relieving property of d-tagatose by modulating the composition of gut microbiota. Int J Mol Sci 2019;20.
Aoki T, Yamaji I, Hisamoto T, Sato M, Matsuda T. Irregular bowel movement in gastrectomized subjects: bowel habits, stool characteristics, fecal flora, and metabolites. Gastric Cancer 2012;15:396-404.
Contributions of each Co-Author
M.W.: conceived and drafted the manuscript; N.W. and Z.Y.: performed quality assessment and statistical analysis; T.W., S.Z., L.D., Z.Z., D.W., P.G., B.Z., Y.Y., G.J., and K.W.: performed data acquisition and reviewed the data; Q.Q. and X.H.: gave critical comments and revised the manuscript. All the authors were involved in the critical revision and final approval of the article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human Rights Statement and Informed Consent
All work was carried out in compliance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Helsinki Declaration in 1975 (revised in 2000).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 381 kb)
Rights and permissions
About this article
Cite this article
Wei, M., Wang, N., Yin, Z. et al. Short-Term and Quality of Life Outcomes of Patients Using Linear or Circular Stapling in Esophagojejunostomy after Laparoscopic Total Gastrectomy. J Gastrointest Surg 25, 1667–1676 (2021). https://doi.org/10.1007/s11605-020-04806-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04806-0