Abstract
The anode biofilm in a microbial fuel cell (MFC) is composed of diverse populations of bacteria, many of whose capacities for electricity generation are unknown. To identify functional populations in these exoelectrogenic communities, a culture-dependent approach based on dilution to extinction was combined with culture-independent community analysis. We analyzed the diversity and dynamics of microbial communities in single-chamber air-cathode MFCs with different anode surfaces using denaturing gradient gel electrophoresis based on the 16S rRNA gene. Phylogenetic analyses showed that the bacteria enriched in all reactors belonged primarily to five phylogenetic groups: Firmicutes, Actinobacteria, α-Proteobacteria, β-Proteobacteria, and γ-Proteobacteria. Dilution-to-extinction experiments further demonstrated that Comamonas denitrificans and Clostridium aminobutyricum were dominant members of the community. A pure culture isolated from an anode biofilm after dilution to extinction was identified as C. denitrificans DX-4 based on 16S rRNA sequence and physiological and biochemical characterizations. Strain DX-4 was unable to respire using hydrous Fe(III) oxide but produced 35 mW/m2 using acetate as the electron donor in an MFC. Power generation by the facultative C. denitrificans depends on oxygen and MFC configuration, suggesting that a switch of metabolic pathway occurs for extracellular electron transfer by this denitrifying bacterium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) show great promise as a method for energy production during wastewater treatment (Logan and Regan 2006a; Lovley 2008; Rittmann et al. 2008). The power output of these systems is primarily affected by the system architecture, but the microbial ecology can be important as well (Logan and Regan 2006b; Rabaey et al. 2007; Rittmann 2006; Xing et al. 2008b). In recent years, power production by MFCs has increased by several orders of magnitude through system architecture improvements that have reduced the internal resistance (Logan and Regan 2006a), such as modifying the reactor configuration (He et al. 2007; Liu and Logan 2004; You et al. 2008; Zuo et al. 2007), improving electrodes (Fan et al. 2007a; Logan et al. 2007), increasing solution conductivity (Cheng and Logan 2007; Fan et al. 2007b; He et al. 2008; Torres et al. 2008), providing flow through a porous anode, and reducing electrode spacing (Cheng et al. 2006b). As a result of these improvements, the microbial community or the specific microorganisms on the anode are now becoming factors in the level of power production. Therefore, it is important to understand the physiology of the exoelectrogenic bacteria and the ecology of the communities on the electrodes and the interplay between system architecture changes and community composition.
Known exoelectrogens primarily fall into several functional groups based on types of anaerobic respiration (Logan 2009). These include dissimilatory metal-reducing bacteria (DMRB) such as Geobacter (Bond and Lovley 2003), Shewanella (Kim et al. 2002), Geopsychrobacter (Holmes et al. 2004c), and Geothrix (Bond and Lovley 2005); sulfate-reducing bacteria (SRB) including Desulfuromonas (Bond et al. 2002) and Desulfobulbus (Holmes et al. 2004a); and nitrate-reducing bacteria (denitrifying bacteria (DNB)) including Pseudomonas (Rabaey et al. 2004) and Ochrobactrum (Zuo et al. 2008). In addition, fermentative bacteria such as Clostridium (Park et al. 2001) and Escherichia coli produce electricity via anaerobic respiration pathways (Zhang et al. 2006). Purple nonsulfur bacteria, nonphotosynthetic Rhodoferax ferrireducens and photosynthetic Rhodopseudomonas palustris DX-1 were also found to produce electricity via anaerobic respiration in an MFC (Chaudhuri and Lovley 2003; Xing et al. 2008b). The current densities and power produced by these isolates vary due to their physiologies, mechanisms of electron transfer, and different MFC architectures used to study them. Mechanisms for extracellular electron transfer include self-produced mediators (Marsili et al. 2008; Newman and Kolter 2000; von Canstein et al. 2008), direct electron transfer via membrane-bound cytochromes (Esteve-Núñez et al. 2008; Myers and Myers 1992; Shi et al. 2007), and nanowires (Gorby et al. 2006; Reguera et al. 2005).
Community analyses of MFC anode biofilms often show the presence of diverse populations whose exoelectrogenic capabilities are still unknown (Logan and Regan 2006a). These communities can be comprised of SRB, DNB, or fermentative bacteria or may consist primarily of uncharacterized bacteria when MFCs are inoculated with wastewater, activated sludge, or rumen bacteria (Aelterman et al. 2006; Choo et al. 2006; Jong et al. 2006; Kim et al. 2004; Kim et al. 2006; Rabaey et al. 2004; Rismani-Yazdi et al. 2007). SRB appear to dominate microbial communities in MFCs enriched with seawater (Bond et al. 2002; Holmes et al. 2004b; Liu et al. 2007; Reimers et al. 2006; Reimers et al. 2007; Ryckelynck et al. 2005; Tender et al. 2002) or river water (Phung et al. 2004). Few studies have reported the prevalence of Geobacter spp. (Choo et al. 2006; Jung and Regan 2007; Kim et al. 2007b; Lee et al. 2003; Xing et al. 2009) or Shewanella spp. (Logan et al. 2005) in MFCs inoculated with wastewaters despite reports that these DMRB can produce high power densities.
In order to isolate and identify unknown exoelectrogens of the microbial community in MFCs, we used PCR–denaturing gradient gel electrophoresis (DGGE) to monitor the diversity of exoelectrogenic communities over time on ammonia-gas-treated and plain anodes made of either graphite fibers or carbon paper. Through dilution-to-extinction experiments using ammonia-treated anodes and a mixture of bacteria from all the anodes, we reduced the diversity of the exoelectrogenic community and isolated an exoelectrogenic bacterium Comamonas denitrificans that was a predominant member of the community.
Materials and methods
MFC construction
Anodes were carbon paper (25 cm2, non-wet-proofed, E-TEK) or a graphite fiber brush 5 cm in diameter and 7 cm in length (PANEX33 160 K, ZOLTEK; Logan et al. 2007). For two of the MFCs, a carbon paper or brush anode was treated using ammonia gas as previously described (Cheng and Logan 2007). Cathodes contained 0.5 mg/cm2 Pt and four PTFE diffusion layers on 30 wt.% wet-proofed carbon cloth (type B-1B, E-TEK; Cheng et al. 2006a). Single-chamber bottle MFCs were made from common laboratory media bottles (320 mL capacity, Corning Inc., NY, USA) as previously described (Logan et al. 2007). A 4-cm-long side tube was set 5 cm from the reactor bottom, with a 3.8-cm-diameter cathode held in place at the end by a clamp between the tube and a separate 4-cm-long tube, providing a total projected cathode surface area of 4.9 cm2 (one side of the cathode). The liquid volume of the chamber was 300 mL. Single-chamber cubic MFCs (4 cm in width) and two-chamber cubic MFCs assembled by joining two cubic MFCs (each 2 cm in width) separated by a cation exchange membrane (CMI 7000, Membranes International Inc, USA) were used to test power generation by pure cultures. For the cubic MFCs, ammonia-treated carbon papers (7 cm2) were used as the anodes and cathodes, and ferricyanide (50 mM K3Fe(CN)6 in 50 mM phosphate buffer solution [PBS]) was used as the catholyte in the two-chamber reactor. All MFCs were autoclaved before use.
MFC operation
The wastewater inoculum was collected from the primary clarifier of the Pennsylvania State University Wastewater Treatment Plant. Four MFCs with different anodes were fed a medium containing 1 g/L of acetate in 50 mM or 200 mM PBS, NH4Cl (0.31 g/L), KCl (0.13 g/L), and metal salt (12.5 mL/L) and vitamin (5 mL/L) solutions (Logan et al. 2007). It is well established that power production is increased with solution conductivity over certain ranges (Liu et al. 2005). We have determined that MFCs do not perform well if started at 200 mM PBS (unpublished results). However, if they are initiated at 50 mM PBS and then switched to 200 mM PBS, power generation is stable and increased compared to the 50 mM PBS (Cheng and Logan 2007). Therefore, we switched from 50 to 200 mM PBS after the first fed-batch cycle and used 200 mM PBS thereafter. Solutions were replaced when the voltage dropped to <50 mV. Open-circuit MFCs with the four different anodes also were operated at the same conditions and then switched to closed-circuit operation (1,000 Ω external resistance) after 85 days of operation for one cycle. C. denitrificans 110 (ATCC 700937) obtained from the American Type Culture Collection and an isolated strain were cultured using nutrient broth (BD 234000) before inoculation into MFCs using the defined medium. All media were autoclaved before use. MFCs were operated in a temperature-controlled room at 30 °C. MFCs containing only sterile medium (without any cells) were used as uninoculated controls.
Dilution to extinction and isolation of dominant exoelectrogen
Dilution to extinction of microbial communities was used to isolate dominant populations in MFCs. A piece of anode (1 cm2 for carbon paper or 0.5 g wet weight for brush anode) was cut and crushed from four MFCs at day 85 and transferred to a common anaerobic tube containing 10 mL of 50 mM PBS solution and glass beads. A cell suspension of ∼5 × 108 cell/mL was obtained by vortexing for 5 min and was serially diluted in 10-fold steps to 10−8 in anaerobic tubes. Samples (5 mL) from the 10−5 to 10−8 dilutions were then transferred to sterile bottle MFCs with ammonia-treated carbon paper anodes. Nutrient medium with 50 mM PBS and operation of MFCs were the same as described above. The anode from the MFC containing the highest dilution that produced electricity was used for the next cycle of dilution to extinction described above (Zuo et al. 2008). Three cycles of dilution to extinction were performed. MFCs containing only sterile medium (without any cells) were used as uninoculated controls.
Community composition and predominant bacteria of anode biofilms were assessed by DGGE after the microbial community diversity was reduced by dilution to extinction. Cells were collected from the anodes and diluted as described above. Agar plates including 3 g/L yeast extract and 5 g/L peptone were used for isolating predominant bacteria from the anode biofilms. Single colonies were transferred to nutrient broth (BD 234000).
Physiological and biochemical characterization
Physiological and biochemical tests of the isolate retrieved through dilution to extinction of anode biofilms were conducted by conventional methods and using Biolog GN2 MicroPlates for examining substrate utilization (Biolog, Inc., Hayward, CA, USA). The denitrification activity of the isolate was determined using Griess reagents (sulfanilamide and N-(1-naphthyl)-ethylene-diamine-dihydrochloride) in anaerobic tubes containing 10 mM nitrate and nutrient broth (BD 234000) or 1 g/L acetate at 30 °C. The ability of cells to respire using hydrous ferric oxide (HFO; 100 mM) was determined using 1 g/L acetate in anaerobic tubes as previously described (Zuo et al. 2008). All tests were performed in duplicate.
Analyses
Internal resistance (R int) was measured using electrochemical impedance spectroscopy with a potentiostat (PC 4/750, Gamry Instrument Inc., PA, USA), with the anode chamber filled with 200 mM PBS and substrate. Cell voltages (V) were recorded across a fixed external resistance (1,000 Ω except as noted) using a multimeter with a data acquisition system Model 2700 (Keithley Instruments, Cleveland, OH, USA). Current (I), power (P = IV), and Coulombic efficiency (CE) were calculated as previously described (Cheng et al. 2006c; Kim et al. 2007a) and normalized by the projected surface area of one side of the cathode.
DNA extraction and PCR amplification
Carbon paper (2 cm2) and brush anodes (1 g, wet weight) were cut from MFCs and fragmented by sterile razors. Genomic DNA was extracted using the PowerSoil™ DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. A fragment of the 16S rRNA gene was amplified by PCR using a pair of universal primers: 968F 5′-AACGCGAAGAACCTTAC-3´ (E. coli 16S rRNA positions 968 to 984) to which a GC clamp was attached (CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCG) at the 5′-terminus, and 1401R, 5′-CGGTGTGTACAAGACCC -3′ (E. coli 16S rRNA gene positions 1385 to 1401; Ren et al. 2007). For 16S rRNA gene amplification of the isolate, a pair of universal primers was used as follows: 8F 5′-AGAGTTTGATCCTGGCTCAG-3′ (E. coli 16S rRNA gene positions 8 to 27) and 1541R 5′-AAGGAGGTGATCCAGCC-3′ (E. coli 16S rRNA gene positions 1525 to 1541; Xing et al. 2008b). PCR amplification was carried out in 50-μL volumes containing 25 μL of 2× GoTaq® Green Master Mix (Promega, Madison, WI, USA), 1 µM of each primer, and 10 ng DNA template. The samples were amplified in an iCycler iQ™ (Bio-Rad Laboratories, Hercules, CA, USA) with an initial denaturation of DNA for 5 min at 94 °C, followed by 30 cycles of 1 min at 94 °C, 30 s at 57 °C (decreasing 0.1 °C per cycle to 54 °C), and 1 min at 72 °C, and then a final extension for 7 min at 72 °C. PCR products were examined by electrophoresis on a 1% (wt/vol) agarose gel containing SYBR Safe™ (Invitrogen, Carlsbad, CA, USA). Blank controls were carried out through all steps.
DGGE analysis
DGGE was performed with a DCode universal mutation detection system (Bio-Rad Laboratories). Approximately 1 µg of PCR products per lane was loaded onto 7% (wt/vol) polyacrylamide (37.5:1 acrylamide/bisacrylamide) gels in a 1× TAE buffer with a denaturing gradient ranging from 30% to 60%. Denaturation of 100% corresponds to 7 M urea and 40% (vol/vol) deionized formamide. The gradient gel was cast with a gradient delivery system (Model 475, Bio-Rad, USA). Electrophoresis was run for 10 min at 30 V and 13 h at 75 V in 1× TAE buffer maintained at 60 °C. Gels were silver stained as previously described (Bassam et al. 1991).
Prominent DGGE bands were excised for sequencing. The gel was crushed in 50 µL TE buffer (10 mM Tris–HCl, 1 mM EDTA [pH 8.0]) and allowed to equilibrate overnight at 4 °C. Then 1 µL of buffer containing DNA was used as the template for a PCR performed under the conditions described above, except that the forward primer lacked the GC clamp. PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA), ligated into vector pCR2.1 using a TOPO TA cloning kit (Invitrogen), and cloned into chemically competent One Shot E. coli cells provided with the cloning kit, as recommended by the manufacturer’s instructions. Plasmids were isolated with the QIAprep Spin Miniprep Kit (QIAGEN) from five randomly selected clone colonies for each band. The plasmid inserts were re-amplified with the GC clamp, and 5 μL of re-amplification product from each clone was subjected to DGGE analysis as described above to check the purity and to confirm the melting behavior of the recovered band. If the bands from the clones were identical with the DGGE parent bands, the inserts were sequenced (ABI 3730XL DNA sequencer, Applied Biosystems, Foster, CA, USA) to determine whether multiple 16S rRNA fragments had co-migrating on the DGGE band (Xing et al. 2008a).
Sequencing and phylogenetic analysis
16S rRNA gene sequences were analyzed in the GenBank database and Ribosomal Database Project II (RDP II, http://rdp.cme.msu.edu). All sequences were examined for chimerism using the CHECK_CHIMERA program at RDP II and BELLEROPHON (http://foo.maths.uq.edu.au/∼huber/bellerophon.pl). A neighbor-joining phylogenetic tree was constructed with the Molecular Evolutionary Genetics Analysis package (MEGA version 4.0) using the Jukes–Cantor algorithm (Tamura et al. 2007). A bootstrap analysis with 1,000 replicates was carried out to assess the tree.
Statistical methods
Statistica 6.0 (StatSoft) was used for principal component analysis (PCA) of the DGGE profiles as previously described (Ren et al. 2007). The DGGE profiles were manually recorded, and a binary matrix was made based on the presence (1) or absence (0) of the bands. The binary data representing the banding patterns were used for PCA. Two-dimensional PCA graphs were drawn to visualize and interpret relative spatial and temporal changes in the microbial community structure among biofilms on the surfaces of different anodes.
Nucleotide sequence accession numbers
The 16S rRNA gene sequences determined in this study have been deposited in the GenBank database under accession numbers EU272905–EU272935.
Results
Power production using different anodes
Power production using the four different anodes (ammonia-treated brush, ammonia-treated carbon paper, untreated brush, and untreated carbon paper) was rapidly achieved during the start-up and acclimation of the reactors (50 mM PBS, 1,000 Ω). Ammonia treatment of the brush anode reduced the acclimation time and increased power production relative to the untreated brush electrode, consistent with previous reports (Cheng and Logan 2007). In contrast, the ammonia pretreatment had little effect on acclimation time for carbon paper anodes. Voltage production with the ammonia-treated brush anode increased between days 2 and 3, approximately 2 days before increases using the untreated brush anode (Fig. 1a). The voltage reached ∼525 mV for the ammonia-treated brush anode by day 3 compared to ∼488 mV for the untreated brush anode by day 6. A slightly higher voltage was also initially produced with the ammonia-treated carbon paper (∼507 mV) than with the untreated paper (∼480 mV; Fig. 1a).
Cell voltages of four MFCs with different anodes. Filled diamonds ammonia-treated brush, empty diamonds untreated brush, filled circles ammonia-treated carbon paper, empty circles untreated carbon paper. Voltage during first 8 days of reactor operation (a) and from day 9 to 100 (b; 1,000 Ω resistor, 1 g/L acetate, 50 mM PBS in first batch and 200 mM PBS thereafter). Dashed lines indicate anode biofilm sampling times (at days 2, 3, 4, 7, 40, 60, and 85)
The solution was replaced after the first cycle, and a 200 mM PBS was used in all subsequent experiments to increase power production (see “Materials and methods”). Voltages reached 520 to 570 mV for all reactors when they were all examined at the same external resistance (1,000 Ω). This voltage was stable for the remainder of the experiments conducted over a total of 100 days (Fig. 1b). Polarization data (obtained on day 85) showed that these systems had substantially different capacities for power generation (Fig. 2). The ammonia-treated brush anode MFC produced a maximum power density of \( {\text{1,76}}0 \pm {\text{7}}0\;{\text{mW}}/{{\text{m}}^{\text{2}}}\;\left( {{\text{15}}0\;\Omega } \right) \) versus 1,390 ± 70 mW/m2 for the untreated brush anode. The carbon paper electrodes produced less power (due to less anode surface area), with \( {\text{1,41}}0 \pm {\text{4}}0\,{\text{mW}}/{{\text{m}}^{\text{2}}}\;\left( {{\text{15}}0\;\Omega } \right) \) for the ammonia-treated paper and \( {\text{1,}}090 \pm 40\;{\text{Mw}}/{{\text{M}}^{\text{2}}}\;\left( {{\text{2}}00\;\Omega } \right) \) for the untreated paper. These maximum power densities are somewhat higher than those previously observed (Logan et al. 2007) likely due to the longer operational time here which allowed for community development to affect power production (Aelterman et al. 2006). In previous tests (conducted over a non-specified period), only 1,430 mW/m2 was achieved with the ammonia-treated brush anode. The CE showed a similar trend as power density with the type of materials, with a maximum CE (37%, 1,000 Ω) for the ammonia-treated brush anode, intermediate values for the ammonia-treated carbon paper (33%) and the untreated brush anode (33%), and the lowest CE (30%) for the untreated carbon paper anode. The improved performance of the ammonia-treated brush anode was reflected by a lower internal resistance (R int) of 40 Ω versus R int = 48 Ω for the untreated brush anode and 57 Ω for the ammonia-treated or untreated carbon paper anode. After 85 days of operation, the open-circuit controls were switched to closed-circuit operation (1,000 Ω external resistance). In the next cycle of operation, they had CEs of 18–20%, which was less than that obtained for the other reactors that were continuously operated under closed-circuit conditions.
Polarization curves of four MFCs with different anodes fed 1 g/L sodium acetate (200 mM PBS) on day 85. Filled diamonds ammonia-treated brush, empty diamonds untreated brush, filled circles ammonia-treated carbon paper, empty circles untreated carbon paper. Error bars are ±SD based on triplicate measurements
Microbial community succession and diversity in MFCs with different anodes
DGGE profiles of 16S rRNA gene fragments (V6-V8 regions) showed that the microbial community structures on the anodes changed over time (Fig. 3). On day 2, when voltage production began to gradually increase (Fig. 1a), prominent changes in banding patterns were observed relative to the inoculum. In the first 7 days, the microbial communities on the surfaces of different anodes changed quickly, especially for the brush anodes. Comparison of the microbial communities over days 7 to 40 showed that the predominant bands continued to change over time. After 40 days, the numbers and densities of bands within the DGGE profiles remained relatively constant.
DGGE profiles of 16S rRNA gene (V6–V8 region) derived from the MFC anode biofilms. Lanes are labeled with sampling time for each MFC. For open-circuit controls, NH3, ammonia-treated anodes; CP carbon paper anodes, B brush anodes. Arrowheads indicate the DGGE bands selected for cloning and sequencing. The bands from different gels which have identical sequences were labeled by the same number
PCA of DGGE band patterns (Fig. 4) indicated clear differences in community structures on the surface of different anodes between the inoculum and the early days of MFC operation compared to those obtained after extended operation at a higher salinity. For the brush-anode reactors, the community structure gradually evolved over the first 40 days of operation, with little change evident over the last 40 to 85 days. In contrast, the microbial communities on the carbon-paper anodes were relatively stable initially, but there were large changes between days 7 and 40. PCA of the four different electrodes after 40 days showed that the communities were stable but different on the electrodes. There were relatively large differences as a result of ammonia treatment for the brush electrodes, while the carbon paper electrodes showed less of an effect of ammonia treatment (Fig 4). Differences in community structures on the surface of different anodes were observed between these MFCs and open-circuit control reactors at the end of the tests (Fig. 4).
To better define the communities, 23 prominent DGGE bands (Fig. 3) were excised and sequenced. The sequences of these bands fell into 31 operational taxonomic units (OTUs) based on 99% minimum similarity as the threshold (Eckburg et al. 2005), with seven bands comprised of 16S rRNA gene fragments from two to three OTUs. The phylogenetic distributions of these OTUs could be divided into five groups (Fig. 5), consisting of Firmicutes (low G+C gram-positive bacteria), Actinobacteria (high G+C gram-positive bacteria), α-Proteobacteria, β-Proteobacteria, and γ-Proteobacteria. The majority of OTUs belonged to Firmicutes (58.1%) and β-Proteobacteria (25.8%). Only 16 OTUs (51.6%) had a sequence similarity >97% with known strains in the GenBank database and Ribosomal Database Project II. Samples from the open-circuit control reactors had some DGGE bands and sequences in common with the closed-circuit systems (Figs. 3 and 5), but the exoelectrogenic capabilities of the bacteria from which these bands were derived cannot be inferred merely from these phylogenetic identifications.
Neighbor-joining dendrogram derived from 16S rRNA gene sequences (V6–V8 region) of predominant bands in DGGE gels. Bootstrap confidence levels greater than 50% are indicated at the nodes (replicate 1,000 times). Numbers in parentheses represent the sequence accession numbers in the GenBank database. Bar indicates 2% divergence. For each tree entry from this study, the number ahead of the hyphen represents the band excised from DGGE gels, and the number behind the hyphen represents the clone from that band. The asterisks indicate sequences that were also retrieved from open-circuit control reactors
These populations inferred from pronounced DGGE bands were classified according to the electron-acceptor versatility reported in the literature of their closest cultivated relatives in the GenBank database (Supplementary Table 1). The electron-acceptor alternatives except for metabolic intermediates (volatile fatty acids) were noted because the substrate (acetate) was not fermentable. Four OTUs (bands 1, 3, 5, and 6) were affiliated with Clostridium sp. capable of iron reduction. Two OTUs (bands 8 and 10) were affiliated with arsenic- or iron-reducing Alkaliphilus. Eight OTUs (bands 15, 16, 17, 18, 19, 20, 21, and 23) were affiliated with denitrifying Pseudomonas, Comamonas, Corynebacterium, Acidovorax, Alcaligenes, and Azospira. One OTU (band 17) was affiliated with Rhodopseudomonas with iron- or sulfate-reduction capabilities. Two OTUs (bands 11 and 13) were related to Clostridium aminobutyricum, which is capable of nitrate reduction. The iron-, sulfate-, and nitrate-reducing capabilities of closest relatives to 14 OTUs (bands 2, 4, 6, 7, 9, 12, 14, 20, 21, 22, and 23) were unknown. Model DMRB such as Geobacter spp. and Shewanella spp. were not found among the OTUs. The OTUs that were common to all MFCs included band 1 for the entire experiment and band 6 after 40 days (Fig. 3), both of which were affiliated with iron-reducing Clostridium sp. The DGGE profiles and PCA showed that all MFCs reached stable performance and community structure after 40 days (Figs. 3 and 4).
Dilution to extinction of exoelectrogenic community
In order to isolate a dominant exoelectrogen from the anode communities, the biofilms on different anodes from four separate MFCs were mixed together, serially diluted to different final concentrations, and re-inoculated into four sterile MFCs each with a different dilution (10−5, 10−6, 10−7, and 10−8). The community on the carbon paper anodes had stabilized more quickly than on the brush anodes (Figs. 3 and 4), so an ammonia-treated carbon paper anode was used for dilution to extinction with several cycles. Figure 6 shows voltage production (1,000 Ω, 50 mM PBS) from three cycles of dilution to extinction. In the first dilution-to-extinction cycle, voltage production of the highest dilution (10−8) produced a much lower voltage (200 mV after 10 days) than the other samples. The biofilm from the anode of the next most dilute sample that produced a high voltage (10−7 dilution, 450–460 mV) was used for the next dilution to extinction test. In this second cycle, the same voltage as obtained in the first cycle was again produced by all reactors except for the 10−8 dilution (only the 10−7 dilution for the second cycle is shown in Fig. 6). However, when the biofilm from this sample was used in a third dilution to extinction test, the maximum voltage produced by the 10−7 diluted was only 340 mV. This suggests that essential members of the community were lost or altered during this last dilution and transfer experiment.
Cell voltages of MFCs inoculated with diluted exoelectrogenic communities (1,000 Ω resistor, 1 g/L acetate, 50 mM PBS). MFCs of the first dilution series (triangles 10−5, inverted triangles 10−6, squares 10−7, empty circles 10−8) were stopped on day 10, and the anode of the 10−7 MFC was used for the second dilution. The 10−7 MFC of the second dilution (diamonds) was sampled on day 20 for the third dilution and then continued to operate through an additional cycle. The 10−7 MFC of the third dilution (filled circles) was sampled on day 40. The arrows represent sample points for the next dilution and community analyses of the anodes
DGGE profiles show that the diversity of the microbial community decreased over the three cycles of dilution to extinction experiments relative to the initial inoculum (Fig. 7). Sequencing of four prominent bands from the communities in the diluted reactors showed four bands (5, 8, 13, and 18) that were also present in the DGGE profiles of the original reactors (Fig. 3). Bands 13 (C. aminobutyricum) and 18 (C. denitrificans) were predominant in the first two cycles, but band 13 disappeared in the third cycle. When bands 5 and 8 appeared in the third cycle, voltage production decreased. These results suggest that populations associated with the two denitrifying bacteria C. aminobutyricum and C. denitrificans (bands 13 and 18) were putative exoelectrogenic bacteria, but that Clostridium sp. and an uncultured bacterium from bands 5 and 8 were not.
DGGE profiles of 16S rRNA gene (V6–V8 region) from the anode biofilms of dilution-to-extinction tests. Lanes are labeled with the dilution cycle. Arrowheads indicate the DGGE bands selected for cloning and sequencing. (Numbering is consistent with corresponding bands in Fig. 3)
Power generation by C. denitrificans
A strain designated as DX-4 was isolated from the anode biofilm of the third dilution to extinction test. Strain DX-4 is a gram-negative, facultative anaerobic, rod-shaped bacterium that is motile with polar flagella and forms a yellow-white colony on nutrient agar plates. Single cells or filaments appeared when grown on nutrient agar plates. Phylogenetic analysis of almost the full-length 16S rDNA gene (1,523 bp) revealed that strain DX-4 was most closely related to C. denitrificans 110 (100% identity). Biochemical tests of C. denitrificans DX-4 showed identical carbon source utilization as strain 110 except that only strain 110 used citrate and only DX-4 used l-ornithine and l-arabinose (Table 1). Strain DX-4 also was capable of denitrification, and it did not show Fe (III) reduction using insoluble iron (HFO) with lactate, acetate, and nutrient broth (yeast extract plus peptone) as carbon sources.
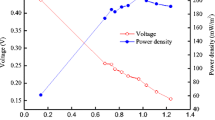
To test the hypothesis that C. denitrificans contributed to power production, two pure cultures of C. denitrificans (strains 110 and DX-4) were inoculated into two-chamber MFCs using ferricyanide as a catholyte and single-chamber MFCs using an air cathode. In the two-chamber reactors, both strains produced a stable voltage of ∼140 mV (1,000 Ω, 50 mM PBS). The maximum power densities produced by the two strains were similar, with 36 ± 0.8 mW/m2 for strain DX-4 and 35 ± 1.2 mW/m2 for strain 110 (Fig. 8). However, both strains did not produce any power in the air-cathode MFCs even though they grew well, presumably due to aerobic respiration metabolism on the surface of the air cathode. The sensitivity of these strains’ exoelectrogenic activity to oxygen would not have been a problem in the two-chamber tests where ferricyanide was used or in a mixed-culture reactor where other microbes could scavenge oxygen leaking in the reactor. Nitrate reduction tests showed that strains 110 and DX-4 of C. denitrificans actively denitrified in open-circuit two-chamber MFCs, but not in open-circuit single-chamber air-cathode MFCs. Power output decreased when nitrate was added into closed-circuit two-chamber MFCs, showing that the change in metabolic pathway affected the power output of C. denitrificans.
Polarization curves of MFCs with carbon paper anodes fed 1 g/L sodium acetate (50 mM PBS). Power density and current density normalized to the anode area are obtained by varying the external circuit resistance (75–3,000 Ω). Filled circles C. denitrificans DX-4, empty circles C. denitrificans 110. Error bars are ±SD based on triplicate measurements
Discussion
MFCs with different anodes showed substantial differences in power production based on polarization data, as well as changes in the composition of the microbial communities. Differences in power output resulted from ammonia gas treatment, but the mechanism by which this treatment improves power has not been clearly established. Increased power did not result from a change in internal resistance, as the two MFCs with carbon paper had the same internal resistance. Instead, power changed due to the surface properties as well as the dominant bacteria. The gas treatment process makes the electrode more positively charged, and thus changes the initial adsorption of exoelectrogens and reduces acclimation time (Cheng and Logan 2007). However, this initial advantage in the rate of colonization would not contribute to improved power output over the long term. Direct cell counts using acridine orange staining did not show an obvious difference in total cell density between ammonia-treated or plain anodes when anode biofilms were well established (85 days; data not shown). However, we cannot assess the fraction of exoelectrogens with this assay. It is clear that gas treatment resulted in differences in the microbial community over time. Thus it appears that the main reason for the increased power was due to the different exoelectrogens that developed on the electrode (Fig. 3) and their specific interactions with the electrode. This effect of the different exoelectrogens on power generation is supported by findings that show pure cultures can produce more (or less) power than mixed cultures, depending on the strain (Nevin et al. 2008; Rabaey et al. 2004; Xing et al. 2008b). Whether increased power results here from reduced contact resistance between bacteria and the surface or it results from favoring the growth of specific exoelectrogens on the electrode cannot be fully resolved at this time. It is only clear that by changing the character of the anode surface, power increased and different bacterial communities evolved over time on these different surfaces.
The approach used here to study changes in microbial communities over time (the use of fixed resistors during inoculation and operation) was chosen to reflect conditions used in most studies of power generation in MFCs (Aelterman et al. 2006; Jong et al. 2006; Rabaey et al. 2004). As a result of this operation mode using a fixed resistance, the current and voltage can vary during the course of the study. However, the voltage observed here from the four reactors varied over a relatively small range (520 to 570 mV). An alternative approach that has been used in some studies is to operate the reactors under identical potentiostatic or galvanostatic modes. The effects of these alternative operational approaches on community development would be interesting to examine in a future study.
All previous community analyses have been based on two-chamber MFCs, where it has been found that the populations primarily belonged to Proteobacteria and Firmicutes. Our results also indicated that the majority of OTUs in these air-cathode MFCs belonged to gram-positive bacteria (Firmicutes and Actinobacteria) and Proteobacteria. Other studies have shown exoelectrogenic capabilities of gram-positive bacteria such as Clostridium spp. (Park et al. 2001; Scala et al. 2006), Propionicimonas sp. (Kim et al. 2006), Enterococcus spp. (Kim et al. 2005; Rabaey et al. 2004), and Desulfitobacterium spp. (Milliken and May 2007). The prevailing theory is that electron mediators are used by gram-positive bacteria for exocellular electron transfer. Therefore, an interaction between these electron shuttles produced by gram-positive and other exoelectrogenic bacteria may exist in the microbial community of MFCs. The role of gram-positive bacteria on power generation in mixed communities needs further investigation.
Several studies have observed a predominance of Geobacter spp. in anodic communities (Choo et al. 2006; Jung and Regan 2007; Kim et al. 2007b), but the contribution of DMRB in MFCs is certainly not universal. DMRB were found to be absent or poorly represented in a number of studies that instead showed communities dominated by SRB, DNB, fermentative bacteria, and uncultured bacteria in the anode biofilms (Aelterman et al. 2006; Jong et al. 2006; Kim et al. 2004; Kim et al. 2006; Rabaey et al. 2004; Rismani-Yazdi et al. 2007). Our results show community members that are phylogenetically related to known DMRB, as well as the predominance of putative exoelectrogenic bacteria phylogenetically related to the known denitrifiers C. denitrificans and C. aminobutyricum. In addition, we demonstrated for the first time exoelectrogenic activity by C. denitrificans using a pure culture. However, Comamonas species have been identified as present in the suspended consortium of an MFC fed with cellulose (Rismani-Yazdi et al. 2007). Our finding of the importance of denitrifying bacteria in some MFCs is consistent with several other studies showing power generation by denitrifying bacteria such as Pseudomonas aeruginosa and Ochrobactrum anthropi (Rabaey et al. 2004; Zuo et al. 2008). Thus, denitrifying bacteria may have an important role in power production in exoelectrogenic communities lacking DMRB. The maximum power density of the Comamonas isolates (35–36 mW/m2) was considerably less than the mixed-culture systems from which they were recovered (1,410 mW/m2 for ammonia-treated carbon paper). While the pure- and mixed-culture experiments were conducted using different reactor configurations (precluding a direct comparison of these numbers), it was clear that the Comamonas pure-culture reactors underperformed compared to mixed cultures. The contribution of these strains to power production within the mixed communities remains unknown, given the potential for synergistic community interactions and that the Comamonas abundance was not determined in either configuration.
Almost all MFC community analyses have reported abundant 16S rRNA gene sequences from previously undescribed bacteria whose exoelectrogenic capabilities are unknown, without providing direct evidence of which members of the population were exoelectrogens. Only a portion of bacteria from the anode biofilm likely contribute to power generation, and this makes it difficult to assess the role of the as-yet-unknown community members due to a deficiency of known exoelectrogenic isolates. Moreover, functional inferences based on 16S rRNA gene comparative analysis are prone to misinterpretation, so we could not make a definitive comparison of exoelectrogens from the different community analyses. Our results showed that community analysis complemented with dilution to extinction was a powerful approach to reveal uncultured exoelectrogenic bacteria. This method effectively screened exoelectrogenic populations by decreasing community diversity via serial dilutions. Zuo et al. (2008) also demonstrated that dilution to extinction coupled with community analysis was a useful method to isolate a previously unknown exoelectrogen.
References
Aelterman P, Rabaey K, Pham HT, Boon N, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40:3388–3394
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555
Bond DR, Lovley DR (2005) Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol 71:2186–2189
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21:1229–1232
Cheng S, Logan BE (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 9:492–496
Cheng S, Liu H, Logan BE (2006a) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8:489–494
Cheng S, Liu H, Logan BE (2006b) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Cheng S, Liu H, Logan BE (2006c) Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 40:364–369
Choo YF, Lee J, Chang IS, Kim BH (2006) Bacterial communities in microbial fuel cells enriched with high concentrations of glucose and glutamate. J Microbiol Biotechnol 16:1481–1484
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M et al (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638
Esteve-Núñez A, Sosnik J, Visconti P, Lovley DR (2008) Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ Microbiol 10:497–505
Fan Y, Hu H, Liu H (2007a) Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J Power Sources 171:348–354
Fan Y, Hu H, Liu H (2007b) Sustainable power generation in microbial fuel cells using bicarbonate buffer and proton transfer mechanisms. Environ Sci Technol 41:8154–8158
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A et al (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA 103:11358–11363
He Z, Shao HB, Angenent LT (2007) Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens Bioelectron 22:3252–3255
He Z, Huang Y, Manohar AK, Mansfeld F (2008) Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochem 74:78–82
Holmes DE, Bond DR, Lovley DR (2004a) Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl Environ Microbiol 70:1234–1237
Holmes DE, Bond DR, O'Neil RA, Reimers CE, Tender LR, Lovley DR (2004b) Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb Ecol 48:178–190
Holmes DE, Nicoll JS, Bond DR, Lovley DR (2004c) Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl Environ Microbiol 70:6023–6030
Jong BC, Kim BH, Chang IS, Liew PWY, Choo YF, Kang GS (2006) Enrichment, performance, and microbial diversity of a thermophilic mediatorless microbial fuel cell. Environ Sci Technol 40:6449–6454
Jung S, Regan JM (2007) Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl Microbiol Biotechnol 77:393–402
Kim HJ, Park HS, Hyun MS, Chang IS, Kim M, Kim BH (2002) A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb Technol 30:145–152
Kim BH, Park HS, Kj K, Kim GT, Chang IS, Lee J et al (2004) Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl Microbiol Biotechnol 63:672–681
Kim GT, Hyun MS, Chang IS, Kim HJ, Park HS, Kim BH et al (2005) Dissimilatory Fe(III) reduction by an electrochemically active lactic acid bacterium phylogenetically related to Enterococcus gallinarum isolated from submerged soil. J Appl Microbiol 99:978–987
Kim GT, Webster G, Wimpenny JWT, Kim BH, Kim HJ, Weightman AJ (2006) Bacterial community structure, compartmentalization and activity in a microbial fuel cell. J Appl Microbiol 101:698–710
Kim JR, Cheng S, Oh SE, Logan BE (2007a) Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ Sci Technol 41:1004–1009
Kim JR, Jung SH, Regan JM, Logan BE (2007b) Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresource Technol 98:2568–2577
Lee J, Phung NT, Chang IS, Kim BH, Sung HC (2003) Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol Lett 223:185–191
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Liu H, Cheng S, Logan BE (2005) Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ Sci Technol 39:5488–5493
Liu JL, Lowy DA, Baumann RG, Tender LM (2007) Influence of anode pretreatment on its microbial colonization. J Appl Microbiol 102:177–183
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381
Logan BE, Regan JM (2006a) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14:512–518
Logan BE, Regan JM (2006b) Microbial fuel cells—challenges and applications. Environ Sci Technol 40:5172–5180
Logan BE, Murano C, Scott K, Gray ND, Head IM (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39:942–952
Logan BE, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 41:3341–3346
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19:564–571
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA 105:3968–3973
Milliken CE, May HD (2007) Sustained generation of electricity by the spore-forming, Gram-positive, Desulfitobacterium hafniense strain DCB2. Appl Microbiol Biotechnol 73:1180–1189
Myers CR, Myers JM (1992) Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bateriol 174:3429–3438
Nevin KP, Richter H, Covalla SF, Johnson JP, Woodard TL, Orloff AL et al (2008) Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ Microbiol 10:2505–2514
Newman DK, Kolter R (2000) A role for excreted quinones in extracellular electron transfer. Nature 405:94–97
Park HS, Kim BH, Kim HS, Kim HJ, Kim GT, Kim M et al (2001) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297–306
Phung NT, Lee J, Kang KH, Chang IS, Gadd GM, Kim BH (2004) Analysis of microbial diversity in oligotrophic microbial fuel cells using 16S rDNA sequences. FEMS Microbiol Lett 233:77–82
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382
Rabaey K, Rodriguez J, Blackall LL, Keller J, Gross P, Batstone D et al (2007) Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 1:9–18
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Reimers CE, Girguis P, Stecher HA, Tender LM, Ryckelynck N, Whaling P (2006) Microbial fuel cell energy from an ocan cold seep. Geobiology 4:123–136
Reimers CE, Stecher HA, Westall JC, Alleau Y, Howell KA, Soule L et al (2007) Substrate degradation kinetics, microbial diversity, and current efficiency of microbial fuel cells supplied with marine plankton. Appl Environ Microbiol 73:7029–7040
Ren N, Xing D, Rittmann BE, Zhao L, Xie T, Zhao X (2007) Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ Microbiol 9:1112–1125
Rismani-Yazdi H, Christy AD, Dehority BA, Morrison M, Yu Z, Tuovinen OH (2007) Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol Bioeng 97:1398–1407
Rittmann BE (2006) Microbial ecology to manage processes in environmental biotechnology. Trends Biotechnol 24:261–266
Rittmann BE, Krajmalnik-Brown R, Halden RU (2008) Pre-genomic, genomic and postgenomic study of microbial communities involved in bioenergy. Nat Rev Microbiol 6:604–612
Ryckelynck N, Stecher HAI, Reimers CE (2005) Understanding the anodic mechanism of a seafloor fuel cell: interactions between geochemistry and microbial ability. Biogeochemistry 76:113–139
Scala DJ, Hacherl EL, Cowan R, Young LY, Kosson DS (2006) Characterization of Fe(III)-reducing enrichment cultures and isolation of Fe(III)-reducing bacteria from the Savannah River site, South Carolina. Res Microbiol 157:772–783
Shi L, Squier TC, Zachara JM, Fredrickson JK (2007) Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol 65:12–20
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR, Lowy DA et al (2002) Harnessing microbially generated power on the seafloor. Nat Biotechnol 20:821–825
Torres CI, Lee HS, Rittmann BE (2008) Carbonate species as OH− carriers for decreasing the pH gradient between cathode and anode in biological fuel cells. Environ Sci Technol 42:8773–8777
von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Xing D, Ren N, Rittmann BE (2008a) Genetic diversity of hydrogen-producing bacteria in an acidophilic ethanol-H2-coproducing system, analyzed using the [Fe]-hydrogenase gene. Appl Environ Microbiol 74:1232–1239
Xing D, Zuo Y, Cheng S, Regan JM, Logan BE (2008b) Electricity generation by Rhodopseudomonas palustris DX-1. Environ Sci Technol 42:4146–4151
Xing D, Cheng S, Regan JM, Logan BE (2009) Change in microbial communities in acetate- and glucose-fed microbial fuel cells in the presence of light. Biosen Bioelectron 25:105–111
You S, Zhao Q, Zhang J, Liu H, Jiang J, Zhao S (2008) Increased sustainable electricity generation in upflow air-cathode microbial fuel cells. Biosen Bioelectron 23:1157–1160
Zhang T, Cui C, Chen S, Ai X, Yang H, Shen P et al (2006) A novel mediatorless microbial fuel cell based on biocatalysis of Escherichia coli. Chem Commun 21:2257–2259
Zuo Y, Cheng S, Call D, Logan BE (2007) Tubular membrane cathodes for scalable power generation in microbial fuel cells. Environ Sci Technol 41:3347–3353
Zuo Y, Xing D, Regan JM, Logan BE (2008) Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell. Appl Environ Microbiol 74:3130–3137
Acknowledgment
This research was supported by a grant from the Air Force Office of Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 64 kb)
Rights and permissions
About this article
Cite this article
Xing, D., Cheng, S., Logan, B.E. et al. Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl Microbiol Biotechnol 85, 1575–1587 (2010). https://doi.org/10.1007/s00253-009-2240-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2240-0