Abstract
In biochemical processes involving filamentous microorganisms, the high shear rate may damage suspended cells leading to viability loss and cell disruption. In this work, the influence of the shear conditions in clavulanic acid (CA) production by Streptomyces clavuligerus was evaluated in a 4-dm3 conventional stirred tank (STB) and in 6-dm3 concentric-tube airlift (ALB) bioreactors. Batch cultivations were performed in a STB at 600 and 800 rpm and 0.5 vvm (cultivations B1 and B2) and in ALB at 3.0 and 4.1 vvm (cultivations A1 and A2) to define two initial oxygen transfer conditions in both bioreactors. The average shear rate (\( \dot{\gamma }_{\rm {av}} \)) of the cultivations was estimated using correlations of recent literature based on experimental data of rheological properties of the broth (consistency index, K, and flow index, n) and operating conditions, impeller speed (N) for STB and superficial gas velocity in the riser (UGR) for ALB. In the same oxygen transfer condition, the \( \dot{\gamma }_{\rm {av}} \) values for ALB were higher than those obtained in STB. The maximum \( \dot{\gamma }_{\rm {av}} \) presented a strong correlation with a maximum consistency index (K max) of the broth. Close values of maximum CA production were obtained in cultivations A1 and A2 (454 and 442 mg L−1) with similar maximum \( \dot{\gamma }_{\rm {av}} \) values of 4,247 and 4,225 s−1. In cultivations B1 and B2, the maximum CA production of 269 and 402 mg L−1 were reached with a maximum \( \dot{\gamma }_{\rm {av}} \) of 904 and 1,786 s−1. The results show that high values of average shear rate increase the CA production regardless of the oxygen transfer condition and bioreactor model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clavulanic acid (CA) is a potent inhibitor of β-lactamase which protects β-lactam antibiotics against hydrolysis by binding irreversibly to the active site of β-lactamase. CA is widely prescribed in conjunction with β-lactamase-susceptible antibiotics, such as penicillins and cephalosporins and the combined effective action make CA very important both clinically and economically [1].
CA is traditionally produced in conventional stirred tank bioreactor by Streptomyces clavuligerus in culture media containing glycerol and soybean derivatives, such as carbon and nitrogen sources [2–7]. S. clavuligerus is a strictly aerobic and filamentous bacterium and its fermentation broths exhibit viscous pseudoplastic non-Newtonian behavior [8]. In bioreactors, the increase in the broth apparent viscosity (μ app) during aerobic fermentations can be partially counterbalanced by changing the operating conditions, namely by increments in the rotational impeller speed (N) or air flow rate (Q) in conventional stirred tank bioreactors or simply by the air flow rate (Q) in bubble column and airlift pneumatic bioreactors, maintaining an adequate oxygen mass transfer. However, high impeller speed (N) or volumetric air flow rate (Q) lead to the formation of high shear zones in bioreactors, causing consequent physical damage to the cells [4]. In most chemical processes, the shear rate is not so important by itself, except for increasing heat and mass transfer. However, in biochemical processes the shear rate is particularly important. Bioprocesses involving the culture of shear-sensitive cells or microorganisms require bioreactors in which the shear level is considerably low. Therefore, knowledge about the effect of the shear rate is essential for the design and operation of bioreactors used in bioprocesses involving shear-sensitive microorganisms. Although the shear rate is an important parameter in bioreactors, it is not easy to characterize. More recently, a new methodology to evaluate the average shear rate (\( \dot{\gamma }_{\rm {av}} \)) in a concentric-tube airlift bioreactor with highly viscous non-Newtonian fluids based on a volumetric oxygen transfer coefficient (k L a) measurement was proposed [9]. The methodology calculates the average shear rate as a function of superficial gas velocity in the riser (U GR), as well as the rheological parameters consistency index (K) and flow index (n), which reflect the fluid behavior. The use of this methodology was recently expanded to determine the shear rate in conventional stirred tank, bubble column, and split airlift bioreactors, as a function of the rheological parameters (K and n) and impeller speed (N) or specific air flow rate (ϕ air), depending on the bioreactor model [10, 11].
Since airlift bioreactors do not use mechanical stirrers, the risk of contamination and energy demand are considerably reduced. Airlift bioreactors require only about one-third of the energy needed for stirred tank reactors. The turbulence of two-phase flow in airlift bioreactors not only produces favorable conditions for mass transfer, it can also be used for suspending solid particles [12]. In order to make a satisfactory choice between different bioreactors in a specific bioprocess, their performances need to be assessed and compared. There is a growing body of literature related to the comparison between airlift and conventional bioreactors for different applications [12–17]. Regarding clavulanic acid production, various studies can be found in the literature related to the improvements in the composition of culture media [6, 8, 18–20], bioreactor operation mode [3, 5, 7, 21–23] and the influence of oxygen supply and agitation condition on CA production by S. clavuligerus in conventional bench scale bioreactor [4]. However, to the best of our knowledge no articles which compare the performances of conventional and non-conventional pneumatic bioreactors on CA production can be found. Therefore, the aim of the present work was to evaluate and compare the effect of the average shear rate on CA production by S. clavuligerus in batch cultures carried out in a conventional stirred tank and concentric-tube airlift bench scale bioreactors.
Materials and methods
Bioreactors
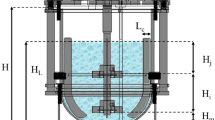
The stirred tank bioreactor (STB) used in the present work was a Bioflo III fermentor (New Brunswick Sci. Co Inc., USA) with 4-dm3 working volume. The STB, with internal diameter (D t ) of 0.17 m and four baffles, was fitted with two 6-flat-blade turbines (Rushton turbine) measuring 0.076 m in diameter (Di) and spaced one impeller diameter apart; each impeller blade (W) was 0.015 m in width. The bioreactor was also provided with a stainless steel ring gas sparger.
A pneumatic concentric-tube airlift bioreactor (ALB) with 6-dm3 working volume was used in this study. The geometrical characteristics and relationships between distances were as follows: H4/De1 = 2.5, Di1/De1 = 0.6, Di2 = Di1 + 10 mm, H1 = H2 and H4/H1 = 10. The holes of the cross-piece type sparger had 0.5 mm diameter and were spaced 5 mm along the length of the rods of the sparger [24].
Both bioreactors were equipped with pH and temperature control systems. The dissolved oxygen concentration was determined using a sterilizable amperometric probe (Mettler-Toledo, model InPro 6800) bearing a Teflon membrane (model T96). Schemes of the stirred tank bioreactor (STB) and concentric-tube airlift bioreactor (ALB) are shown in Fig. 1.
Determination of the volumetric oxygen transfer coefficient (k L a)
The volumetric oxygen transfer coefficient (k L a) was determined by the dynamic pressure-step method (DPM) [25]. In this experimental method, the pressure in the vessel was changed abruptly by approximately ±15 kPa, and an increase in the dissolved oxygen concentration (C e ) from C e0 to C es in the bulk liquid phase occurred regardless of the gas flow pattern. Equation 1 was fitted to the experimental data (C e as a function of time) and k L a values were estimated using a procedure that performs a non-linear regression by the least square method.
where C e0 is the electrode signal at the initial condition, when t = t 0.
The time constant of the oxygen probe (k e ) was calculated from the inverse of the response time (τ e ). The response to a unit step function, in triplicate, gave the calculation of k e = 0.215 s−1, assuming the first-order response.
Determination of the average shear rate (\( \dot{\gamma }_{\rm {av}} \))
The average shear rate (\( \dot{\gamma }_{\rm {av}} \)) in the airlift (ALB) and stirred tank (STB) bioreactors were evaluated by recent methods proposed by Cerri et al. [9] and Campesi et al. [10], respectively. Both methods are based on analogical analysis and the volumetric oxygen transfer coefficient (k L a) was chosen as a characteristic parameter, since the oxygen transfer occurs through the interfacial area of the air bubbles and is distributed evenly in the bioreactor.
For ALB, the average shear rate was obtained by Eq. 2, valid U GR > 0.05 m s−1 [9].
where U GR is superficial gas velocity in the riser, defined as the volumetric gas flow rate (Q) divided by cross-sectional area of the riser (A R).
For STB, the average shear rate was obtained by Eq. 3, valid for specific air flow rate ϕ air = 0.5 vvm [10].
Microorganism and culture media
Streptomyces clavuligerus ATCC 27064 was used throughout this study. It was maintained in a 10% v/v glycerol solution at −70 °C.
The seed medium, based on the one used by Reading and Cole [26], contained (in g L−1 distilled water): glycerol, 15.0; bacto peptone, 10.0; K2HPO4, 2.5, MgSO4·7H2O, 0.75; MnCl2·4H2O, 0.001; FeSO4·7H2O, 0.001; ZnSO4·7H2O, 0.001; and 3-(N-morpholino) propanesulfonic acid (MOPS) buffer, 21 (100 mM), pH 6.8. The inoculum medium contained (in g L−1 distilled water): glycerol, 15.0; soybean protein isolated, 10.0 (15% w/v of total nitrogen); malt extract, 10.0; yeast extract, 1.0; K2HPO4, 2.5, MgSO4·7H2O, 0.75; MnCl2·4H2O, 0.001; FeSO4·7H2O, 0.001; ZnSO4·7H2O, 0.001; and 3-(N-morpholino) propanesulfonic acid (MOPS) buffer, 21 (100 mM), pH 6.8. The production medium had the same composition as the inoculum medium, except that no MOPS buffer was used and silicone antifoam (0.08 mL L−1) was added.
Evaluation of oxygen transfer conditions in ALB and STB
Firstly, the volumetric oxygen transfer coefficient (k L a) was evaluated in ALB and STB using water and air as a liquid and gas phase, respectively. In ALB, k L a was obtained as a function of the specific air flow rate (ϕ air) and in STB as a function of the rotational impeller speed (N), at ϕ air of 0.5 and 1.0 vvm, respectively. This procedure was performed in order to define two different initial oxygen transfer conditions for both bioreactors in the cultivations. Only at the beginning of the cultivation it is possible to define and compare the oxygen transfer condition in both bioreactors, because it varies with the operating conditions (N and ϕ air) and with the rheology of the fermentation broth, which change during cultivation. Thus, water was chosen as the liquid phase because in the beginning of the cultivation, the rheological properties of fermentation broth in both bioreactors are similar to that of water.
Cultivation procedure
Cell suspensions from cryotubes (3.5 mL) with a concentration of ca. 5 g L−1 were inoculated with 50 mL of the seed medium in a 500-mL Erlenmeyer flask and incubated in a rotary shaker (New Brunswick Sci.) at 30 °C and 250 rpm for 24 h. Erlenmeyer flasks of 500 mL filled with 45 mL of the inoculum medium were inoculated with 5 mL of the cultivated seed medium and incubated in a rotary shaker at 30 °C and 250 rpm for 24 h. The contents of the inoculum flasks corresponding to 10% v/v were then poured into the bioreactors, making a total of 6-dm3 of fermentation broth for ALB and 4-dm3 for STB. All batch cultivations were conducted in a batch mode at 30 °C and the pH was automatically controlled at 6.8 ± 0.1 by adding 2 M HCl or 1 M NaOH solution and the dissolved oxygen concentration was monitored by a sterilized galvanic electrode (Mettler-Toledo InPro6000 Series). Samples of 25 mL were withdrawn every 6 h.
The glycerol concentration (Cs) was determined by high performance liquid chromatography (HPLC) using NaOH (1 mM) solution as the mobile phase. The equipment was operated at 80 °C with a flow rate of 1 mL min−1. A Shodex KS 802 (Lonpak) column was utilized.
The CA concentration (Cp) was determined by HPLC as described by Foulstone and Reading [27]. Samples were centrifuged at 17,500×g for 5 min at 4 °C, filtered in 0.22 μm membrane, and diluted in order to adjust the CA concentration in the range from 0 to 40 mg L−1. Imidazole reagent was prepared by dissolving 8.25 g of imidazole (Sigma-Aldrich) in 24 mL of deionized water. The pH was adjusted to 6.8 with addition 10 M hydrochloric acid and the volume was completed to 40 mL with deionized water. Four milliliter of the sample to be assayed was added to 1 mL of imidazole reagent, and, after a 10 min reaction period at 30 °C, 20 to 40 μL was injected into the HPLC unit. The C-18 μ-Bondapack (Waters) column was used as the stationary phase and the mobile phase was composed of 94% KH2PO4 0.1 M (pH 3.2) and 6% methanol (v/v). The detection of the CA derivative occurred at 311 nm. The CA from the Pharmaceutical product Clavulin (Glaxo-SmithKline Farmacêutica, Rio Janeiro, Brazil) was used as the standard.
In order to evaluate the effect of the average shear rate on the cultivations, the power law model (\( \tau = K \times \dot{\gamma }^{n} \)) was adjusted to the experimental data of shear stress (\( \tau \)) as a function of shear rate (\( \dot{\gamma } \)). Rheological parameters of the broth, consistency index (K) and flow index (n), were determined over cultivation time using a digital concentric-cylinders rheometer (Brookfield Engineering Laboratories Inc., USA), model LV-DVIII+.
Two batch cultivations of S. clavuligerus were performed in ALB at ϕ air of 3 and 4.1 vvm (A1 and A2). In STB, two batch cultivations were carried out at a specific air flow rate (ϕ air) of 0.5 vvm and rotational impeller speeds (N) of 600 and 800 rpm, respectively, for cultivations B1 and B2. The first (A1 and B1) and second (A2 and B2) cultivations set in ALB and STB bioreactors were performed at the same initial volumetric oxygen transfer coefficient (k L a), measured in triplicate using distilled water as a liquid phase. All cultivations were carried out in duplicate. The operational conditions are presented in Table 1.
Results and discussion
The oxygen transfer was evaluated in ALB and STB using water and air as a liquid and gas phase, respectively. Figure 2 illustrates the experimental values of volumetric oxygen transfer coefficient (k L a) obtained in ALB as a function of the specific air flow rate (ϕ air) and in STB as a function of the rotational impeller speed (N), at ϕ air of 0.5 and 1.0 vvm, respectively. It can be observed that the difference among the k L a values obtained in ALB operated at 3.0 < ϕ air (vvm) <5.0, and in STB operated in the range of N from 600 to 1,000 rpm, were very close. This shows the high oxygen transfer capacity of the ALB model used in the present work [24], due to its geometric characteristics and specific sparger. Thus, the operational conditions of the batch cultivations shown in Table 1 were defined based on initial k L a values of 0.038 s−1 (A1 and B1) and 0.056 s−1 (A2 and B2), respectively.
Results in terms of glycerol concentration (C G), consistency index (K), flow index (n), and CA concentration (C CA) of the cultivations carried out at initial k L a value of 0.038 s−1 (A1 and B1) are illustrated in Figs. 3 and 4, where similar glycerol consumptions of approximately 15 g L−1 in 30 h can be observed, indicating that similar cell growth occurred in both cultivations. The minimal dissolved oxygen concentrations were of 31 and 36% in cultivations A1 and B1, respectively (data not shown), showing that oxygen limitation did not occur in both cultivations. In the cultivation A1, the microorganism was exposed to more drastic shear conditions, reflecting in a much lower value of maximum consistency index of the broth (K max = 14.3 dyne cm−2 sn). As a result, the maximum CA production of 454 mg L−1 was higher than that obtained in the cultivation B1 conducted in the same oxygen transfer condition. In cultivation B1, the microorganism was less affected by the shear conditions, due to the lowest rotational impeller speed (N = 600 rpm) used. As a result, the highest value of the maximum consistency index (K max) of 36.1 dyne cm−2 sn was reached, and a maximum CA production of 269 mg L−1 was much lower than that found in cultivation A1.
Results in terms of glycerol concentration (C G), consistency index (K), flow index (n), and CA concentration (C CA) of the cultivations carried out at initial k L a value of 0.056 s−1 (A2 and B2) are shown in Figs. 5 and 6. Glycerol consumptions were similar (~15 g L−1 in 36 h). The minimal dissolved oxygen concentrations were 51 and 63% in cultivations A2 and B2, respectively (data not shown), showing once more that oxygen limitation did not occur in the cultivations at initial k L a = 0.056 h−1. In cultivation B2, a maximum value of the consistency index (K max = 27.1 dyne cm−2 sn) and a maximum CA concentration of 404 mg L−1 were found. In the cultivation A2, the high shear condition led to a maximum value of consistency index of 11.9 dyne cm−2 sn, the lowest among them. However, this extreme shear condition was responsible for a high CA production of 442 mg L−1.
For comparison, Fig. 7 illustrates the time course of average shear rate (\( \dot{\gamma }_{\rm {av}} \)) calculated by the correlations proposed by Cerri et al. [9] and Campesi et al. [10] for the airlift (ALB) and stirred tank (STB) bioreactors, respectively, throughout the cultivations performed at different oxygen transfer conditions. Furthermore, Table 1 summarizes the main results obtained in the cultivations performed in the ALB and STB bioreactors. The cultivations in ALB were performed at constant ϕ air, and in STB at constant N and ϕ air, which defined two same initial k L a conditions for both bioreactor configurations evaluated with water as liquid phase. The power inputs were not measured, but it is known that in STB it is much higher than in ALB. It can be observed that in the same oxygen transfer condition, the \( \dot{\gamma }_{\rm {av}} \) values for the airlift bioreactor (ALB) were higher than values obtained in the conventional bioreactor (STB), breaking an important paradigm related to the operation and performance of bioreactors. Similar behavior was observed by Thomasi et al. [11], which compared the average shear rate values obtained in pneumatic bioreactors with those obtained by Campesi et al. [10] in a conventional stirred tank bioreactor. The results indicate that the CA production was highly affected by the average shear rate (\( \dot{\gamma }_{\rm {av}} \)), regardless of the oxygen transfer condition chosen, compatible with the results reported for CA production in STB [4]. Figures 3, 4, 5, 6 show the time course of rheological parameters K and n in the cultivations A1, B1, A2 and B2. In all cultivations, the flow index (n) present a small variation along the time and consistency index (K) increase over time until the glycerol exhaustion. Then, K decreases over time due to cell lysis. It can be observed that the behavior of the average shear rate (\( \dot{\gamma }_{\rm {av}} \)) accompanies the consistency index (K) behavior. In the cultivations A1 and A2, \( \dot{\gamma }_{\rm {av}} \) reached close maximum values of 4,247 and 4,225 s−1, the highest among them and the highest and close maximum CA productions of 454 and 442 mg L−1 were obtained. On the other hand, in cultivation B1, \( \dot{\gamma }_{\rm {av}} \) reached a maximum value of 904 s−1, the lowest among them and the lowest CA production of 269 mg L−1 was obtained. In cultivation B2 performed at 800 rpm, the maximum average shear rate of 1,786 s−1 was about twice as high as the maximum \( \dot{\gamma }_{\rm {av}} \) obtained in cultivation B1. As a result, the maximum CA production reached the value of 402 mg L−1, approximately 50% higher than the production obtained in cultivation B1. According to Rosa et al. [4], it is known from the literature that during the biosynthesis of β-lactam antibiotics, such as penicillins, intermediates such as 6-aminopenicillanic acid (6-APA) are synthesized in the Golgi complex and transferred to the periplasmic space between the membrane and cell wall, remaining there until secretion [28]. CA is a β-lactam and, as such, the fact that its production is directly related to shear conditions may be explained by the higher stress on the cell wall promoting CA liberation into the medium. Moreover, a strong correlation was observed between the maximum \( \dot{\gamma }_{\rm {av}} \) and maximum consistency index (K max). The higher the shear rate, the higher the fragmentation level of the hyphae and the lower the consistency index of the broth (K), a variable related to the structural health of the mycelia.
In the recent literature, work can be found regarding the comparison between the performance of conventional and pneumatic bioreactors in important bioprocesses. Xu et al. [15] have investigated the production of exopolysaccharides (EPS) by Paecilomyces tenuipes C240 in a stirred tank (STB) and airlift (ALB) bioreactors both with 3-L working volume operated at 2 vvm. The optimal impeller speed (N) for the production of EPS in the STB was 150 rpm with the mycelial morphology of hairy pellets, where the final concentration and the specific production rate of EPS were 2.33 g L−1 and 0.312 g g−1 h−1, respectively. However, the maximum concentration of biomass (21.1 g L−1) in the STB was obtained at a high impeller speed of 300 rpm. The specific production rate of EPS (0.456 g g−1 h−1) in the ALB was significantly higher than that achieved in the STB, in which the typical morphological form of mycelium was a loose clump. The authors attributed the higher EPS production to advantageous morphological features of the fungus defined by lower shear forces in ALB. Other works related to the production of lipase [14] and endo and exo-polygalacturonase [17], and phenol degradation [16] have a similar approach. However, unlike the present work, none of the studies evaluate and compare the effect of the shear conditions in both STB and ALB in the bioprocess investigated.
Conclusion
Due to its geometric characteristics and specific sparger, the airlift bioreactor used in the present work presented a high oxygen transfer capacity compared with a conventional stirred tank bioreactor. Usually, at moderate ϕ air bench scale pneumatic bioreactors not present high oxygen mass transfer when compared with conventional stirred and aerated tank bioreactor. In this work, in the range of ϕ air from 3 to 5 vvm, the ALB presented k L a values close to those obtained in STR in the range from 600 to 1,000 rpm at ϕ air of 0.5 vvm.
In the same oxygen transfer condition, the average shear rate (\( \dot{\gamma }_{\rm {av}} \)) for the airlift bioreactor (ALB) was higher than \( \dot{\gamma }_{\rm {av}} \) obtained in the conventional bioreactor (STB), breaking an important paradigm related to the operation and performance of bioreactors.
The average shear rate (\( \dot{\gamma }_{\rm {av}} \)) presented a strong correlation with the CA production and with the maximum consistency index (K max). The higher the shear rate, the higher the fragmentation level of the hyphae and the lower the consistency index of the broth (K). However, these results cannot be generalized to all bioreactor scales. Additional researches should be performed in pilot and industrial scales.
CA production by Streptomyces clavuligerus was strongly affected by shear conditions. The results clearly show that high values of the average shear rate increase the CA production in both bioreactor types. Clavulanic acid production in ALB was higher than in STB in the same oxygen transfer condition, indicating that airlift bioreactor is potentially suitable for use in CA production, having lower installation and operational costs.
References
Lynch HC, Yang Y (2004) Degradation products of clavulanic acid promote clavulanic acid production in cultures of Streptomyces clavuligerus. Enzyme Microb Technol 34:48–54
Mayer AF, Deckwer WD (1996) Simultaneous production and decomposition of clavulanic acid during Streptomyces clavuligerus cultivations. Appl Microbiol Biotechnol 45:41–46
Roubos JA, Krabben P, Luiten RGM, Verbruggen HB, Heijnen JJ (2001) A quantitative approach to characterizing cell lysis caused by mechanical agitation of Streptomyces clavuligerus. Biotechnol Progr 17:336–347
Rosa JC, Baptista-Neto A, Hokka CO, Badino AC (2005) Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst Eng 27:99–104
Teodoro JC, Baptista-Neto A, Cruz-Hernández IL, Hokka CO, Badino AC (2006) Influence of feeding conditions on clavulanic acid production in fed-batch cultivation with medium containing glycerol. Appl Microbiol Biotechnol 72:450–455
Ortiz SCA, Hokka CO, Badino AC (2007) Utilization of soybean derivatives on clavulanic acid production by Streptomyces clavuligerus. Enzyme Microb Technol 40:1071–1077
Teodoro JC, Baptista-Neto A, Araujo MLGC, Hokka CO, Badino AC (2010) Influence of glycerol and ornithine feeding on clavulanic acid production by Streptomyces clavuligerus. Braz J Chem Eng 27:499–506
Gouveia ER, Baptista-Neto A, Hokka CO, Badino AC (2000) Studies on the rheology and oxygen mass transfer in the clavulanic acid production by Streptomyces clavuligerus. Braz J Chem Eng 17:827–834
Cerri MO, Futiwaki L, Jesus CDF, Cruz AJG, Badino AC (2008) Average shear rate for non-Newtonian fluids in a concentric-tube airlift bioreactor. Biochem Eng J 39:51–57
Campesi A, Cerri MO, Hokka CO, Badino AC (2009) Determination of the average shear rate in a stirred and aerated tank bioreactor. Bioprocess Biosyst Eng 32:241–248
Thomasi SS, Cerri MO, Badino AC (2010) Average shear rate in three pneumatic bioreactors. Bioprocess Biosyst Eng 33:979–988
Träger M, Qazi GN, Onken U, Chopra CL (1989) Comparison of airlift and stirred reactors for fermentation with Aspergillus niger. J Ferment Bioeng 68:112–116
Siedenberg D, Gerlach SR, Weigel B, Schugerl K, Giuseppin MLF, Hunik J (1997) Production of xylanase by Aspergillus awamori on synthetic medium in stirred tank and airlift tower loop reactors: the influence of stirrer speed and phosphate concentration. J Biotechnol 56:103–114
Burkert JFM, Maldonado RR, Maugeri Filho F, Rodrigues MI (2005) Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J Chem Technol Biotechnol 80:61–67
Xu CP, Kim SW, Hwang HJ, Yun JW (2006) Production of exopolysaccharides by submerged culture of an enthomopathogenic fungus, Paecilomyces tenuipes C240 in stirred-tank and airlift reactors. Bioresour Technol 97:770–777
Saravaman P, Pakshirajan K, Saha P (2008) Performance of batch stirred tank bioreactor and internal loop airlift bioreactor in degrading phenol using Pseudomonas spp.—a comparative study performance. J Environ Prot Sci 2:81–86
Fontana RC, Polidoro TA, Silveira MM (2009) Comparison of stirred tank and airlift bioreactors in the production of polygalacturonases by Aspergillus oryzae. Bioresour Technol 100:4493–4498
Gouveia ER, Baptista-Neto A, Badino AC, Hokka CO (2001) Optimisation of medium composition for clavulanic acid production by Streptomyces clavuligerus. Biotechnol Lett 23:157–161
Wang YH, Yang B, Ren J, Dong ML, Liang D, Xu AL (2005) Optimization of medium composition for the production of clavulanic acid by Streptomyces clavuligerus. Process Biochem 40:1161–1166
Maranesi GL, Baptista-Neto A, Hokka CO, Badino AC (2005) Utilization of vegetable oil in the production of clavulanic acid by Streptomyces clavuligerus ATCC 27064. World J Microbiol Biotechnol 21:509–514
Baptista-Neto A, Hirata DB, Cassiano Filho LCM, Bellão C, Badino AC, Hokka CO (2005) A study on clavulanic acid production by Streptomyces clavuligerus in batch, fed-batch and continuous processes. Braz J Chem Eng 22:557–563
Baptista-Neto A, Teodoro JC, Cassiano Filho LCM, Badino AC, Hokka CO (2005) Comparisons between continuous and batch processing to produce clavulanic acid by Streptomyces clavuligerus. Braz Arch Biol Technol 45:97–104
Lavarda SCS, Hokka CO, Araujo MLGC (2008) Clavulanic acid production processes in a tower bioreactor with immobilised cells. Biochem Eng J. 39:131–136
Badino AC, Hokka CO, Cerri MO (2006) Pneumatic reactor with an inner and a transparent outer cylinder gas injection for circulation and temperature control of the enclosed reaction mixture. Brazilian Patent BR200404703-A
Blažej M, Annus J, Markŏs J (2004) Comparison of gassing-out and pressure-step dynamic methods for kLa measurement in an airlift reactor with internal loop. Chem Eng Res Des 82:1375–1382
Reading C, Cole M (1977) Clavulanic acid: a beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother 11:852–857
Foulstone M, Reading C (1982) Assay of amoxicillin and clavulanic acid, the components of Augmentin, in biological fluids with high performance chromatography. Antimicrob Agents Chemother 22:753–762
Hersbach GJM, Van der Beek LP, Van Dijck PWM (1984) The penicillins: properties, biosynthesis and fermentation. In: Vandamme EJ (ed) Biotechnology of industrial antibiotics. Marcel Dekker, New York
Acknowledgments
The authors gratefully acknowledge FAPESP (Grant Proc. no. 2011/23807-1), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Grant Proc. 478472/2011-0 (Brazil) for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerri, M.O., Badino, A.C. Shear conditions in clavulanic acid production by Streptomyces clavuligerus in stirred tank and airlift bioreactors. Bioprocess Biosyst Eng 35, 977–984 (2012). https://doi.org/10.1007/s00449-012-0682-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0682-8