Abstract
Background
Laparoscopy offers some evidence of benefit compared to open rectal surgery. Robotic rectal surgery is evolving into an accepted approach. The objective was to analyze and compare laparoscopic and robotic rectal surgery learning curves with respect to operative times and perioperative outcomes for a novice minimally invasive colorectal surgeon.
Methods
One hundred and six laparoscopic and 92 robotic LAR rectal surgery cases were analyzed. All surgeries were performed by a surgeon who was primarily trained in open rectal surgery. Patient characteristics and perioperative outcomes were analyzed. Operative time and CUSUM plots were used for evaluating the learning curve for laparoscopic versus robotic LAR.
Results
Laparoscopic versus robotic LAR outcomes feature initial group operative times of 308 (291–325) min versus 397 (373–420) min and last group times of 220 (212–229) min versus 204 (196-211) min—reversed in favor of robotics; major complications of 4.7 versus 6.5 % (NS), resection margin involvement of 2.8 versus 4.4 % (NS), conversion rate of 3.8 versus 1.1 (NS), lymph node harvest of 16.3 versus 17.2 (NS), and estimated blood loss of 231 versus 201 cc (NS). Due to faster learning curves for extracorporeal phase and total mesorectal excision phase, the robotic surgery was observed to be faster than laparoscopic surgery after the initial 41 cases. CUSUM plots demonstrate acceptable perioperative surgical outcomes from the beginning of the study.
Conclusions
Initial robotic operative times improved with practice rapidly and eventually became faster than those for laparoscopy. Developing both laparoscopic and robotic skills simultaneously can provide acceptable perioperative outcomes in rectal surgery. It might be suggested that in the current milieu of clashing interests between evolving technology and economic constrains, there might be advantages in embracing both approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Initially, laparoscopic rectal surgery was plagued with skepticism on technical feasibility and oncologic appropriateness. The early focus of laparoscopic rectal surgery was therefore on sphincter eradicating surgery [1–6]. With evolving technology and skill set, laparoscopic low anterior resection (LAR) with total mesorectal excision (TME) is emerging as a technique with several advantages when compared to the sphincter preserving open procedure. Based on several moderately sized randomized control trials, compared to open surgery, the sphincter preserving laparoscopic approach to rectal cancer has not only been shown to have equivalent short- and long-term oncologic outcomes, it was also found to provide statistically significant benefits with respect to shorter hospital stay, earlier return of bowel function, reduced blood loss, number of blood transfusions, lower rates of wound infection, intra-abdominal postoperative bleeding as well as decreased rate of obstructions due to late intestinal adhesions [7–9].
Robotic colorectal surgery using the da Vinci system offers further theoretical advantages to laparoscopic surgery. These advantages include depth perception of the 3D vision system, articulating instrument dexterity, tremor filtering, and more favorable surgeon ergonomics. Several authors therefore reported on the system’s potential benefits during TME in a narrow pelvis. These potential reported benefits include decreased surgeon fatigue, better identification of the neural structures, easier dissection of the inferior mesenteric vessels, and less cumbersome splenic flexure mobilization [10, 11]. Whether these theoretical benefits of robotic rectal surgery translate into significant favorable patient outcomes still remains to be determined and is a subject of registered randomized clinical trials such as the international ROLARR trial (NCT01196000) and the COLRAR trial (NCT01423214) out of South Korea. However, current research based on multiple observational studies indicates at least equivalency between laparoscopic and robotic surgery on most critical perioperative outcomes such as operative complication rates, resection margins, and lymph node harvest [10, 12–14]. Based on a 3 year follow-up, Baek et al. [15] reported that robotic rectal surgery can be carried out safely in terms of recurrence and survival rates. Furthermore, a trend toward earlier bladder function recovery in robotic rectal surgery has been recently published [16].
With some of the benefits of minimally invasive surgery (MIS) and the majority of rectal resections still being performed using the open technique, surgeons and administrators face difficult decisions with respect to investing time and other resources into new technology. Surgeons performing rectal cancer surgery using open techniques might be interested in feasibility of transitioning into MIS techniques. They might be further interested in laparoscopic versus robotic outcomes and learning curves in a surgeon without prior extensive laparoscopic experience. Increased cost associated with robotic surgery is an important factor when considering this technology. This is a complex and rapidly changing issue which has been addressed in several other publications [17–20] and is beyond the scope of this report. The purpose of this manuscript is twofold; first, to analyze and compare laparoscopic versus robotic rectal surgery learning curves with respect to operative times and surgical outcomes for a novice minimally invasive colorectal surgeon and second, to offer a possible pathway for an academic surgeon who primarily trained in open rectal surgery and who is interested in adopting minimally invasive rectal surgery.
Methods
Study description
In order to investigate learning curves for laparoscopic versus robotic rectal surgery, the first author (GM) identified, via tips from established colorectal surgeons and e-mail communications, a surgeon (BSM) who was reported to have adopted robotic surgery at the onset of his career. The author travelled from North America to Korea to audit the surgeon’s MIS rectal cases. From 2008 to 2011, data of 106 laparoscopic and 92 robotic LAR rectal surgery cases were entered into a database and reviewed for analysis. These were all consecutive cases deemed to be candidates for MIS surgery. Combined resections were not included. All patients were provided with available information on both techniques and were offered to choose from the two approaches. Decision on whether the laparoscopic or robotic technique was performed was based solely on patient’s choice of a procedure and patient’s possession of robotic surgery insurance coverage or ability to pay for the extra cost associated with robotic surgery. All surgeries were performed by a single academic colorectal surgeon (BSM) who was primarily trained in open colorectal surgery and whose prior training included general MIS training in laparoscopic appendectomy, cholecystectomy, and hernia repair. The surgeon further trained in a two-year university affiliated colorectal surgery fellowship program (Severance Hospital, Yonsei University Health System, Seoul, Korea). During his fellowship training, he participated in about 700 open and 150 laparoscopic colonic resections as well as about 50 cases of open rectal cancer surgery. In 2008, the surgeon joined as a faculty member of the division of colorectal surgery at the same hospital where he trained. He has completed formal laparoscopy and robotic surgery courses and started MIS rectal cancer surgery practice under the institutional support from experts in both laparoscopic and robotic surgery. His mentors included gastrointestinal (GI) surgeons who had a combined experience of about 100 robotic GI cancer surgeries. After the initial 10 laparoscopic and 10 robotic cases under the institutional supervision, the institution privileged him to perform LARs independently without a mentor. This series covers the beginning of his MIS colorectal practice. Patient characteristics and outcome measures were analyzed. Outcome measures included operative times, postoperative complications, resection margin involvement, rates of open conversion, lymph node harvest, estimated blood loss, and days to discharge. The data were separated into three groups representing initial 31 cases, middle 31 cases, and the latest cases (44 for the laparoscopic cases and 30 for the robotic cases). Without the presence of any other obvious grouping criteria, three nearly equal groups allow for most statistically relevant comparisons with lessened potential for introducing data grouping bias.
Operative time plots were generated to evaluate learning curve with respect to the speed of individual parts of the operation. Total surgical time was separated into four distinct time intervals which included (1) the extracorporeal operative time, (2) inferior mesenteric artery (IMA) operative time, (3) splenic flexure operative time, and (4) TME operative times. Extracorporeal time included port placement, robot docking/re-docking, if applicable, rectal anastomosis time, and wound closure.
Total operation time was defined as skin-to-skin time as retrieved from the operative record. Entire operative procedure was segmented into 4 steps according to following definitions: (1) IMA operative time was defined as the interval from the opening of retroperitoneum at the level of aortic bifurcation to the ligation of IMA; (2) splenic flexure operative time as the interval from the end of IMA time to the end of splenic flexure mobilization; (3) TME operative time as the intracorporeal interval from splenic flexure mobilization to rectal transection (excluding re-docking time for the robotic surgery); and (4) extracorporeal time was calculated as total operative time minus (IMA operative time + splenic flexure operative time + TME operative time), thus including port placement, robot docking/re-docking, if applicable, rectal anastomosis, and wound closure. All times were precisely clocked with postoperative review of video footage. Statistics used involved determination of means with 95 % confidence intervals calculated using an Excel spread sheet based on a Student’s T-Distribution. Non-overlapping 95 % confidence intervals, corresponding to p-values of less than 0.05, were employed to identify statistical significance. P-values can be calculated from confidence intervals and conversely. However, confidence intervals offer easier comparison across more than two groups of data. Only very restricted statements about effect strength are possible on the basis of p-values. Confidence intervals provide an adequately plausible range for the true value related to the measurement of the point estimate. With confidence intervals, statements are therefore possible on the direction of the effects, as well as its strength and the presence of a statistically significant result [21].
CUSUM plots were used for evaluating the learning curve with respect to patient important outcomes for laparoscopic and robotic resections. CUSUM plots are commonly used in assessing learning progress and proficiency in medical field across many specialties including colorectal surgery [22–24]. They can be a valuable tool in rapidly depicting unfavorable trends. The CUSUM plots are a visual representation of cumulative failures and successes where the plot starts at zero and goes down with a success or up with a failure. In their simplest form, as used in this manuscript, the plots will go down by a fraction consistent with established acceptable failure rate and up by a fraction consistent with a success rate. For example, if an acceptable rate of major complication is 10 %, the graph will go down only by 0.1 unit with a success and up by 0.9 unit with a failure. Failure is therefore depicted more dramatically than a success. While plot centered on the zero line indicates a rate consistent with established acceptable failure rate, upward and downward sloping plots are indicative of less and more favorable rates, respectively. In this manuscript, CUSUM plots were used to track the rates of major complications and the rates of positive margins for both robotic and laparoscopic techniques. To create CUSUM plots, acceptable failure rates must be established. The leak rate in LAR varies greatly but is accepted to be around 10 % regardless of approach used [25]. The general rate of major complications also varies greatly and is reported between 12 and 22 % [7, 26]. The acceptable rate of positive resection margins is not clearly established. The circumferential resection margin (CRM) rate has been reported between 1 and 28 % [27]. Larger size studies, involving significant stage III proportions similar to the series presented in this manuscript, report positive CRM resection margins of 8–22 % [27]. The most conservative acceptable rates for major complications and positive resection margins of 10 and 8 %, respectively, were therefore used in generating the CUSUM plots featured in this report.
Surgical techniques description
The LAR was carried out a minimum of 5 cm below distal tumor edge or, if not possible, to the levator complex. All anastomoses below 5 cm from anal verge and patients who have received neo-adjuvant chemo-radiation therapy were covered by a diverting ileostomy. Although some minor adjustments were made during the initial period of ~30 cases, the following is a general description of the surgical techniques used.
Laparoscopic LAR
(1) SET-UP: Patient is in supine lithotomy position. A 10-mm 30o camera through transumbilical port, three 5-mm ports in left upper quadrant (LUQ), left lower quadrant (LLQ), and right upper quadrant (RUQ), and one 12-mm port in right lower quadrant (RLQ) is used. (2) Management of IMA: Skeletonization of IMA is performed from its root just above hypogastric nerve fibers for maximal nodal yield using endoscopic dissectors, spatula cautery, and an advanced energy device such as EnSeal (Ethicon). This is followed by ligation and transection of sigmoid artery and preservation of left colic artery if technically as well as oncologically feasible. Otherwise, IMA is ligated at its root. (3) Splenic flexure mobilization and (4) Pelvic TME: Both are performed in a standard fashion, maintaining general surgical principles. (5) Bowel resection, specimen retrieval, and anastomosis: Intracorporeal transection is performed using an articulated endostapler which is inserted through the 12-mm RLQ port. Specimen retrieval is done through a 3-cm transumbilical incision using a wound protector. This is followed by standard laparoscopically assisted end-to-end stapled anastomosis.
Robotic LAR
(1) Set-up: Totally robotic double docking approach is used. Patient is in supine lithotomy position using standard DaVinci S or Si instrumentation (Intuitive). The trocars are placed as per Fig. 1. In the Fig. 1, the R1, R2, R3 trocar placement corresponds to first docking stage used for IMA mobilization and splenic flexure takedown. R1′, R2′, R3′ refers to the second docking stage trocar placement for the pelvic TME. Surgeon controls the R1 monopolar scissors using the right hand and the R2 bipolar grasper using the left hand. The R3 grasper is used only intermittently for static retraction. (2) Management of IMA and (3) Splenic flexure mobilization are performed using R1–R3 instrumentation observing same principles described for laparoscopic surgery. (4) Pelvic TME is performed using R1′–R3′ (following robot re-docking and patient re-positioning) in a standard fashion, maintaining general surgical principles. (5) Bowel resection, specimen retrieval, and (6) Anastomosis are performed in the same fashion as described for laparoscopic LAR, except the R3′ port is used to fire the endostapler.
Results
Data summarizing preoperative parameters and operative outcomes are shown in Table 1 and Table 2, respectively. The preoperative data in Table 1 feature a lack of stage IV disease in the robotic group—this is statistically significant only for the initial 31 cases and is addressed in the discussion section of this manuscript. The outcome data in Table 2 feature laparoscopic versus robotic LAR total operative times (including port placement and robot docking/re-docking) of 308 (291–325) min versus 397 (373–420) min for the initial group of cases—in favor of laparoscopy—and 220 (212–229) min versus 204 (196–211) min for the last group of cases—reversed in favor of robotics. The data further feature laparoscopic versus robotic major perioperative complications of 4.7 versus 6.5 % (not statistically significant—NS), minor complications of 12.3 versus 12.0 (NS), resection margin involvement (both distal and circumferential) of 2.8 versus 4.4 % (NS), rate of conversion to open surgery of 3.8 versus 1.1 % (NS), conversion rate of 3.8 versus 1.1 (NS), lymph node harvest of 16.3 versus 17.2 (NS), estimated blood loss of 231 versus 201 cc (NS), and days to discharge of 9.9 versus 9.6 days (NS). There were no perioperative deaths. Major complications included leaks/intra-abdominal abscesses, and a case requiring same admission laparotomy for non-resolving obstruction due to a small bowel adhesion. All, even minor, abdominal abscesses were included in this group, assuming that they might have represented small leaks. Minor complications observed included ileus, urinary retention, wound infection, development of ascites as well as a case of pneumonia. Conversions were due to bleeding not readily controlled by an MIS approach.
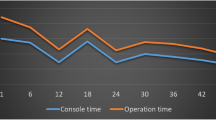
Figures 2, 3, 4, 5, 6 depict operating time curves for entire operations and their individual components. Although total operative time curves (Fig. 2) did not reveal a flat plateau even after the 106, respectively 92 cases, the initially fast rate of operative time improvement slowed down after about 55 cases for both approaches. In this study, the total operative time curves show that after initial 41 cases, the robotic surgery was faster than laparoscopic surgery. Time curves of individual components of the procedure are depicted in Figs. 3, 4, 5, and 6. Their significance is discussed in the discussion section of this manuscript.
CUSUM plots of major complications, Figs. 7 and 8, and CUSUM plots of resection margin involvement, Figs. 9 and 10, visually depict each failure as a spike and because they lie below the zero line, they demonstrate acceptable perioperative surgical outcomes from the beginning of the surgeon’s MIS rectal surgery practice.
Major complication CUSUM plot—Laparoscopy; failure defined as any major complication observed and described in Table 1, assuming acceptable failure rate of 10 %
Major complication CUSUM plot—Robotics; failure defined as any major complication observed and described in Table 1, assuming acceptable failure rate of 10 %
Discussion
Paucity of reports exists on learning curves involving robotic rectal surgery. The available literature comes from investigators with prior extensive laparoscopic experience [24, 28–30]. Their vision and insight are invaluable. However, by the time, these investigators adopt robotic technology; they are likely near their laparoscopy skill plateau, making a direct comparison of robotic surgery with laparoscopy difficult. Covering a surgeon who simultaneously adopts laparoscopic and robotic surgery at the beginning of his minimally invasive career, the series presented in this report provides a unique insight on MIS learning curves and allows for direct comparisons.
Although operative time curves did not reveal a flat plateau even after the 106 laparoscopic or after the 92 robotic cases, the initial fast rate of operative time improvement decreased after about 55 cases for both approaches. For laparoscopic LAR, in comparison with previous findings of operative time curve stabilization after about 50 cases [31], these results show somewhat similar, although a bit slower, trend. There are no reports directly looking at reaching operative plateau times in robotic rectal surgery without prior extensive laparoscopic experience. Our results show more cases needed to reach operative time proficiency than the 15–25 cases reported by Bokhari et al. [24]. However, this comparison is less than ideal as these are highly skilled laparoscopists reporting on their robotic experience with distal colorectal cancer where only 30 % of cases consisted of LAR. Akmal et al. [30] recently reported on 80 robotic TME cases without significant TME operating time learning curve. Again, their data originate from a team with an extensive laparoscopic experience. Their data might, thus, suggest that laparoscopic skills are transferable to robotics rather than robotic TME has significantly attenuated learning curve.
The data presented in this manuscript further show that initially longer total operative times for robotic surgery improve rapidly and after 41 cases become faster than those for laparoscopic surgery. From the analysis of individual operative time components, this can be clearly attributed to rapid extracorporeal and TME operative time learning curves. Indeed, in this data set, the extracorporeal time stabilizes and becomes comparable to its laparoscopic equivalent by case number 41. Based on TME operative time curves, robotic approach to TME becomes faster early on by case number 21 and continues improving beyond. Furthermore, based on TME times generated for the summary data table, laparoscopic TME never shows statistically shorter times, while robotic TME clearly shows statistically lower operative times for all but the initial cohort. This is consistent with recent research at a more basic level which concluded that robotic assistance improves performance with complex tasks such as knot tying while decreasing operator workload [32]. While tying a knot is not an ideal surrogate to tasks more specific to colorectal manipulation, it provides some evidence of benefit at a more fundamental level—evidence often sought in other fields of medicine such as drug design where it is desirable to link medication’s clinical efficacy with its mode of action.
Except for a brief initial period when laparoscopic splenic mobilization is somewhat faster, IMA and splenic flexure operative time curves indicate that there is not much time difference for these tasks—perhaps indicating that robotic approach does not provide as much assistance with less complex tasks. A valid argument can therefore be made for a hybrid technique. Considering equivalency between the two techniques and our short robotic docking/re-docking times, we chose the totally robotic approach to save resources by not having to use additional laparoscopic instruments and to continue further optimizing our technique.
Laparoscopy versus robotics rate of major complications of 4.7 (0.7–8.8) % versus 6.5 (1.5–11.6) % and resection margin involvement of 2.8 (0.0–6.0) % versus 4.4 (0.2–8.5) % observed in this study are acceptable results when compared to previously published data which were discussed in detail earlier in this report [7, 25–27]. These results are further accentuated by the fact that population presented in this manuscript included >25 % stage III disease across all cohorts and the fact that all, even relatively minor intra-abdominal abscesses, were assumed to be anastomotic leaks. Furthermore, the CUSUM plots indicate that developing both laparoscopic and robotic skills simultaneously provides acceptable outcomes in rectal surgery from the beginning of the study and might therefore be a viable approach to MIS for an academic surgeon who primarily trained in open colorectal surgery.
One of the limitations of this study is the lack of stage IV disease patients in the robotic cohorts. This is an honest disclosure and does not represent surgeon bias. This is due to economics-based decisions made by patients and their insurers with respect to financing robotic surgery when facing the burden of additional costs associated with further therapy. However, the absence of stage IV disease in the robotic cohorts was statistically insignificant in all but the initial cohort. A deviation from data originating in other centers is the patients’ lower BMI and low proportion of neoadjuvant therapy. This is not a reflection of specially selected cohorts, but merely a reflection of our general population’s lower BMI and the fact that our institution had stricter indications for preoperative chemo-radiation (locally advanced cancers, tumor-infiltrating endorectal fascia on MRI or suspicious lymph nodes in lateral pelvic walls on MRI). All patients deemed candidates for an MIS surgery were offered a robotic resection, but ultimately the robotic cohort placement was dictated by the patient’s ability and willingness to cover the robotic co-pay. This, again, is an honest disclosure, and we understand that this might be a major distraction of the study. On the other hand, this selection did not result in any major variation in the two groups’ pre-operative parameters. Other limitations of this study include the fact that this is a single surgeon experience and that there are likely additive or even synergistic learning effects transferring from laparoscopy to robotics and vice versa. On the other hand, the fact that this is a single surgeon experience performing both procedures at the onset of his career allows for a unique head-to-head comparison where many variables and effects stay similar for both techniques.
We do not necessarily view robotics as a brand new operative strategy but as an additional tool making MIS easier and more precise. Still, the burden of superiority proof for robotics will continue to linger until long-term randomized trials materialize. Furthermore, the higher cost of robotics versus laparoscopy still remains an issue for administrators and surgeons when deciding on adopting MIS technologies [17–20]. This manuscript is intended to contribute as a guide during the decision making process on which technology to adopt. Based on the data presented in this study, developing both laparoscopic and robotic skills simultaneously provides acceptable results with learning curve potentially favoring robotics. It can be argued that in current state of flux, developing both techniques simultaneously offers itself as a possible approach in rectal cancer, balancing clashing interests between trying to improve technology and outcomes on one side and on the other side facing economic constraints and burden of superiority proof.
References
Larach SW, Salomon MC, Williamson PR, Goldstein E (1993) Laparoscopic assisted abdominoperineal resection. Surg Laparosc Endosc 3(2):115–118
Chindasub S, Charntaracharmnong C, Nimitvanit C, Akkaranurukul P, Santitarmmanon B (1994) Laparoscopic abdominoperineal resection. J Laparoendosc Surg 4(1):17–21
Darzi A, Lewis C, Menzies Gow N, Guillou PJ, Monson JR (1995) Laparoscopic abdominoperineal excision of the rectum. Surg Endosc 9(4):414–417
Ramos JR, Petrosemolo RH, Valory EA, Polania FC, Pecanha R (1997) Abdominoperineal resection: laparoscopic versus conventional. Surg Laparosc Endosc 7(2):148–152
Iroatulam AJ, Agachan F, Alabaz O, Weiss EG, Nogueras JJ, Wexner SD (1998) Laparoscopic abdominoperineal resection for anorectal cancer. Am Surg 64(1):12–18
Fleshman JW, Wexner SD, Anvari M, La Tulippe JF, Birnbaum EH, Kodner IJ et al (1999) Laparoscopic versus open abdominoperineal resection for cancer. Dis Colon Rectum 42(7):930–939
Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 250(1):54–61
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97(11):1638–1645
Trastulli S, Cirocchi R, Listorti C, Cavaliere D, Avenia N, Gullà N, Giustozzi G, Sciannameo F, Noya G, Boselli C (2012) Laparoscopic versus open resection for rectal cancer: a meta-analysis of randomized clinical trials. Colorectal Dis 14(6):277–296
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16(6):1480–1487
Mirnezami AH, Mirnezami R, Venkatasubramaniam AK et al (2010) Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis 12(11):1084–1093
Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L (2009) Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS 13(2):176–183
Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B (2010) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 24(11):2888–2894
Park JS, Choi GS, Lim KH, Jang YS, Jun SH (2010) Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 17(12):3195–3202
Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A (2010) Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg 251(5):882–886
Kim JY, Kim NK, Lee KY, Huh H, Min BS, Kim JW (2012) A comparative study of voiding and sexual function after Total Mesenteric excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19(8):2485–2493
Baek JH, Pastor C, Pigazzi A (2011) Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc 25:521–525
Kim NK, Kang J (2010) Optimal total mesorectal excision for ectal cancer: the role of robotic surgery from an expert’s view. J Korean Soc Coloproctology 26:377–387
Delaney CP, Lynch AC, Senagore AJ, Fazio VW (2003) Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46:1633–1639
Rawlings AL, Woodland JH, Vegunta RK, Crawford DL (2007) Robotic versus laparoscopic colectomy. Surg Endosc 21:1701–1708
Hommel G, Röhrig B, Blettner M (2009) Confidence Interval or P-Value? Dtsch Arztebl Int 106(19):335–339
Lim TO, Soraya A, Ding LM, Morad Z (2002) Assessing doctors’ competence: application of CUSUM technique in monitoring doctor’s performance. Int J Qual Health Care 14(3):251–258
Colquhoun PHD (2008) CUSUM analysis of J-pouch surgery reflects no learning curve after board certification. Can J Surg 51(4):296–299
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860
Taflampas P, Christodoulakis M, Tsiftis DD (2009) Anastomotic Leakage after low anterior resection for rectal cancer: facts, obscurity, and fiction. Surg Today 39:183–188
Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG (2006) Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol 13(3):413–424
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26(2):303–312
Spinoglio G, Summa M, Priora F, Quarati R, Testa S (2008) Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum 51(11):1627–1632
Hellan CM, Anderson C, Ellenhorn JDI, Paz B, Pigazzi A (2007) Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol 14(11):3168–3173
Akmal Y, Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 2012; Surg Endosc. 2012 Mar 22 [Epub ahead of print],PMID: 22437950
Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N (2011) Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc 25:2972–2979
Stefanidis D, Wang F, Korndorffer JR, Dunne JB, Scott DJ (2010) Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 24(2):377–382
Acknowledgement
We would like to express our gratitude to Mr. Martin Morris, Librarian, Medical Library, Royal Victoria Hospital, McGill University, Montreal, Canada for assistance with literature search and Mrs. Myo Jeong Kim, MS with Dr. Dong Wook Kim, PhD from the Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, South Korea for their help with statistical analysis. We also acknowledge and appreciate editing services of Mr. Shaun Fawcett of Final Draft Consulting, Montreal.
Disclosure
Drs. George Melich, Young Ki Hong, Jieun, Kim, and Sender Liberman have no conflicts of interest or financial ties to disclose. Drs. Hyuk Hur MD, Seung Hyuk Baik, Nam Kyu Kim, and Byung Soh Min have received in the past travel and speaking honoraria from Intuitive Surgical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melich, G., Hong, Y.K., Kim, J. et al. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29, 558–568 (2015). https://doi.org/10.1007/s00464-014-3698-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3698-0