Abstract
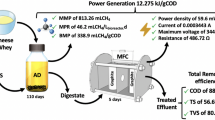
An important pollutant produced during the cheese making process is cheese whey which is a liquid by-product with high content of organic matter, composed mainly by lactose and proteins. Hydrogen can be produced from cheese whey by dark fermentation but, organic matter is not completely removed producing an effluent rich in volatile fatty acids. Here we demonstrate that this effluent can be further used to produce energy in microbial fuel cells. Moreover, current production was not feasible when using raw cheese whey directly to feed the microbial fuel cell. A maximal power density of 439 mW/m2 was obtained from the reactor effluent which was 1000 times more than when using raw cheese whey as substrate. 16S rRNA gene amplicon sequencing showed that potential electroactive populations (Geobacter, Pseudomonas and Thauera) were enriched on anodes of MFCs fed with reactor effluent while fermentative populations (Clostridium and Lactobacillus) were predominant on the MFC anode fed directly with raw cheese whey. This result was further demonstrated using culture techniques. A total of 45 strains were isolated belonging to 10 different genera including known electrogenic populations like Geobacter (in MFC with reactor effluent) and known fermentative populations like Lactobacillus (in MFC with cheese whey). Our results show that microbial fuel cells are an attractive technology to gain extra energy from cheese whey as a second stage process during raw cheese whey treatment by dark fermentation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
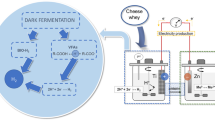
Cheese whey is a liquid by-product that remains after the cheese making process and represent up to 95% of the initial milk volume and is considered an important pollutant partially due to its high chemical oxygen demand (0.8–102 gCOD/L) [1]. Dry matter in cheese whey accounts up to 7% and is composed by lactose, proteins, salts, lipids, lactic acid, citric acid and nitrogenated compounds such as urea and uric acids [2]. The typical treatment for this wastewater are anaerobic lagoons, but more efficient technologies as nutrient and water recovery or anaerobic digestion have been explored and applied at full scale [1]. One interesting alternative for cheese whey treatment is dark fermentation where biohydrogen is produced, which we and other authors have demonstrated feasible [3–10]. However, this option presents some limitations like strong variations in hydrogen yields and the incomplete degradation of organic matter remaining as volatile fatty acids which indicates that further research is necessary to obtain a stable and efficient system [4, 9, 11].
Within bioelectrochemical systems, microbial fuel cells (MFC) are emerging as a new alternative for energy recovery during treatment of wastewaters. In MFCs the organic matter is converted to CO2, protons and electrons by microorganisms as part of their metabolism using the anode electrode as a final electron acceptor. The released electrons are transferred to a current collector and migrate through an external resistor to the cathode generating electric current. At the cathode, oxygen reduction to water is catalyzed using the electrons and protons produced on the anode [12]. In a MFC, the development of a biofilm on the anode surface is essential for an improved bio-catalysis and electron transfer to get efficient current production. Microbial communities developed on MFC anodes are very diverse and no typical MFC microbial community has been described [13]. Several phylogenetic groups have been found to be predominant in MFCs, however, some genera like Geobacter and Pseudomonas are commonly found in mixed culture MFC anodes and are associated with current production due to their feasibility of current generation under axenic conditions [14]. Nevertheless, highly diverse communities are detected on anodes [15, 16] and therefore, predicting electrical power production performances through microbial ecology analysis is still a challenge.
MFCs have been proposed as a possible technology to harness energy from the remaining organic matter after the dark fermentation process for effluents like cellulose, organic solid wastes, cane molasses, crude glycerol and sucrose [17–21]. To our knowledge, the effluent of dark fermentation of cheese whey has never been used for energy production in MFCs. But, several studies report the use of cheese whey to produce energy in MFCs generally with low coulombic efficiencies [22–27]. A predominance of alternative pathways instead of current production were inferred through the low coulombic efficiencies obtained and cheese whey sterilization has been recommended to improve MFC performance [23, 24]. No analysis of the microbial communities associated to anodes of cheese whey fed MFCs have been reported which is relevant to understand the scarce coulombic efficiencies obtained.
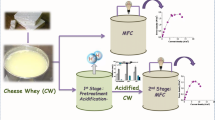
Our main focus was to study the feasibility of energy production with effluent produced by dark fermentation of cheese whey in microbial fuel cells and to compare its performance with two microbial fuel cell, one fed with cheese whey and a control fed with acetate. We intend to explain the performance of the microbial fuel cells, in terms of current production and coulombic efficiency, through the analysis of the microbial communities developed on the anodes and the isolation of bacteria from the anodes.
Materials and methods
MFC set-up and operation
Three single chamber air cathode MFCs containing graphite felt anodes were built using acrylic according to Liu and Logan (28). Each MFC consisted of a 4 cm long by 3 cm in diameter chamber with a measured working volume of 25 mL after installing the electrodes. Graphite felt anodes (7 cm2) (Alfa Aesar) were connected to a 3 cm titanium wire as current collector. The cathodes (7 cm2 total exposed surface area) were made by applying a platinum catalyst (0.36 mg Pt/cm2, Electrodes and more-US) and a Nafion membrane layer (Dupont) on the liquid-facing side of a carbon cloth (Fuel Cell Earth). Five PTFE (SIGMA–Aldrich) diffusion layers were added on the air-facing side [29]. An external resistance of 200 Ω was used to connect anode and cathode electrodes as 218 Ω was shown to produce maximum power in a similar MFC fed with acetate [30]. Medium was recirculated using a peristaltic pump (i150, iPumps-UK) with a flow of 3 mL/min between the anodic chamber and an auxiliary 120 mL flask.
MFCs were inoculated (10% v/v) with liquid from the anodic chamber of a tubular MFC operated in LabMet, Belgium. This MFC was inoculated with activated sludge and fed with acetate for more than 2 years. The liquid from the anodic chamber was stored at 4 °C for 6 months before using it as inoculum in the MFCs. All MFCs were initially fed with mineral medium containing (per liter) 5.6 g Na2HPO4·H2O, 3 g K2HPO4, 0.5 g NaCl, 1 g NH4Cl, 0.24 g MgSO4, 0.011 g CaCl2, 1 mg FeSO4, 0.07 mg ZnCl2, 0.1 mg MnCl2, 0.006 mg H3BO3, 0.002 mg CuCl2·2H2O, 0.024 NiCl2·6H2O, 0.036 mg Na2Mo4·2H2O, 0.238 mg CoCl2·6H2O and 2.5 gCOD/L of sodium acetate as organic substrate. Sodium acetate was replaced by complex substrates (2.5 gCOD/L) when the voltage response after each sodium acetate addition was similar (aprox. 2–3 months). The complex substrates used were raw cheese whey for MFC 1 and the effluent from a biohydrogen producing reactor fed with cheese whey for MFC 2 (full description of the these substrates can be found in the following section). The complex substrates were previously diluted in the mineral medium to reach a concentration of 2.5 gCOD/L for MFC 1 and MFC 2, respectively. A third MFC was operated with sodium acetate (2.5 gCOD/L) as substrate throughout the whole experiment and was used as control MFC. A complete operation cycle was considered from the feeding start until a cell potential value below 5 mV. MFCs were operated at 30 °C in a thermostatically controlled room and the initial pH was 7.2. Samples (1 mL) were taken from the anodic chamber of the MFCs for reducing sugars and volatile fatty acids measurements.
Complex substrates used to fuel the MFCs
Raw cheese whey was provided from a dairy factory in Canelones, Uruguay. The average composition of the cheese whey was as follows: chemical oxygen demand (COD) 67,000 mg/L (standard deviation 6000 mg/L, 66 samples); total nitrogen 1335 mgN/L; total phosphorus 310 mg/L; and pH 4.7 (standard deviation 0.9, 66 samples) [3]. The concentration of reducing sugars was 54.7 g/L (61.4 gCOD/L assuming reducing sugars as lactose) which represents 92% of the total COD. The main volatile fatty acid (VFA) was lactic acid with a concentration of 0.59 g/L. The biohydrogen reactor effluent was obtained from a lab-scale biohydrogen producing reactor fed with the aforementioned raw cheese whey operated at the BioProA laboratory (Engineering Faculty, University of the Republic in Uruguay). The COD of the reactor effluent was 25,000 mg/L (measured by BioProa group according to standard methods [31]). No lactose was detected in the reactor effluent and the main VFAs were acetic (8.7 g/L) and butyric (6.6 g/L). Both complex substrates were homogenized, dispensed in 20 mL falcon tubes and stored at −20 °C until use.
Chemical analyses
Volatile fatty acids concentration was determined by high performance liquid chromatography (HPLC) with a Rezex ROA-Organic Acid H+ (8%) (300 × 7.8 mm) column (Phenomenex) and a diode arrange detector (Waters 2998) at the HPLC platform of the Biological Research Institute “Clemente Estable” (Uruguay). Prior to the analysis protein content was precipitated for each sample adding a final concentration of 1 M perchloric acid (Sigma Aldrich) and centrifuged 5 min at 5000g. The pellet was discarded and the supernatant was filtered through 0.22 µm filter (Sartorius) Reduced sugar concentration was measured by 3,5-dinitrosalicylic acid (DNS) method [32] and the calibration curve was performed using lactose.
Electrochemical measurements and coulombic efficiency calculation
Cell potential was registered every 15 min using a datalogger (Keithley, USA) connected to a personal computer. The coulombic efficiency (CE), the ratio between the charge in coulombs recovered and total charge in coulombs in the substrate, was calculated as previously described for the best operation cycle after adding the complex substrate [33]. Polarization curves were performed in a three arrange electrodes using a PGZ 301 Voltalab potentiostat and the Voltamaster 4 software (Radiometer Analytical, France) using the working cell volume (25 mL) to calculate the power density by cubic meter or the anode geometrical area to calculate the power density by square meter. The reference and counter electrode were connected to the cathode and the working electrode was connected to the anode. Before performing polarization curves, the cell was operated under open circuit conditions for a period of 15 min. The cell resistance was gradually decreased to obtain the short circuit value. The power density was calculated assuming an ohmic like system.
Microbial community analysis
DNA extraction and amplicon sequencing
Microbial communities of the bio-anode biofilm were analyzed at the end of the experiments. Anode graphite felts were washed with sterile distilled water, chopped in fine pieces and the genomic DNA was extracted using the Power Soil DNA Kit (Mo Bio laboratories, Carlsbad, CA, USA) as described by the manufacturer instructions. DNA was dehydrated with 95% ethanol and submitted to the Institute for Agrobiotechnology Rosario (INDEAR, Rosario, Argentina) for 454-pyrosequencing analysis (Roche Genome Sequencer FLX Titanium system). A fragment of 16S rRNA genes, corresponding to V3 and V4 regions, were amplified with the primer set 515 forward and 806 reverse [34]. Sequences were analyzed using the Quantitative Insights into Microbial Ecology (QIIME) software [35]. Chimera detection was performed with USEARCH 6.1 software. De novo OTUs picking and taxonomic assignment were defined using the UClust algorithm on the basis of 97% sequence similarity. The identity of the sequences assigned using local BLAST tool with Greengenes database reference sequences. Sequence alignments were performed with PyNAST. Alpha (Dominance and Shannon indexes) and Beta (weighted-Unifrac and principal component analysis) diversity analysis were performed with the QIIME software. Raw sequences were deposited at NCBI-SRA database (Accession number SRX958299).
Anodic bacteria isolation and 16S rRNA gene sequencing
Anode samples from MFC 1 (raw cheese whey), MFC 2 (biohydrogen reactor effluent) and the control MFC (acetate) were suspended in sterile PBS buffer and treated with sonication (15 s, 200 kHz) to detach bacteria from the anodes.
1mL from the anode suspensions were inoculated into 25 mL vials with 10 mL of different media (Table 1). The vials were sparged with nitrogen gas (99.99%, The Linde Group, Uruguay) to ensure anaerobic conditions. Successive dilutions were made and then two strategies were followed. (1) Direct plate isolation from dilutions 10−5 to 10−8; (2) dilution to extinction. Vials were incubated until growth was observed and plate isolation was performed from the last vial showing growth. For isolation, the media were supplemented with agar (1.3%, Difco). Plates were incubated under aerobic and anaerobic conditions. For anaerobic conditions, anaerobic bags (Anaerocult® A mini, Merck, Germany) were used. Different media were selected to cover diverse physiological groups, focusing on anaerobic respiring bacteria which includes several exoelectrogenic bacteria. Fusibacter media (DSM 853) was included due to the high abundance of Fusibacter on anodes detected by 16S rRNA gene amplicon sequencing. Geobacter media (DSM 579) was included as several species within Geobacter are exoelectrogenic. Media and culture conditions are shown in Table 1.
Colony PCR was performed to amplify the 16S rRNA gene by suspending a single colony in 100 μL of sterile Milli-Q water and heated for 15 min at 100 °C and then frozen at −20 °C for 20 min. The suspension was centrifuged for 15 min at 12,000 rpm and 2 μL were used for the PCR reaction. PCR was performed using the general Bacteria primers, 27F and 1492R as described previously [3, 36]. PCR products were verified in 1% agarose gel and sequenced in Macrogen Sequencing Service (Korea). Sequences were deposited at NCBI GeneBank database under the following accession numbers: N1 KX898513, N2 KX898514, N3 KX898515, N4 KX898516, N5 KX898517, N6 KX898518, N7 KX898519, N8 KX898520, N9 KX898521, N10 KX898522, N11 KX898523, N12 KX898524, N14 KX898525, O4 KX898526, O22 KX898527, O23 KX898528, O26 KX898529, FE1 KX898530, FE2 KX898531, FE5 KX898532, FE7 KX898533, FE8 KX898534, O1 KX898535, O2 KX898536, O3 KX898537, O5 KX898538, O6 KX898539, O7 KX898540, O8 KX898541, O9 KX898542, O10 KX898543, O11 KX898544, O12 KX898545, O13 KX898546, O14 KX898547, O15 KX898548, O16 KX898549, O17 KX898550, O18 KX898551, O19 KX898552, O20 KX898553, B KX898554, F KX898555, NAR KX898556, NV KX898557.
Phylogenetic analysis
Phylogenetic trees were constructed with sequences from the most abundant OTUs presented in Table 2 and one phylogenetic tree with the 16S rRNA gene sequences of the isolated strains.
The phylogenetic trees was performed using the software MEGA 6 [37]. Sequences were aligned using clustalW together with sequences downloaded from GeneBank database (National Center for Biotechnology Information-NCBI, http://www.ncbi.nlm.nih.gov/). The phylogenetic relationship between sequences was determined using the Neighbor-Joining method [38], and the bootstrap consensus tree was inferred from 1000 replicates [39]. For the OTUs 225 positions were considered while for the isolated strains the positions considered were 493.
Results
Electrochemical performance of the microbial fuel cells
After changing the influent from acetate to the complex substrate, a higher potential was obtained for MFC 2 (fed with biohydrogen bioreactor effluent) compared to MFC 1 (fed with raw cheese whey) (Fig. 1). The maximum cell potential obtained for MFC 2 was 0.209 V in the first operation cycle with reactor effluent but dropped to 0.142 and 0.097 V in the second and third cycles, respectively (Fig. 1). MFC 1 reached only 0.035 V in the first feed cycle with raw cheese whey and this potential was never exceeded in subsequent cycles (Fig. 1). The control MFC (fed with acetate) reached almost a constant potential close to 0.2 V in every successive feed cycle (Fig. 1). Polarization curves performed at the last cycle of operation showed that MFC 2 produced higher optimal current density than MFC 1 (Table 2). However, both produced less optimal current density than the control. When comparing power densities, MFC 2 produced 1000 times more power density than MFC 1 and the control produced a maximum power density 1.2 times higher than MFC 2 (Table 2). The coulombic efficiency (CE) (calculated from the current measured during MFC operation) obtained for MFC 2 was 24%, whereas for MFC 1 the CE was 14% but, the COD removal in MFC 1 was only 42%. Both CEs were lower than the CE of the control MFC (46%) (Table 2).
Potential response for MFC 1 (fed with raw cheese whey) and MFC 2 (fed with biohydrogen reactor effluent) compared to the control MFC (fed with acetate) in consecutive batch cycles. Time 0 indicates the moment when the influent was changed from acetate to the complex substrate. Arrows indicate consecutive substrate additions
To determine if the current was related to the consumption of organic compounds present in the complex substrates reducing sugars and volatile fatty acids were measured. Reducing sugars were the main substrate at the beginning of the operation cycle in MFC 1 and were consumed producing VFAs (mainly acetic) which remained in the MFC until the end of the batch cycle (Fig. 2a). In MFC 2 no reducing sugars were detected in the substrate and the main VFAs present initially were acetic, butyric and propionic acids. After one operation cycle, all measured VFAs were completely consumed. The pH in MFC 1 decreased reaching a value of 4.7 at the end of the batch cycle while pH remained close to 7 until the end of the experiment in MFC 2 (Fig. 2).
Volatile fatty acids (VFA) concentration, lactose concentration (measured as reducing sugars) and pH during one batch cycle for MFC 1 (a) and MFC 2 (b). Lactose is only shown for MFC 1 as it was not detected in MFC 2. pH (open square), Lactose (plus symbol), Acetic acid (closed square), Propionic acid (closed circle), Lactic acid (asterisk), Isobutyric acid (multiple symbol), Butyric acid (closed triangle). For the control MFC acetate was completely consumed (data not shown)
Microbial community analysis of anodes
To understand the differences in the electrochemical performance of the MFCs through the anode microbial communities, we performed 16S rRNA gene based high throughput sequencing analysis. A total of 4877, 5334 and 2673 high-quality reads (average length of 225 bp) were obtained for MFC 1, MFC 2 and control MFC, respectively. The number of OTUs at 3% distance were 211 for MFC 1, 213 for MFC 2 and 155 for the control MFC. Alpha diversity indexes calculated from pyrosequencing data showed that the microbial community on the anode from MFC 2 presented higher diversity (Shannon indexes) and evenness (Dominance index) than the microbial communities of the anodes from the MFC1 and control MFC (MFC 1: 2.879 and 0.193; MFC 2: 3.616 and 0.049 and control MFC 3.073 and 0.097, Shannon and Dominances indexes, respectively).
Weighted UniFrac analysis followed by principal component analysis showed that the microbial communities from the anode of MFC 2 and control MFC were phylogenetically more similar than the community from the anode of MFC 1 (Fig. 3a). The microbial communities from MFC 2 and control MFC anodes share 43 OTUs while only 16 OTUs are shared by the microbial communities of MFC 1 anodes and the control MFC anodes (Fig. 3b). This indicates a strong selective pressure of the substrates used on the microbial communities developed on the anodes.
a Weighted UniFrac analysis with principal component analysis (PCA) of pyrosequencing results for MFC 1 (fed with raw cheese whey), MFC 2 (fed with biohydrogen reactor effluent), the control MFC (fed with acetate) and the inoculum. The percentage of variation explained is presented on each axis. b Overlap of OTUs in bacterial communities from MFC 1, MFC 2 and control MFC. The number in parentheses indicates the total number of OTUs in the community
Microbial community composition at phylum level and class level of anodes from MFC 1 (fed with raw cheese whey), MFC 2 (fed with biohydrogen reactor effluent) and the control MFC (fed with acetate), obtained by 16S rRNA gene pyrosequencing. Phyla or classes with relative abundances below 1% are grouped and named “Other”
The OTUs analyzed at phylum level were grouped into 28 phyla being the main phyla Proteobacteria and Firmicutes (Fig. 4). Firmicutes was clearly predominant in MFC 1 anodes accounting for 73% of the total sequences, whereas both Proteobacteria and Firmicutes were predominant in MFC 2 anodes (31 and 42%, respectively) and the control MFC anodes (29 and 38%) (Fig. 4). The composition within Proteobacteria showed a predominance of the classes Alpha, Beta, Gamma and Delta Proteobacteria on the anodes of all MFCs and Firmicutes was dominated by Bacilli and Clostridia. However, relative abundances of each class changed depending on the MFC substrate (Fig. 4). Main OTUs within Protebacteria were different in the different MFC anodes indicating the strong influence of the substrate used. One OTU (429) predominant in the control MFC (19.2%) and in MFC 2 (6.1%) was, according to the phylogenetic tree, closely related to Geobacter anodireducens (Table 3; Figure S1). Several species within the genus Geobacter have been shown to be exoelectrogenic bacteria and OTU 429 might be relevant for current production in MFC 2 and the control MFC. OTU 123 classified according to the phylogenetic tree within the genus Pseudomonas, was more abundant in MFC 2 than OTU 429 (8.7 vs 6.1%) (Table 3; Figure S1). Pseudomonas aeruginosa has been reported as electroactive and the genus Pseudomonas has been frequently found, using rRNA 16S amplicon sequencing, on anodes of MFCs indicating that OTU 123 might also be related to current production in MFC 2 [40].
In MFC 2 and the control MFC, predominant OTUs within Firmicutes were classified, according to the phylogenetic analysis as belonging to the genera Fusibacter, Clostridium, Dethiosulfatibacter and the family Peptostreptococaceae. (Table 3; Figure S1). A completely different community was detected on the anode of MFC 1 where the predominant OTUs were classified as belonging to the genera Lactobacillus, Streptococcus and Clostridium (Table 3; Figure S1).
The microbial communities developed on the anodes differ strongly from the microbial community of the inoculum used, presenting high abundance of three OTUs classified within the phylum Fusobacteria, and the genera Rhodopseudomonas and Arcobacter which were not present in the microbial communities of the MFC anodes indicated a strong influence of the substrate and the MFC operation on the anode community selection (Table 3; Figure S1).
Isolation of bacteria from the anodes
45 bacterial strains were isolated from the different anodes using different culture media and incubation conditions. From the anodes of MFC 2, 19 isolates were obtained and classified according to the phylogenetic analysis to the genera Pseudomonas, Paracoccus, Raoultella, Geobacter, Achromobacter, Ochrobactrum and Lactobacillus (Table 4; Figure S2). From MFC 1, 11 strains belonging to the genera Pseudomonas, Lactobacillus and Comamonas and one strain that could only be classified as belonging to the family Enterobacteriaceae were isolated (Table 4; Figure S2). From the control MFC, 13 strains were isolated belonging to the genera Pseudomonas, Paracoccus and Ralstonia (Table 4; Figure S2). Most genera detected by the culture approach were also detected by high throughput sequencing with the exception of the genera Raoultella and Ralstonia which were isolated but not detected in any of the anode samples by amplicon sequencing. All the genera detected by the isolation procedure presents at least one species reported as being able to produce current in pure culture with the exception of Achromobacter (Table 4).
Discussion
This is the first report where the effluent from a biohydrogen reactor fed with raw cheese whey was used to produce current in a MFC. The maximum power density, as well as CE values obtained were in the range reported for other MFCs using dark fermentation effluent (Table 5). VFAs present in the influent were consumed completely and pH values were constant throughout the operation of the MFC. After changing the substrate from acetate to the reactor effluent the potential decreased, until the third operation cycle, indicating that the anodic microbial community had to adapt to the new operation condition. When using cheese whey directly to feed the MFC, a very low maximum power density and CE were obtained, similarly to other MFCs using raw cheese whey [25, 26] (Table 5). Endogenous fermenting bacteria present in the cheese whey are introduced in the MFC favouring fermentation processes instead of current generation. The low diversity obtained for MFC 1 might be explained by an outgrowth of lactic acid bacteria which ferment lactose with a concomitant acidification of the medium. These microorganisms are also known to produce antimicrobial compound as bacteriocins, and might in this way compete successfully with electrogenic microorganisms [41]. In MFC 1 lactose was consumed and acetate concentration increased up to a concentration of 0.5 g/L with a concomitant drop in the pH to values lower than 5 indicating that fermentation of lactose occurred. In MFC 2 the lactose was consumed in the hidrogenogenic reactor and no fermentable sugars were fed into the MFC 2. Acetate remained at the end of the cycle and current production was lost indicating that the acetate was not used by electrogenic bacteria in this MFC. If the pH would have been controlled more efficiently, probably the acetate would have been consumed and the performance of MFC1 increased. Zhang et al. [42] operated a similar MFC with acetate at four different pHs (4, 5, 6 and 7). The authors demonstrated that the performance decreases when lowering the pH but acetate was still consumed. Moreover, the performance of the MFCs was restored when increasing the pH in all MFCs except at pH4 probably due to an irreversible damage to the biofilm. The successful current production when using the effluent from dark fermentation of cheese whey and the poor current production using raw cheese whey indicates that the combination of biohydrogen production from cheese whey and current generation from the effluent of the reactor in a MFC is a viable option to completely treat cheese whey obtaining energy in the forms of hydrogen and current.
High predominance of known exoelectrogenic bacteria were detected on the anodes of MFC 2 and the control MFC anodes while fermenting bacteria were enriched on the anode of MFC 1 explaining the MFC performances. In MFC 2 and in the control MFC, a high proportion of reads classified within the genus Geobacter were observed. Moreover, two strains belonging to Geobacter were isolated from the anode of MFC 2. Several species within the genus Geobacter (G. sulfurreducens, G. metallireducens and G. anodireducens) are known electrogenic microorganisms [43–45]. Other genera detected on MFC 2 anodes might play a role in power production, such as Pseudomonas and Thauera. P. aeruginosa has been reported as electrogenic in pure culture [40] and Thauera humireducens strain SgZ-1, isolated from a MFC anode, was able to use organic acids (acetate, propionate, pyruvate, and lactate) but not fermentable sugars (glucose and sucrose) as electron donors in both anthraquinone-2,6-disulfonate (AQDS) and Fe(III) reductions [46]. However, it has not been tested for current production in pure culture and iron reduction does not directly imply electron transfer to anodes [47]. Several strains isolated from all anodes were affiliated to Pseudomonas and according to the phylogenetic analysis, most strains isolated from MFC 2 form a separate cluster which might indicate a particular role in current generation in this MFC. The ability of current generation in pure culture should be determined to confirm that these strains are electrogenic.
Interestingly, one strain isolated form MFC 2 (strain NAR) presents a 16S rRNA gene sequence closely related to Raoultella ornithinolytica and Raoultella electrica. Kimura et al. [48] demonstrated that R. electrica is able to produce current from glucose in an H-type MFC. According to the 16S rRNA gene sequence, strain NAR is more similar to R. ornithinolytica than to R. electrica but due to the similarities in 16S rRNA gene sequences within members of the Klebsiella/Raoultella complex, sequencing of other genes like gyrA, rpoB and parC would be needed for species-level identification. Current generation in pure culture using other substrates than glucose would give more information about the role of this strain in current production from biohydrogen reactor effluent. No sequence closely related to the 16S rRNA sequence of Raoultella was detected by pyrosequencing indicating that combining culture methods with high throughput sequencing are complementary techniques. Cultivation approach has no PCR bias and we might even detect bacteria that are in very low proportion but are favoured by the culture media.
In MFC 1, a high proportion of fermenting microorganisms were detected by pyrosequencing on the anode classified within the genera Clostridium and Lactobacillus. Members of these genera are able to ferment lactose producing volatile fatty acids decreasing the pH which might hamper the growth of electrogenic bacteria [49]. Moreover, several strains affiliated to Lactobacillus were isolated from MFC 1. Even though Lactobacillus species are fermentative, electricity production by Lactobacillus pentosus has been shown in a microbial fuel cell fed with synthetic dairy wastewater [50]. The sequences from the Lactobacillus strains isolated in this work were closely related to L. rhamnosus and L. casei both extendedly used as fermentation starters in dairy industry [51]. To understand more on the role of Lactobacillus in dairy effluent MFCs current production of these strains should be studied in pure culture.
The relative abundance of sequences related to Fusibacter in MFC 2 and the control MFC anodes was surprisingly high. It has been reported that member of the genera Fusibacter ferment several sugars to acetate, H2 and CO2, or reduce thiosulfate and elemental sulfur [52]. Sulfur compounds can be present in the feeding of MFC 2 as proteins containing amino acids with S can be present in cheese whey, and therefore, in the biohydrogen reactor effluent (which is used to feed MFC 2) as cheese whey is used to produce the biohydrogen. Moreover, amino acids with S can be also present in biomass from cell decay. These compounds together with organic substrates coming from the decay of biomass could explain the presence of Fusibacter on the anodes. But, their role on the anodes is not yet clear and must be further investigated. An effort to isolate Fusibacter from the anodes using Fusibacter media was performed but, no strain isolated using this media was classified within the genus Fusibacter.
One of the problems of biohydrogen production from raw cheese whey is the stability of the process. We have demonstrated previously the difficulties in obtaining a stable hydrogen production, mainly due to the negative effect of non-hydrogen producing fermenters and the low predominance of high-yield hydrogen producing organisms [3, 4]. Interestingly, when using cheese whey as substrate in a MFC, a high proportion of Clostridium (39%) were selected on the anode. The presence of Clostridium is generally associated with a good performance of biohydrogen production by dark fermentation [53]. Then, the application of a MFC could be a way to select this high-yield hydrogen microorganism for further hydrogen production. Moreover, the removal of organic acids which are a source of instability and low yields in dark fermentation may also result in higher hydrogen production. An alternative could be a combination of both processes in a single device operated intermittently as a MFC and as a biohydrogen producing reactor by dark fermentation. More work is necessary to investigate the way to combine these two processes to achieve a stable process and higher energy recovery.
In the present work, the differences in the electrochemical performance of two MFCs were explained through the analysis of the anode microbial communities. Electroactive populations were enriched on anodes of MFCs fed with biohydrogen reactor effluent while fermentative populations were predominant on the MFC anode fed directly with raw cheese whey. Effluent from dark fermentation of cheese whey was successfully used to produce current in MFC. But, fermentative metabolism prevails in the cheese whey fed MFC resulted in low power production and acidification which hampered the use of the VFAs produced for current production. Our results demonstrate that MFC might be an attractive technology to be applied as a second stage process during raw cheese whey treatment by dark fermentation process, improving energy recovery or within biohydrogen reactors to stabilize the process.
References
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445–446:385–396. doi:10.1016/j.scitotenv.2012.12.038
Vymazal J (2014) Constructed wetlands for treatment of industrial wastewaters: a review. Ecol Eng 73:724–751. doi:10.1016/j.ecoleng.2014.09.034
Castelló E, García y Santos C, Iglesias T et al (2009) Feasibility of biohydrogen production from cheese whey using a UASB reactor: links between microbial community and reactor performance. Int J Hydrogen Energy 34:5674–5682. doi:10.1016/j.ijhydene.2009.05.060
Castelló E, Perna V, Wenzel J et al (2011) Microbial community composition and reactor performance during hydrogen production in a UASB reactor fed with raw cheese whey inoculated with compost. Water Sci Technol 64:2265–2273. doi:10.2166/wst.2011.706
Perna V, Castelló E, Wenzel J et al (2013) Hydrogen production in an upflow anaerobic packed bed reactor used to treat cheese whey. Int J Hydrogen Energy 38:54–62. doi:10.1016/j.ijhydene.2012.10.022
Venetsaneas N, Antonopoulou G, Stamatelatou K et al (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour Technol 100:3713–3717. doi:10.1016/j.biortech.2009.01.025
Davila-Vazquez G, Cota-Navarro CB, Rosales-Colunga LM et al (2009) Continuous biohydrogen production using cheese whey: Improving the hydrogen production rate. Int J Hydrogen Energy 34:4296–4304. doi:10.1016/j.ijhydene.2009.02.063
Yang P, Zhang R, McGarvey J a., Benemann JR (2007) Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int J Hydrogen Energy 32:4761–4771. doi:10.1016/j.ijhydene.2007.07.038
Cota-Navarro CB, Carrillo-Reyes J, Davila-Vazquez G et al (2011) Continuous hydrogen and methane production in a two-stage cheese whey fermentation system. Water Sci Technol 64:367. doi:10.2166/wst.2011.631
Ghimire A, Frunzo L, Pirozzi F et al (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95. doi:10.1016/j.apenergy.2015.01.045
Azbar N, Çetinkaya Dokgöz FT, Keskin T et al (2009) Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int J Hydrogen Energy 34:7441–7447. doi:10.1016/j.ijhydene.2009.04.032
Rabaey K, Rodríguez J, Blackall LL et al (2007) Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 1:9–18. doi:10.1038/ismej.2007.4
Clauwaert P, Aelterman P, Pham TH et al (2008) Minimizing losses in bio-electrochemical systems: the road to applications. Appl Microbiol Biotechnol 79:901–913. doi:10.1007/s00253-008-1522-2
Sharma M, Bajracharya S, Gildemyn S et al (2014) A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim Acta 140:191–208. doi:10.1016/j.electacta.2014.02.111
Shun’ichi I, Suzuki S, Norden-krichmar TM et al (2013) Identifying the microbial communities and operational conditions for optimized wastewater treatment in microbial fuel cells. Water Res 47:7120–7130. doi:10.1016/j.watres.2013.07.048
Koch C, Popiel D, Harnisch F (2014) Functional redundancy of microbial anodes fed by domestic wastewater. Chem Electrochem 1:1923–1931. doi:10.1002/celc.201402216
Wang A, Sun D, Cao G et al (2011) Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour Technol 102:4137–4143. doi:10.1016/j.biortech.2010.10.137
Vázquez-Larios AL, Solorza-Feria O, Vázquez-Huerta G et al (2011) Effects of architectural changes and inoculum type on internal resistance of a microbial fuel cell designed for the treatment of leachates from the dark hydrogenogenic fermentation of organic solid wastes. Int J Hydrogen Energy 36:6199–6209. doi:10.1016/j.ijhydene.2011.01.006
Pandit S, Balachandar G, Das D (2014) Improved energy recovery from dark fermented cane molasses using microbial fuel cells. Front Chem Sci Eng 8:43–54. doi:10.1007/s11705-014-1403-4
Chookaew T, Prasertsan P, Ren ZJ (2014) Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. N Biotechnol 31:179–184. doi:10.1016/j.nbt.2013.12.004
ElMekawy A, Srikanth S, Vanbroekhoven K et al (2014) Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J Power Sources 262:183–191. doi:10.1016/j.jpowsour.2014.03.111
Kelly PT, He Z (2014) Understanding the application niche of microbial fuel cells in a cheese wastewater treatment process. Bioresour Technol 157:154–160. doi:10.1016/j.biortech.2014.01.085
Tremouli A, Antonopoulou G, Bebelis S, Lyberatos G (2013) Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour Technol 131:380–389. doi:10.1016/j.biortech.2012.12.173
Stamatelatou K, Antonopoulou G, Tremouli A, Lyberatos G (2011) Production of gaseous biofuels and electricity from cheese whey. Ind Eng Chem Res 50:639–644. doi:10.1021/ie1002262
Antonopoulou G, Stamatelatou K, Bebelis S, Lyberatos G (2010) Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem Eng J 50:10–15. doi:10.1016/j.bej.2010.02.008
Kassongo J, Togo C a (2010) The potential of whey in driving microbial fuel cells: a dual prospect of energy recovery and remediation. Afr J Biotechnol 9:7885–7890. doi:10.5897/AJB10.1066
Ghasemi M, Ahmad A, Jafary T, Azad AK (2016) ScienceDirect Assessment of immobilized cell reactor and microbial fuel cell for simultaneous cheese whey treatment and lactic acid/electricity production. Int J Hydrogen Energy:2–10. doi:10.1016/j.ijhydene.2016.04.136
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8:489–494. doi:10.1016/j.elecom.2006.01.010
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662
Rice EW, Baird RB, D, Eaton LA, Clesceri LS (1995) Standard methods for the examination of water and wastewater. 19th ed
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Logan BE, Hamelers B, Rozendal R et al (2006) Microbial fuel cells†¯: methodology and technology†. Environ Sci Technol 40:5181–5192
Caporaso JG, Lauber CL, Walters WA et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108:4516–4522
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high- throughput community sequencing data. Nat Methods 7:335–336. doi:10.1038/nmeth0510-335
Wheeler Alm EW, Oerther DB, Larsen N et al (1996) The oligonucleotide probe database. The Oligonucleotide Probe Database. Appl Environ Microbiol 62:3557–3559
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Saitou N, Nei M (1987) The neighbor-joining method†¯: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1985) Phylogenis and the comparative method. Am Nat 125:1–15
Rabaey K, Boon N, Siciliano SD et al (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382. doi:10.1128/AEM.70.9.5373
Gomes SD, Fuess LT, Mañunga T et al (2016) Bacteriocins of lactic acid bacteria as a hindering factor for biohydrogen production from cassava flour wastewater in a continuous multiple tube reactor. Int J Hydrogen Energy 41:8120–8131. doi:10.1016/j.ijhydene.2015.11.186
Zhang L, Li C, Ding L et al (2011) Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J Chem Technol Biotechnol 86:1226–1232. doi:10.1002/jctb.2641
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. doi:10.1128/AEM.69.3.1548
Sun D, Wang A, Cheng S et al (2014) Geobacter anodireducens sp. nov., an exoelectrogenic microbe in bioelectrochemical systems. Int J Syst Evol Microbiol 64:3485–3491. doi:10.1099/ijs.0.061598-0
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485. doi:10.1126/science.1066771
Ma C, Yu Z, Lu Q, Zhuang L (2015) Anaerobic humus and Fe (III) reduction and electron transport pathway by a novel humus-reducing bacterium, Thauera humireducens SgZ-1. Appl Microbiol Biotechnol 99:3619–3628. doi:10.1007/s00253-014-6254-x
Richter H, Lanthier M, Nevin KP, Lovley DR (2007) Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl Environ Microbiol 73:5347–5353. doi:10.1128/AEM.00804-07
Kimura Z, Chung KM, Itoh H, et al (2014) Raoultella electrica sp. nov., isolated from anodic biofilms of a glucose-fed microbial fuel cell. 1384–1388. doi:10.1099/ijs.0.058826-0
Schleifer K-H (2009) Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes [sic] Gibbons and Murray 1978, 5). Bergey’s Manual® Syst Bacteriol SE-3, vol 5, pp 19–1317. doi:10.1007/978-0-387-68489-5_3
Vilas Boas J, Oliveira VB, Marcon LRC et al (2015) Effect of operating and design parameters on the performance of a microbial fuel cell with Lactobacillus pentosus. Biochem Eng J 104:34–40. doi:10.1016/j.bej.2015.05.009
Cogan TM, Beresford TP, Steele J et al (2007) Invited review†¯: advances in starter cultures and cultured foods. J Dairy Sci 90:4005–4021. doi:10.3168/jds.2006-765
Hania W Ben, Fraj B, Postec A et al (2016) Fusibacter tunisiensis sp. nov., isolated from an anaerobic reactor used to treat olive-mill wastewater. Int J Syst Evol Microbiol 62:1365–1368. doi:10.1099/ijs.0.034603-0
Etchebehere C, Castelló E, Wenzel J et al (2016) Microbial communities from 20 different hydrogen-producing reactors studied by 454 pyrosequencing. Appl Microbiol Biotechnol 100:3371–3384. doi:10.1007/s00253-016-7325-y
Kim N, Choi Y, Jung S, Kim S (2000) Development of microbial fuel cells using Proteus vulgaris. Bull Korean Chem Soc 21:44–48
Rezaei F, Xing D, Wagner R et al (2009) Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl Environ Microbiol 75:3673–3678. doi:10.1128/AEM.02600-08
Feng C, Li J, Qin D et al (2014) Characterization of exoelectrogenic bacteria Enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PLoS One. doi:10.1371/journal.pone.0113379
Xia X, Cao X, Liang P, Huang X (2010) Electricity generation from glucose by a Klebsiella sp. in microbial fuel cells. Appl Microbiol Biotechnol 87:383–390. doi:10.1007/s00253-010-2604-5
Zhang L, Zhou S, Zhuang L et al (2008) Microbial fuel cell based on Klebsiella pneumoniae biofilm. Electrochem Commun 10:1641–1643. doi:10.1016/j.elecom.2008.08.030
Zhang T, Cui C, Chen S et al (2008) The direct electrocatalysis of Escherichia coli through electroactivated excretion in microbial fuel cell. Electrochem commun 10:293–297. doi:10.1016/j.elecom.2007.12.009
Huang J, Zhu N, Cao Y, Peng Y (2015) Exoelectrogenic bacterium phylogenetically related to Citrobacter freundii, isolated from anodic biofilm of a microbial fuel cell. Appl Biochem Biotechnol 175:1879–1891. doi:10.1007/s12010-014-1418-9
Rabaey K, Boon N, Höfte M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39:3401–3408
Xing D, Cheng S, Logan BE, Regan JM (2010) Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl Microbiol Biotechnol 85:1575–1587. doi:10.1007/s00253-009-2240-0
Zuo Y, Xing D, Regan JM, Logan BE (2008) Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell. Appl Environ Microbiol 74:3130–3137. doi:10.1128/AEM.02732-07
Kiely PD, Call DF, Yates MD et al (2010) Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera. Appl Microbiol Biotechnol 88:371–380. doi:10.1007/s00253-010-2757-2
Nishio K, Kimoto Y, Song J, et al (2014) Extracellular electron transfer enhances polyhydroxybutyrate productivity in Ralstonia eutropha. Environ Sci Technol Lett 1:40–43
Acknowledgements
The authors thank Marcela Martínez and Juan A. Abin from the HPLC platform at the Biological Research Institute “Clemente Estable” for chemical analysis of the samples. The authors also thank Dr. Nico Boon from LabMet (University of Gent, Belgium) for providing electroactive biomass and helpful suggestions on MFC operation. This work was funded by project ANII FSE 067 and ANII FSE 6432. J. Wenzel and L. Fuentes were funded ANII grants for postgraduate students in Uruguay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wenzel, J., Fuentes, L., Cabezas, A. et al. Microbial fuel cell coupled to biohydrogen reactor: a feasible technology to increase energy yield from cheese whey. Bioprocess Biosyst Eng 40, 807–819 (2017). https://doi.org/10.1007/s00449-017-1746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1746-6