Abstract
Mastication is controlled by central pattern generator in the brainstem and can be modulated by volition. The aim of this study was to investigate the effect of chewing well on swallowing. Twenty-six healthy participants were instructed to eat 8, 12, and/or 16 g of steamed rice with barium sulphate under the following two conditions: chewing freely task (CF; chewing naturally in their usual manner) and chewing well task (CW; chewing the food with a request to “chew well”). We evaluated bolus transport and swallowing movement using videofluoroscopy and electromyography of the masseter, suprahyoid and thyrohyoid muscles. The chewing time and pharyngeal transit time (PTT) at the first swallow showed high reproducibility in both CF and CW. PTT for CW was significantly shorter and longer than CF in 12 and 16 g, respectively. In 12 g, CW increased the pharyngeal bolus velocity and decreased thyrohyoid EMG activity during swallowing compared with CF. In 16 g, the difference between CW and CF in the estimated swallowed bolus volume was positively correlated with that in upper esophageal sphincter transit duration. We speculate that CW modulates PTT during swallowing depending on the mouthful volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastication is the first step of digestion. During mastication, solid foods are broken down from large particles into small particles and mixed with saliva to form a bolus before swallowing. Although masticatory rhythm and pattern are controlled by central pattern generator in the brainstem [1], they can be modulated by volition [2,3,4].
The interaction between mastication and swallowing is acknowledged in humans and animals. At rest, the swallowing reflex is readily evoked by mechanical or chemical stimulation to the oropharyngeal region in humans [5] and even in anesthetized animals [6]. When human subjects eat solid foods, a triturated bolus accumulates in the oropharynx during mastication and before swallowing; this is known as stage II transport [7, 8]. Consistent with this, animal studies have revealed the inhibitory effect of activation of the cortical masticatory area on swallowing initiation [9,10,11,12]. Studies of healthy humans found that stage II transport starts at four-fifths of the masticatory sequence and the number of stage II transport cycle is associated with the duration of swallowing [13, 14]. Palmer et al. reported that stage II transport was delayed and transport to the valleculae was inhibited under the command of a swallow by investigator in cookie chewing [4], suggesting that volition alters initiation of swallowing.
The relationship between masticatory function and systemic disease has received much attention. Fushida et al. reported that lower masticatory performance increases the risk of developing metabolic syndrome [15]. In addition, Yamazaki et al. suggested that higher masticatory performance decreases the risk of developing diabetes [16]. The importance of the oral function for safe eating has been emphasized [17]. About half of non-hospitalized choking victims aged 18 to 64 years were defective or partial dentition without dental prostheses, suggesting that reduced masticatory performance increases the risk of choking [18]. Because the condition of the food broken down by mastication affects swallowing, chewing might change the pharyngeal bolus transit during swallowing and relate the risk of aspiration and pharyngeal bolus residue in patients with dysphagia. Furuya et al. reported that longer chewing with volition alters bolus transport during swallowing, resulting in easier swallowing [3]. Although chewing well is generally recommended in both young and old, it remains unclear how this affects swallowing depending on mouthful volumes. The aim of the present study was to investigate the effect of chewing well on swallowing among three mouthful volumes.
Materials and Methods
On the basis of our and other previous studies [19,20,21], we determined the mouthful volumes in this study. Because we assumed that the volume of 16 g of rice is too large for women [20], only men were recruited. Since we selected the vaguely instructions “chewing well”, we doubted the intrasubject reproducibility. Thus, we firstly evaluated the reproducibility of food bolus transit time using 12 g rice which is reported as the volume of rice in a single natural bite [21].
Participants
Twenty-six healthy men (mean age ± standard deviation (SD): 30.1 ± 8.5 years, range: 20–62 years) participated in this study. Before starting the study, a dentist confirmed that no participants had abnormalities in the number or position of their teeth, temporomandibular disorders, or masticatory problems. No participants complained of coughing, swallowing, or speech problems, and none had histories of respiratory disease, neuromuscular disorders, or stroke.
Electromyography (EMG) and Videofluoroscopic (VF) Images
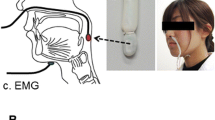
Surface EMGs were recorded from the right side of the masseter (Mas) muscle and bilateral sides of the suprahyoid (SH) and thyrohyoid (TH) muscles. The main contributors to the SH EMG are the mylohyoid, the anterior belly of the digastric, and the geniohyoid muscles [22]. Disposable electrodes (NM-31; Nihon Kohden, Japan) were attached to the skin with an interpolar distance of 2 cm and were positioned at the center of the Mas muscle, the anterior belly of the digastric muscle, and the TH muscle. Before recording, we confirmed Mas, SH, and TH muscle activation during biting, opening the mouth, and swallowing, respectively. Signals were amplified (AB-611 J; Nihon Kohden) and filtered (low cut: 15 Hz; high cut: 1 kHz). Lateral views of the VF images were simultaneously recorded to evaluate the feeding sequence (ARCADIS Avantic, Siemens Healthineers, Germany). The sampling rate was 2 kHz for the EMGs and 30 Hz for the VF images, and these data were converted via an analogue-to-digital converter (Power Lab 8/35; AD Instruments, USA). Signals from the EMGs and VF images were stored on a computer through the PowerLab software package (LabChart 8 with Video Capture Add-On; AD Instruments).
Data Collection
All participants were instructed not to eat or drink for at least 1 h prior to each experiment. During the recording, they were seated comfortably in a chair. A steel ball 11 mm in diameter was attached to their chin for distance correction in the VF images. Initially, they were instructed to perform maximum opening mouth, maximum biting and swallowing voluntarily twice in this order as the reference activity for the SH, Mas, and TH muscles, respectively.
Next, participants were instructed to eat steamed rice (8, 12, and/or 16 g, Sato No Gohan, Sato Foods, Japan) with barium sulphate (20%) under the following two conditions: chewing freely task (CF: chewing and swallowing the food in their usual manner) and chewing well task (CW: chewing the food with a request to “chew well” followed by swallowing in their own manner). In initial experiment, we evaluated the reproducibility of food bolus transit time during chewing and swallowing for six participants using 12 g mouthful volume with following trial order: 1st CF, 2nd CF, 1st CW, 2nd CW. Because we confirmed good reproducibility of chewing time and pharyngeal transit time (PTT) in CW and CF trials and needed to reduce radiation exposure, we investigated CF and CW once in each mouthful volume for another twenty participants. Out of them, eight participants also joined the preliminary single swallow experiment to evaluate the validity of the measurement of the food bolus area in the VF images. In this experiment, participants were instructed to freely chew the steamed rice with barium sulphate (2, 4, 6, 8 and 10 g) and attempt single swallows per bolus.
Before eating, all participants were asked to swallow their saliva. When finished eating, they lifted right hand and each trial was ended after examiner’s confirmation of no food residue in their mouth. The time interval between trials was at least 1 min, and the participants rinsed their mouths with distilled water between trials whenever they wished. After all the experiments, we asked twenty participants which mouthful volume (8, 12 or 16 g) was closest to their usual mouthful volume.
Data Analysis
The results are presented as the mean ± SD. Three dentists (A.O., T.T. and T.S.) analyzed VF images, who were well trained and had > 5 years of clinical experience in dental practice and dysphagia rehabilitation. VF images were divided into the following three stages, in accordance with previous studies [3, 4]. Stage I transport time: From when the head of food passed the incisors until the food was transported to the molar region for chewing, Chewing time: From the start of mastication until the rapid hyoid movement in an antero-superior direction (Onset of the first pharyngeal swallow), PTT: From the first rapid hyoid movement in an antero-superior direction until the trailing edge of the food bolus passed the entrance of upper esophageal sphincter (UES) as defined by reaching the level of the vocal cords [7]. PTT was further subdivided into before (pre-UES transit duration: Pre-UTD) and after (UES transit duration: UTD) the head of the bolus passed the entrance of the UES.
To measure the hyoid movement during the first swallow, the anterior-inferior ridge of the hyoid bone and the anterior-inferior corner of the C4 vertebra were defined as the hyoid bone position and the origin, respectively. The line running from the origin and the anterior inferior corner of the C2 vertebra was used as a vertical axis and the line passing through the origin perpendicular to the vertical axis was used as a horizontal axis. The distance from the hyoid position at the start of rapid movement in an anterior-superior direction to the position in the most anterior-superior aspect was measured with Image J 1.53e software (National Institutes of Health, USA). The distance from the bolus head position to the entrance of the UES at the onset of the first swallow was also measured with Image J 1.53e software. The pharyngeal bolus velocity was calculated by dividing the distance the bolus head position to the entrance of the UES at the first swallow by Pre-UTD.
To measure the swallowed bolus volume, the food bolus at the onset of each swallow and the pharyngeal residue after each swallow were checked frame by frame using VF images. After each image was imported into Adobe Photoshop CS6 software (Adobe Systems, USA) and optimized for brightness and contrast, the outlines of the food bolus at the onset of each swallow and the oropharyngeal residue after each swallow were drawn and measured with Image J 1.53e software as the food bolus area. In a preliminary experiment, the food bolus area was normalized to the food bolus area for 10 g in each participant. The estimated swallowed bolus volume at the first swallow was calculated using the following formula with the measurement of the food bolus area.
EMG activity was defined as active when the rectified and smoothed EMG signal was more than the mean + 3SD of the background activity gained from the 5 s stable period with a rapid elevation and drop. The duration, peak amplitude, and integral area of the filtered EMG bursts in bilateral SH and TH muscles during the first swallow were measured. The peak amplitude and area were normalized to the average values for two voluntary swallows. Averaged EMG data between right and left sides were used.
Tests for normality and equality of variances were initially performed for the statistical analysis to determine whether to use parametric or nonparametric tests. A paired t-test or Wilcoxon’s rank sum test was used to analyze differences between two groups. The correlation coefficient was evaluated using Pearson’s correlation. Differences were considered significant at p < 0.05.
Results
Mouthful Volume
Six participants ate 12 g of steamed rice with barium sulphate in their own manner (CF) or chewing the food with a request to “chew well” (CW) two times each. Next, twenty participants ate 8, 12, and 16 g of steamed rice with barium sulphate in CF and CW conditions. After eating three mouthful volumes of steamed rice, eleven of twenty (55%) participants answered that 12 g fitted their one mouthful volume. Seven (35%) and two (10%) participants answered 16 g and 8 g, respectively.
Effect of Chewing Well on Food Bolus Transport
Typical recordings for CF and CW are shown in Fig. 1. Following large SH EMG bursts with mouth opening, the food passed the incisors and was transported to the molar region. Subsequently, Mas and SH EMG bursts were reciprocally observed during chewing followed by a swallow accompanied by TH EMG bursts. No participants showed laryngeal penetration and aspiration in all tasks. Many participants did not show pharyngeal residue after tasks. Chewing time was markedly longer for CW than CF (Fig. 1). Using the VF images, we analyzed food bolus transport. Initially, we evaluated the reproducibility of each stage (stage I transport time, chewing time, PTT) for CF and CW in 12 g (Table 1). The stage I transport time for CF and CW were short and quite varied, in that intraclass correlation coefficient (ICC) between first and second trials was low in CF and CW. On the other hand, the ICC of chewing time and PTT between first and second trials were high in CF and CW. There was no significant difference between first and second trials of all stages in both conditions. Next, we compared food bolus transit time between CF and CW (Fig. 2). The stage I transport time did not differ between CF and CW in all mouthful volumes. The chewing time was significantly longer for CW than CF in all mouthful volumes. On the other hand, the PTT was affected by CW depending on the mouthful volume. In 8 g, the PTT did not differ between CF and CW. In 12 g, the PTT for CW was significantly shorter than CF. Conversely, the PTT for CW was significantly longer than CF in 16 g. Subsequently, we subdivided PTT into before (Pre-UTD) and after (UTD) the head of the bolus passed the entrance of the UES in 12 and 16 g (Fig. 3). In 12 g, Pre-UTD and UTD were significantly shorter for CW than CF. In 16 g, UTD was significantly longer for CW.
Example of simultaneous recording of electromyographs and videofluoroscopy (VF) images from one participant. This participant ate 12 g rice under the chewing freely (CF) and chewing well (CW) conditions. The chewing time in CF (left) was 13.25 s and that in CW (right) was 33.82 s. Two swallows (arrowheads) were evoked in CF and one swallow was evoked in CW. Vertical dotted lines represent the passage of food between the upper and lower incisors (a), food transportation to the molar region (b), the passage of the bolus head on the lower border of the mandible (c), the start of rapid hyoid movement in an antero-superior direction (onset of pharyngeal swallow) (d), and the passage of the trailing edge of the food bolus on the entrance of the upper esophageal sphincter (e). The lower panel shows VF images of (a) to (e) for CW. Rt-Mas: right side of masseter muscle; Rt-SH: right side of suprahyoid muscle; Lt-SH: left side of suprahyoid muscle; Rt-TH: right side of thyrohyoid muscle; Lt-TH: left side of thyrohyoid muscle
Comparison of bolus transit time between chewing freely task (CF) and chewing well task (CW). Stage I transport time (Stage I) did not differ between CF and CW (n = 20, Wilcoxon’s rank sum test, p = 0.121 for 8 g; paired t-test, p = 0.065 for 12 g, paired t-test, p = 0.523 for 16 g). CW significantly extended chewing time (Chewing) compared with CF in all mouthful volumes (n = 20, paired t-test, p < 0.001 for 8 g, 12 g and 16 g). CW significantly shortened and extended pharyngeal transit time (PTT) compared with CF in 12 and 16 g, respectively (n = 20, paired t-test, p = 0.638 for 8 g, paired t-test, p < 0.001 for 12 g; paired t-test, p = 0.007 for 16 g). **p < 0.01, ***p < 0.001
Comparison of the time from the first rapid hyoid movement in an antero-superior direction until the head of the bolus passed the upper esophageal sphincter (pre-UES transit duration: Pre-UTD) and the time from when the head of the bolus passed the UES until the trailing edge of the bolus passed the UES (UES transit duration: UTD) between chewing freely task (CF) and chewing well task (CW) in 12 and 16 g. Pre-UTD and UTD for CW was significantly shorter than CF in 12 g (n = 20, paired t-test, p = 0.002 for Pre-UTD; paired t-test, p = 0.031 for UTD). On the other hand, UTD for CW was significantly longer than CF in 16 g (n = 20, paired t-test, p = 0.487 for Pre-UTD; paired t-test, p = 0.009 for UTD). *p < 0.05, **p < 0.01
We developed the following three hypotheses for the effect of CW on PTT: CW modulates the first swallowing movement (first hypothesis), changes the bolus head position at the onset of first swallow (second hypothesis), and alters the bolus volume at the first swallow (third hypothesis). To investigate them, we further analyzed the EMG activity and VF images.
Swallowing Movement and the Position of the Food Bolus
To evaluate the effect of CW on the swallowing movement, we analyzed SH and TH EMG activity (Fig. 4) and hyoid movement (Fig. 5A) of the first swallow. The relative integral area and duration of SH EMG did not differ between CF and CW in all mouthful volumes. On the other hand, the area of TH EMG for CW was significantly smaller than CF in 12 g but not 8 and 16 g. Consistent with this data, the TH EMG duration for CW was significantly shorter than CF in 12 g. The relative maximum amplitude of SH and TH EMG activity did not differ between CF and CW in all mouthful volumes (SH: 145 ± 66% for CF and 143 ± 66% for CW in 8 g, 159 ± 85% for CF and 150 ± 61% for CW in 12 g, 166 ± 78% for CF and 142 ± 80% for CW in 16 g; TH: 143 ± 61% for CF and 154 ± 85% for CW in 8 g, 136 ± 51% for CF and 131 ± 61% for CW in 12 g, 144 ± 99% for CF and 154 ± 105% for CW in 16 g, n = 20, paired t-test, p > 0.05). The correlation analysis revealed that the difference between CW and CF in the TH EMG was not correlated with the difference between them in the PTT (n = 20, Correlation Coefficient = 0.165, Pearson’s correlation, p = 0.486). The hyoid distance from the start of the rapid movement to the anterior–superior maximum position did not differ between CF and CW in all mouthful volumes. Next, we evaluated the position of the food bolus at the first swallow (Fig. 5B). The distance from the bolus head position to the entrance of the UES at the onset of the first swallow did not differ between CF and CW in all mouthful volumes.
Comparison of the relative integral area (A) and duration (B) of the electromyographic bursts of the first swallow between chewing freely task (CF) and chewing well task (CW). The integral area of TH EMG in CW was significantly smaller than CF in 12 g (n = 20, SH: paired t-test, p = 0.939 for 8 g, paired t-test, p = 0.299 for 12 g, Wilcoxon’s rank sum test, p = 0.571 for 16 g; TH: paired t-test, p = 0.144 for 8 g, paired t-test, p = 0.037 for 12 g, Wilcoxon’s rank sum test, p = 0.784 for 16 g). The duration of TH EMG in CW was significantly shorter than CF in 12 g (n = 20, SH: paired t-test, p = 0.508 for 8 g, paired t-test, p = 0.194 for 12 g, paired t-test, p = 0.804 for 16 g; TH: paired t-test, p = 0.069 for 8 g, paired t-test, p = 0.049 for 12 g, paired t-test, p = 0.460 for 16 g). *p < 0.05. SH: suprahyoid muscle; TH: thyrohyoid muscle
Comparison of hyoid movement during first swallowing (A) and the bolus head position at the onset of the first swallow (B) between chewing freely task (CF) and chewing well task (CW). A: There was no significant difference between CF and CW for all mouthful volumes (n = 20, paired t-test, p = 0.475 for 8 g; paired t-test, p = 0.574 for 12 g; paired t-test, p = 0.443 for 16 g). B: There was no significant difference between CF and CW for all mouthful volumes (n = 20, paired t-test, p = 0.204 for 8 g; paired t-test, p = 0.257 for 12 g; paired t-test, p = 0.305 for 16 g)
Quantitative Measures of the Estimated Swallowed Bolus Volume
To investigate the validity of quantitative measures of the estimated swallowed bolus volume using VF images, we first evaluated the estimated swallowed bolus volume at a single swallow per bolus in eight participants. The data recorded from one participant was discarded, because he could not swallow 10 g of steamed rice at one time. The food bolus area was positively correlated with mouthful volume (Fig. 6A). The relative food bolus area was significantly positively correlated with mouthful volume with a high coefficient of determination (Fig. 6B). We also estimated the inter-rater reliability using the ICC. The ICC for the two raters (AO and TT) was 0.945, indicating excellent reliability. On the basis of these results, we made a formula to calculate the estimated swallowed bolus volume at the first swallow. CW significantly increased the estimated food bolus than CF in 16 g, although CW did not change it in 8 and 12 g (Fig. 7).
Correlation between bolus area and mouthful volume A: Pixel-based quantitative measures of bolus area at the onset of the swallow (n = 7, Correlation Coefficient = 0.909, Pearson’s correlation, p < 0.001). B: Relative quantitative measures of bolus area at the onset of the swallow. The relative food bolus area was normalized to the food bolus area for 10 g in each participant (n = 7, Correlation Coefficient = 0.967, Pearson’s correlation, p < 0.001)
Comparison of the estimated swallowed bolus volume at the first swallow between chewing freely task (CF) and chewing well task (CW). A: Videofluoroscopic images at the onset of the first swallow (left), after the first swallow (middle), and at the onset of the second swallow (right). Dashed lines (black) indicate the outlines of the food bolus at the onset of the first (left) and second (right) swallows and the oropharyngeal residue after the first swallow (middle). This participant ate 12 g of steamed rice in two swallows. B: There was no significant difference between CF and CW in estimated swallowed bolus volume for 8 and 12 g (n = 20, paired t-test, p = 0.140 for 8 g; paired t-test, p = 0.127 for 12 g). Estimated swallowed bolus volume was significantly larger in CW than CF in 16 g (n = 20, paired t-test, p < 0.001). ***p < 0.001
Pharyngeal Bolus Velocity and Relationship Between Estimated Swallowed Bolus Volume and PTT
Because CW shortened Pre-UTD and UTD compared with CF in 12 g, we speculate that CW accelerates pharyngeal bolus velocity at the first swallow. Pharyngeal bolus velocity was significantly faster for CW than CF in 12 g (Fig. 8A). In contrast, the velocity did not differ between them in 16 g (247 ± 77 mm/sec for CF, 237 ± 113 mm/sec for CW, n = 20, paired t-test, p = 0.746). Next, we speculate that the increased swallowed bolus volume in CW extends the UTD in 16 g. The correlation analysis revealed that the difference between CW and CF in the estimated swallowed bolus volume was significantly positively correlated with the difference between them in the UTD in 16 g (Fig. 8B). In contrast, those parameters did not correlate in 12 g (n = 20, Pearson’s correlation, Correlation Coefficient = 0.143, p = 0.546).
Pharyngeal bolus velocity and relationship between estimated swallowed bolus volume and upper esophageal sphincter transit duration (UTD). A: Comparison of the pharyngeal bolus velocity between chewing freely task (CF) and chewing well task (CW) in 12 g. Pharyngeal bolus velocity was significantly faster for CW than CF (n = 20, paired t-test, p = 0.012). B: Correlation of the difference in the estimated swallowed bolus volume between the CF and CW and that of UTD in 16 g. (n = 20, Correlation Coefficient = 0.703, Pearson’s correlation, p < 0.001). *p < 0.05
Discussion
In the present study, we demonstrated the different effects of CW on the pharyngeal bolus transit during swallowing among three mouthful volumes. The chewing time and PTT showed good reproducibility in both CF and CW. The PTT for CW was significantly shorter and longer than CF in 12 and 16 g, respectively. The pharyngeal bolus velocity in CW was significantly faster than CF in 12 g. The difference between CW and CF in the estimated swallowed bolus volume was positively correlated with the difference between them in UTD in 16 g. These results suggest that CW shortens PTT via the acceleration of pharyngeal bolus velocity in 12 g and extends the PTT via the increased swallowed volume in 16 g. We speculate that CW modulates PTT during swallowing depending on the mouthful volume.
Appropriate Mouthful Volume
Over half of participants considered that 12 g fitted their one mouthful volume in the present study. Okada et al. reported that the volume of sushi rice in a single natural bite was approximately 12 g [21]. We speculate that 8, 12, and 16 g of steamed rice would correspond to small, appropriate, and large volumes for healthy adult men, respectively. Consistent with a previous study [21], most of the rice in 8 and 12 g were swallowed in CF and CW conditions, and those did not significantly differ between them. In 16 g, however, about 60% volume in CF and 80% volume in CW were swallowed in the first swallow and those significantly differed. One participant could not swallow 10 g rice at one time in the preliminary experiment. Even in healthy participants, the bolus volume they can swallow might differ based on their eating habits and swallowing ability. Indeed, the fitted mouthful volume varied among participants.
Different Effects of CW on Food Bolus Transport Among Three Mouthful Volumes
Since stage I transport time was short, it is easy to imagine that the time varied and represented low ICC between repetitive two trials. On the other hand, chewing time and PTT showed high ICC, indicating good reproducibility. Consistent with a previous study [3], chewing time was significantly longer for CW than CF in all mouthful volumes. On the other hand, CW did not change the bolus head position at the onset of first swallowing. Although a previous study reported that longer chewing with volition extended the duration of the post stage II chewing sequence [3], the bolus head position at the onset of the first swallow might not depend on the chewing time in healthy participants. However, this may be not the case in older people and patients with dysphagia [23].
CW shortened and extended the PTT in 12 and 16 g, respectively. We discuss the modulatory effect of CW on PTT. The results of shortened PTT in 12 g was consistent with Furuya’s study. The authors reported that longer duration of chewing with volition shortened the PTT using agar jelly [3]. We described that CW shortened Pre-UTD and UTD and accelerated pharyngeal bolus velocity during first swallowing in 12 g. We can imagine that one of the reasons for reduction of PTT by CW in 12 g might be the alteration in the physical property of the bolus after chewing well, such as hardness, cohesiveness and adhesiveness. SH EMG activity includes both voluntary and involuntary components during swallowing [24], whereas TH EMG activity is involved in involuntary component of swallowing, i.e. pharyngeal phase of swallowing [25]. We observed that CW decreased TH EMG activity during swallowing than CF in 12 g. Although only half of participants answered 12 g steamed rice for fitted their one mouthful volume, it might be appropriate volume which CW change into the proper triturated bolus and facilitate the pharyngeal bolus passage. With decreased TH EMG activity, this might be related to the subjective easiness of swallowing by CW [3]. Although modulation of TH EMG activity by CW might affect bolus transit, we thought that TH EMG change by CW is not main cause of PTT reduction by CW based on the correlation analysis between TH EMG activity and PTT. Another possible reason might be the tongue manipulation. The importance of tongue driving pressure during swallowing has been shown in a comparison between healthy participants and patients with restricted tongue motion [26, 27]. Huckabee and Steele reported the emphasizing tongue-to-palate contact during effortful swallowing generated greater orolingual and pharyngeal pressure compared with inhibiting tongue-to-palate emphasis [28]. Increasing tongue driving force may contribute to shortened PTT. In the future study, we should evaluate the bolus transit during swallowing with tongue motion and pharyngeal pressure among different mouthful volumes.
During mastication, mechanoreceptors in the periodontal ligaments and gingival increase salivary secretion via masticatory-salivary reflex [29] and the saliva changes the structure of food bolus including lubrication and cohesiveness [30]. There is no doubt that the estimated swallowed volume did not reflect the real weight, because the weight of the mixed saliva needs to be considered. Nevertheless, the strong positive linear relationship between the relative food bolus area and the mouthful volume indicates that the calculation of the estimated food volume at the first swallow is relatively valid. Our results suggest that CW extends the PTT in 16 g mouthful volume via increased first swallowed volume. Previous studies support our speculation. For instance, the food bolus volume has been shown to modulate swallowing more than the bolus viscosity [31]. Additionally, the larger swallowed bolus volume causes a longer UES opening duration [32, 33]. Although longer chewing changes the physical property of the bolus which is decreased hardness and increased the cohesiveness and water content of with mixed saliva [34], we speculate that the effect of CW on pharyngeal bolus transit during swallowing is more affected by the swallowed bolus volume than the physical property of the bolus in the case of large mouthful volume. In fact, two out of two (100%) participants who decreased swallowed bolus volume in CW compared with CF recorded a shortened UTD in CW (Fig. 8B), although averaged swallowed bolus volume in CW was significantly larger than CF in 16 g (Fig. 7B). In human liquid swallowing, the duration and integral area of the SH EMG and hyoid movement during swallowing has been shown to increase with increasing the bolus volume [35,36,37,38]. Although CW in 16 g increased the swallowed bolus volume, we did not find any change in EMG activity and hyoid movement. One possibility for the discrepancy between the present results and previous studies is that the increase in volume in this study was quite small compared with that of previous studies—approximately 3 g for this study and 10 ml or more for previous studies. Another possibility is that the different food type (liquid vs solid) may have affected the results. Additionally, some researchers have described differences in hyoid velocity and temporal EMG activity among swallowing-related muscles during swallowing under certain experimental conditions [39, 40]. Further precise analyses in swallowing kinematics will be needed in future studies.
Limitations
There are several limitations in this study. First, this study includes only small number of healthy male participants. Therefore, we were unable to investigate any difference in sex and age. Masticatory functions are known to vary with age and gender [41]. We must consider the investigation for older population, subjects with dental prosthesis including a denture or patients with dysphagia in the future study. This is useful to discuss the importance of mouthful volume and chewing behavior on safe eating. Second, we used only steamed rice which is relatively soft and sticky as a test food. The foods with different hardness and cohesiveness might bring different results. Third, we instructed just “chew well” for CW tasks. We selected this vaguely request to participants because dentists and other medical staffs often advise patients or older people to “chew well” at mealtimes. The meaning of chewing freely and chewing well might depend on participants, i.e. subjective, and affect the results. Fourth, we evaluated the immediate effect following CW instruction. A recent study reported that the use of a wearable device for measuring masticatory behaviors increases the number of chews for a rice in healthy participants [42]. We should investigate how to chew and swallow the food after masticatory behavior modification. Fifth, the test food contained barium sulphate and the number of trials was small to reduce radial exposure. Because of these methodological limitations, we have to consider the difference between experimental condition and meal situation. Nevertheless, our results suggest that appropriate mouthful volume accelerates pharyngeal bolus transit and large mouthful volume increased swallowed bolus volume under chewing well condition. This might emphasize the importance of mouthful volume and chewing behavior on swallowing dynamics.
Data Availability
All data generated or analyzed during this study are available from the corresponding author T.T. upon reasonable request.
Abbreviations
- CW:
-
Chewing well
- CF:
-
Chewing freely
- EMG:
-
Electromyography
- PTT:
-
Pharyngeal transit time
- ICC:
-
Intraclass correlation coefficient
- Mas:
-
Masseter
- SD:
-
Standard deviation
- SH:
-
Suprahyoid
- TH:
-
Thyrohyoid
- UES:
-
Upper esophageal sphincter
- VF:
-
Videofluoroscopy
References
Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol. 1971;215:1–13.
Ashiga H, Takei E, Magara J, Takeishi R, Tsujimura T, Nagoya K, Inoue M. Effect of attention on chewing and swallowing behaviors in healthy humans. Sci Rep. 2019;9:6013.
Furuya J, Hara A, Nomura T, Kondo H. Volitional chewing with a conscious effort alters and facilitates swallowing during feeding sequence. J Oral Rehabil. 2014;41:191–8.
Palmer JB, Hiiemae KM, Matsuo K, Haishima H. Volitional control of food transport and bolus formation during feeding. Physiol Behav. 2007;91:66–70.
Shingai T, Miyaoka Y, Ikarashi R, Shimada K. Swallowing reflex elicited by water and taste solutions in humans. Am J Physiol. 1989;256:R822–6.
Kitagawa JI, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol-Regul Integr Compar Physiol. 2002;282:R1342–7.
Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14:31–42.
Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7:187–200.
Amri M, Lamkadem M, Car A. Effects of lingual nerve and chewing cortex stimulation upon activity of the swallowing neurons located in the region of the hypoglossal motor nucleus. Brain Res. 1991;548:149–55.
Lamkadem M, Zoungrana OR, Amri M, Car A, Roman C. Stimulation of the chewing area of the cerebral cortex induces inhibitory effects upon swallowing in sheep. Brain Res. 1999;832:97–111.
Tsujimura T, Tsuji K, Ariyasinghe S, Fukuhara T, Yamada A, Hayashi H, Nakamura Y, Iwata K, Inoue M. Differential involvement of two cortical masticatory areas in modulation of the swallowing reflex in rats. Neurosci Lett. 2012;528:159–64.
Tsujimura T, Tsuji K, Magara J, Sakai S, Suzuki T, Nakamura Y, Nozawa-Inoue K, Inoue M. Differential response properties of peripherally and cortically evoked swallows by electrical stimulation in anesthetized rats. Brain Res Bull. 2016;122:12–8.
Hiraoka T, Palmer JB, Brodsky MB, Yoda M, Inokuchi H, Tsubahara A. Food transit duration is associated with the number of stage II transport cycles when eating solid food. Arch Oral Biol. 2017;81:186–91.
Yamashita S, Sugita D, Matsuo K. Relationship between stage II transport and number of chewing strokes as mastication progresses. Physiol Behav. 2013;122:100–3.
Fushida S, Kosaka T, Nakai M, Kida M, Nokubi T, Kokubo Y, Watanabe M, Miyamoto Y, Ono T, Ikebe K. Lower masticatory performance is a risk for the development of the metabolic syndrome: the Suita study. Front Cardiovasc Med. 2021;8: 752667.
Yamazaki T, Yamori M, Asai K, Nakano-Araki I, Yamaguchi A, Takahashi K, Sekine A, Matsuda F, Kosugi S, Nakayama T, Inagaki N, Bessho K. Nagahama Study Collaboration G: Mastication and risk for diabetes in a Japanese population: a cross-sectional study. PLoS ONE. 2013;8: e64113.
Feinberg MJ. Radiographic techniques and interpretation of abnormal swallowing in adult and elderly patients. Dysphagia. 1993;8:356–8.
Berzlanovich AM, Fazeny-Dorner B, Waldhoer T, Fasching P, Keil W. Foreign body asphyxia: a preventable cause of death in the elderly. Am J Prev Med. 2005;28:65–9.
Maeda R, Takei E, Ito K, Magara J, Tsujimura T, Inoue M. Inter-individual variation of bolus properties in triggering swallowing during chewing in healthy humans. J Oral Rehabil. 2020;47:1161–70.
Nakamichi A, Matsuyama M, Ichikawa T. Relationship between mouthful volume and number of chews in young Japanese females. Appetite. 2014;83:327–32.
Okada A, Honma A, Nomura S, Yamada Y. Oral behavior from food intake until terminal swallow. Physiol Behav. 2007;90:172–9.
Palmer PM, Luschei ES, Jaffe D, McCulloch TM. Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech Lang Hear Res. 1999;42:1378–91.
Park HS, Kim DK, Lee SY, Park KH. The effect of aging on mastication and swallowing parameters according to the hardness change of solid food. J Texture Stud. 2017;48:362–9.
Ertekin C, Kiylioglu N, Tarlaci S, Turman AB, Secil Y, Aydogdu I. Voluntary and reflex influences on the initiation of swallowing reflex in man. Dysphagia. 2001;16:40–7.
Pearson WG Jr, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia. 2012;27:445–51.
McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98:71–8.
Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108:1418–26.
Huckabee ML, Steele CM. An analysis of lingual contribution to submental surface electromyographic measures and pharyngeal pressure during effortful swallow. Arch Phys Med Rehabil. 2006;87:1067–72.
Anderson DJ, Hector MP, Linden RWA. The effects of unilateral and bilateral chewing, empty clenching and simulated bruxism, on the masticatory-parotid salivary reflex in man. Exp Physiol. 1996;81:305–12.
Mosca AC, Chen JS. Food-saliva interactions: mechanisms and implications. Trends Food Sci Technol. 2017;66:125–34.
Ferris L, Doeltgen S, Cock C, Rommel N, Schar M, Carrion S, Scholten I, Omari T. Modulation of pharyngeal swallowing by bolus volume and viscosity. Am J Physiol Gastrointest Liver Physiol. 2021;320:G43–53.
Cock C, Jones CA, Hammer MJ, Omari TI, McCulloch TM. Modulation of upper esophageal sphincter (UES) relaxation and opening during volume swallowing. Dysphagia. 2017;32:216–24.
Nascimento WV, Cassiani RA, Santos CM, Dantas RO. Effect of bolus volume and consistency on swallowing events duration in healthy subjects. J Neurogastroenterol Motil. 2015;21:78–82.
Kochi I, Takei E, Maeda R, Ito K, Magara J, Tsujimura T, Kulvanich S, Inoue M. Changes of bolus properties and the triggering of swallowing in healthy humans. J Oral Rehabil. 2021;48:592–600.
Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–87.
Ertekin C, Aydogdu I, Yuceyar N, Pehlivan M, Ertas M, Uludag B, Celebi G. Effects of bolus volume on oropharyngeal swallowing: an electrophysiologic study in man. Am J Gastroenterol. 1997;92:2049–53.
Miyaoka Y, Ashida I, Kawakami S, Tamaki Y, Miyaoka S. Activity patterns of the suprahyoid muscles during swallowing of different fluid volumes. J Oral Rehabil. 2010;37:575–82.
Moon IY, Yi CH, Park IW, Yong JH. Effects of sitting posture and bolus volume on activation of swallowing-related muscles. J Oral Rehabil. 2020;47:577–83.
Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1985;86(1663–1669):1999.
Ueda N, Nohara K, Kotani Y, Tanaka N, Okuno K, Sakai T. Effects of the bolus volume on hyoid movements in normal individuals. J Oral Rehabil. 2013;40:491–9.
Woda A, Foster K, Mishellany A, Peyron MA. Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiol Behav. 2006;89:28–35.
Uehara F, Hori K, Hasegawa Y, Yoshimura S, Hori S, Kitamura M, Akazawa K, Ono T. Impact of masticatory behaviors measured with wearable device on metabolic syndrome: cross-sectional study. JMIR Mhealth Uhealth. 2022;10: e30789.
Acknowledgements
We thank Helen Jeays, BDSc AE, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was supported in part by a grant from JSPS KAKENHI (Grant Numbers 20K10069 to T.T.) and a grant from Food and Health Science Yazuya to T.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical Approval and Consent
The Ethics Committee of Niigata University approved this study (approval number: 2020-0173). The participants provided written informed consent, and the study was performed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okubo, A., Tsujimura, T., Ueha, R. et al. Chewing Well Modulates Pharyngeal Bolus Transit During Swallowing in Healthy Participants. Dysphagia (2024). https://doi.org/10.1007/s00455-024-10689-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00455-024-10689-z