Abstract
Clavulanic acid (CA), a potent β-lactamase inhibitor, is produced by a filamentous bacterium. Here, the effect of DO and shear, expressed as impeller tip velocity, on CA production was examined. Cultivations were performed in a 4 L fermentor with speeds of 600, 800 and 1,000 rpm and a fixed air flow rate (0.5 vvm). Also, cultivation with automatic control of dissolved oxygen, at 50% air saturation, by varying stirrer speed and using a mixture of air and O2 (10% v/v) in the inlet gas, and a cultivation with fixed stirrer speed of 800 rpm and air flow rate of 0.5 vvm, enriched with 10% v/v O2, were performed. Significant variations in CA titer, CA production rate and O2 uptake-rate were observed. It was also found that the DO level has no remarkable effect on CA production once a critical level is surpassed. The most significant improvement in CA production was related to high stirrer speeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A variety of Gram-positive and Gram-negative pathogenic bacteria exhibit an antibiotic resistance mechanism based on their ability to produce β-lactamases, which deactivate penicillins and cephalosporins by hydrolyzing their β-lactam ring. The use of antibiotics to control infectious diseases is greatly hindered by this kind of resistance. Clavulanic acid (CA) is a β-lactam antibiotic with low antibacterial activity; it is, however, a potent inhibitor of the β-lactamases produced by many pathogenic microorganisms resistant to β-lactam antibiotics. The combination of CA with amoxycillin is the most successful example of the use of a β-lactam antibiotic sensitive to β-lactamase together with an inhibitor of these enzymes [1].
CA is traditionally produced by Streptomyces clavuligerus, filamentous and strictly aerobic bacterium, in conventional agitated and aerated bioreactors. Although Streptomyces sp. can grow as pellets or freely dispersed mycelia, the mycelial form is preferred in industry [2]. Fermentation broths containing filamentous microorganisms frequently exhibit highly viscous pseudoplastic non-Newtonian behavior, affecting the bioreactor performance [3]. The increase in the apparent viscosity of the broth (μap) during aerobic fermentations can be partially counterbalanced by changing the operating conditions, namely, by increments in impeller speed (N) and air flow rate (Q), maintaining an adequate oxygen mass transfer. However, high impeller speeds (N) lead to the formation of high shear zones in the neighborhood of the impellers, with consequent physical damage to the cells and a reduction in the process productivity [4].
The influence of dissolved oxygen (DO) level and shear condition on biomass and product yields in specific processes involving S. clavuligerus have been reported in the literature.
Yegneswaran et al. [5] investigated the influence of reduced oxygen on growth and antibiotic production in batch cultivations of S. clavuligerus utilizing defined medium. During the growth phase (first 50 h), antibiotic biosynthesis was not affected by reduced oxygen. The antibiotic concentration remained stable when air was used to supply oxygen. However, under reduced oxygen concentration (air plus N2 in the inlet gas) antibiotic concentration dropped to one third of its highest value. Their study focused on total antibiotic production, measured as cephalosporin C.
The effect of DO level on cephamycin C production by S. clavuligerus, in a 10 L bioreactor, was studied by Rollins et al. [6]. During the growth phase, in complex media, control of DO at 50 and 100% saturation increased the rate of specific cephamycin C production, two and threefold, respectively, compared to the experiments without DO control. The improvement of the cephamycin C production was confirmed by increases in the specific activities of two enzymes in the antibiotic biosynthetic pathway, deacetoxycephalosporin C synthase and isopenicillin N synthase, 2.3 and 1.3 times higher, respectively, observed when the DO was maintained at 100% saturation in the cultivations [7].
In similar work, Yegneswaran et al. [8] studied the growth and production of cephamycin C at different DO levels during S. clavuligerus fermentation. A 2.4-fold increase in the final cephamycin C concentration was observed when the DO was controlled at 100% saturation level during the growth phase, compared with cultivations without DO control. The enhancement of the cephamycin C production was independent of DO level during the stationary phase, in the range of 50–100% saturation.
While a few experimental studies, as indicated above, report DO effects on S. clavuligerus cultivations, there is no published work dealing with this effect on CA production.
The effects of agitation on morphology, cell growth and product yield have been investigated for several processes involving filamentous microorganisms. However, shear effects are often not generic and seem to depend upon the strain, the medium composition and preculture conditions. Few studies have been published that investigate, specifically, the influence of shear conditions on cultures of S. clavuligerus.
Tarbuck et al. [9] reported the effect of stirrer speed on CA production both in batch and fed-batch fermentations in a 10 L fermentor. S. clavuligerus was grown in the complex medium proposed by Reading and Cole [10], composed of glycerol (20 g L−1), malt extract (10 g L−1) and bacteriological peptone (10 g L−1). When the stirrer speed was increased from 350 to 500 rpm in batch cultivations, it was observed that CA production decreased. A maximal CA concentration of only 36 mg L−1 was obtained at an air flow-rate of 0.25 vvm and stirrer speed of 350 rpm.
Belmar-Beiny and Thomas [2] studied the influence of stirrer speed on biomass, morphology and CA yield in S. clavuligerus batch cultivations in a 5 L bioreactor utilizing the same medium as Tarbuck et al. [9]. Experiments were conducted with specific air flow of 0.5 vvm and stirrer speeds (N) of 490, 990 and 1,300 rpm, corresponding to 1.7, 3.5 and 4.6 m s−1 impeller tip speeds (v tip), respectively. They concluded that growth and CA production (ca. 200 mg L−1) were essentially independent of stirrer speed.
Large et al. [11] investigated the effect of impeller tip speed (v tip), within the range of 1.9–3.8 m s−1, on growth and production of CA by S. clavuligerus in a 5 L batch-scale process, utilizing a medium containing modified starch (10 g L−1), soyaflour (20 g L−1), vegetable oil (23 g L−1) and phosphate (1.2 g L−1). It was observed that when the tip speed was changed from 1.9 to 2.8 m s−1, biomass production increased, while a further increase to 3.8 m s−1 was found to be detrimental to cell growth. Lipase activity decreased when stirrer speed increased and an optimum impeller tip speed of 2.4 m s−1 was found for CA production presented as relative values.
Sebastine et al. [12] examined the viability of S. clavuligerus during batch fermentation in a fermentor of 5 L working volume at 500 rpm. They observed that the cell viability, measured with the aid of an image analysis system, decreased from 100 to 64% during the rapid growth phase. CA production started during the stationary phase and the percentage of viability remained constant at approximately 75%, decreasing to 53% at the end of the fermentation. No relationship between the CA production rate and the viability, measured as percentage or the total count of viable cells, was observed.
Recently, Roubos et al. [13] investigated the effect of mechanical agitation speed on the lysis of S. clavuligerus cells, utilizing a quantitative approach. Observations of ten batch cultivations in a bioreactor containing 20–30 L defined medium showed that the organism is very sensitive to the shear which results from the increasing stirrer speed required to maintain the DO concentration above 50% air saturation.
Thus, while Belmar-Beiny and Thomas [2] indicated only a slight dependence of CA production on stirrer speed, Large et al. [11] found an optimum tip speed for CA production; in the recent work of Sebastine et al. [12] and Roubos et al. [13], there is no mention of any relationship between shear conditions and CA production.
As pointed out by Large et al. [11], it is often difficult to distinguish the effects of changes in the stirrer speed from the concomitant changes in the DO concentration, in batch fermentations of filamentous microorganisms. Ideally, the DO should be held constant, independently of stirrer speed changes, by blending oxygen or nitrogen into the air feed [2].
In the present study, the effect of oxygen supply and shear conditions on CA production by S. clavuligerus was investigated. Six batch cultivations were studied in a 4 L conventional bioreactor, containing a complex culture medium. Experiments were carried out at various stirrer speeds and oxygen supply rates.
2 Materials and methods
2.1 Microorganism and culture media
Vegetative cells of S. clavuligerus ATCC 27064, stored in cryotubes (glycerol 10% v/v) at −70°C, were used throughout this work. Seed medium contained (in g L−1 distilled water): glycerol, 10; bacto peptone, 10; malt extract, 10; yeast extract, 1.0; K2HPO4, 2.5; MgSO4·7H2O, 0.75; MnCl2·4H2O, 0.001; FeSO4·7H2O, 0.001; ZnSO4·7H2O, 0.001; MOPS buffer, 21 (100 mM), pH 6.8.
Inoculum medium contained (in g L−1 distilled water): glycerol, 15; Samprosoy 90NB (soybean protein hydrolyzate from Bunge Alimentos S.A., Esteio RS, Brazil), 10; malt extract, 10; yeast extract, 1.0; K2HPO4, 2.5; MgSO4·7H2O, 0.75; MnCl2·4H2O, 0.001; FeSO4·7H2O, 0.001; ZnSO4·7H2O, 0.001; MOPS buffer, 21 (100 mM), pH 6.8. Production medium had the same composition as inoculum medium, except that MOPS buffer was withdrawn and silicone antifoam (0.08 mL L−1) added.
2.2 Fermentation equipment and cultivation procedure
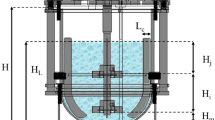
Six batch cultivations were performed in Bioflo III fermentor (New Brunswick Sci. Co. Inc., USA) with a 4 L working volume. The bioreactor, with internal diameter (D t) of 0.17 m and four baffles was fitted with two six-flat-blade turbines (Rushton turbine) measuring 0.076 m in diameter (D i), spaced one impeller diameter apart and one impeller diameter from the bottom, was provided with a stainless steel ring gas sparger.
Cell suspensions from cryotubes (3.5 mL), with a concentration of ca. 5 g L−1, were inoculated into 50 mL seed medium in a 500 mL Erlenmeyer flask and incubated in a rotary shaker (New Brunswick Sci.) at 28°C, 250 rpm, for 24 h. Eight Erlenmeyer flasks of 500 mL with 45 mL of inoculum medium were inoculated with 5 mL of the cultivated seed medium and incubated in a rotary shaker at 28°C, 250 rpm for 24 h. The whole contents of the flasks (400 mL) were transferred to the fermentor with 3.6 L of production medium, making a total of 4 L fermentation broth. All six cultivations were conducted in batch mode at 28°C and the pH was automatically controlled at 6.8±0.1 by adding 4 M HCl or 2 M NaOH solution. The cultivations were carried out at different shear and oxygen-feed conditions. The impeller tip speeds (v tip=πD i N) ranged from 1.2 to 4.0 m s−1 and the specific flow rate (ϕ) of air \(({\text{oxygen mole fraction, }}y_{{\text{O}}_2 }^{\text{i}} = 0.21)\) or air enriched with pure oxygen \((y_{{\text{O}}_2 }^{\text{i}} = 0.30)\) was fixed at 0.5 vvm (2 NL min−1). Run BC6 was performed with automatic DO control. The DO concentration was kept at 50% air saturation by automatically varying the impeller speed (N). Table 1 presents the operating conditions of the batches performed in this study.
The DO concentration was measured by a sterilizable galvanic electrode (Mettler-Toledo InPro6000 Series). Oxygen uptake rate (OUR) was determined by the gas balance method, assuming the reactor to be completely mixed. Accurate measurements of oxygen \((y_{{\text{O}}_2 } )\) and carbon dioxide \({\text{(}}y_{{\text{CO}}_2 } )\) mole fractions in the outlet gas were furnished by gas analyzers from Beckman Industrial (model 755) and Fuji Electric (model IR-730), respectively. Air and oxygen flow rates were measured using the ColeParmer mass flow meters (models 33116-40 and 33116-22).
Samples of ca. 20 mL were withdrawn each 3 h approximately. An amount of 10 mL aliquots of the fermentation broth was centrifuged at 3,720 g, 5°C for 20 min.
2.3 Analytical methods
Cell growth was evaluated indirectly by measuring the broth rheological parameter K (consistency index) of the “power law” model, using a Brookfield concentric-cylinders rheometer. Recent publications have taken the view that the consistency index (K) is the best parameter from which to infer cell growth in broth containing insoluble particles, as is the case of complex fermentation broths. It can be related also to the morphology of filamentous microorganisms [15, 16].
Glycerol concentration was determined by high-performance liquid chromatography (HPLC). NaOH (1 mM) solution was used as mobile phase. The equipment was operated at 34°C with a flow rate of 1 mL min−1 . A Shodex 802 column (Lonpak, division of Millipore) was utilized.
The CA concentration was determined by HPLC, as described by Foulstone and Reading [14]. These results were checked by bioassay with Klebsiella pneumoniae ATCC 29665.
3 Results and discussion
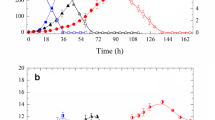
Results of each batch cultivations are plotted in Fig. 1a–d, where the time course of glycerol concentration (C Gly), consistency index (K), DO concentration and CA concentration (C CA), are shown, respectively.
Oxygen limitation took place during cultivation BC1, performed with a low stirrer speed (300 rpm). From the very beginning of this batch, DO dropped rapidly, leveling out at values bellow 10% air saturation, and even lower, near zero, towards the end of the cultivation. As a consequence, no CA was produced in this batch. During all the other experimental runs, irrespective of the aeration and agitation conditions utilized, CA production occurred. It was initiated whenever the glycerol concentration (C Gly) reached values below 12.5 g L−1, in agreement with the results obtained by Baptista Neto et al. [17].
Table 2 summarizes the main results obtained (maximum consistency index, K max, CA concentration, C CAmax, CA productivity, P CAmax and the minimum disssolved oxygen observed, DOmin) and the main operating conditions \({\text{(tip speed, }}v_{{\text{tip}}} {\text{, oxygen mole fraction in the inlet gas, }}y_{{\text{O}}_2 }^{\text{i}} )\) in the six cultivations. Batch experiment BC3 produced the highest values of K, although the initial glycerol concentration was the lowest, indicating that, under these operating conditions, 800 rpm and 0.5 vvm of pure air, cell growth was enhanced. The strategy of supplying the culture with air enriched with oxygen did not lead to good results in terms of cell growth, as can be observed comparing the results of BC3 and BC5. The highest value of K attained during BC5 was about half that found in BC3. Comparing the two batches performed with oxygen enriched air supply, BC5 and BC6, it can be observed that, although the DO level was kept above 40% air saturation, cell growth did not achieve the highest values. In BC4, the intense shear conditions utilized during the batch, 1,000 rpm and v tip=4.0 m s−1, negatively affected cell growth, as shown in Fig. 1b. The profile of K indicates cell damage due to the high shear stress, making cell growth proceed slowly, so that K values similar to those in BC6 are reached only within 51 h. This negative effect of the shear conditions can also be seen in Fig. 1a, where a slower glycerol consumption rate in BC4 is evident, causing glycerol depletion only after 48 h of growth. This slow glycerol consumption may account for the higher CA production, by providing a steady supply of the C3 unit of the CA molecule.
The OUR was calculated from the mass balance of O2 in the inlet and outlet gases (data not shown). During the growth phase, the OUR of BC2, BC3, BC4 and BC5 reached maximum values, between 20 and 25 mmol L−1 h−1, and remained on this plateau value for up to 24 h. For BC6, the maximum value lay between 28 and 30 mmol L−1 h−1, but this level of uptake was maintained for only 6 h.
In all runs, the onset of CA production was associated with the time when OUR attained its maximum value (data not shown), regardless of the cultivation conditions. This phenomenon makes the OUR a useful parameter for process monitoring and control. Besides, a rise in the minimum dissolved oxygen concentration (DOmin) was accompanied by enhanced CA production, as can be verified by comparing BC2, BC3 and BC4 (DOmin = 12, 21 and 28%, respectively). However, when oxygen-enriched air was supplied (BC5), no further increase in CA production was observed. Indeed, the strategy of controlling the DO concentration at 50% air saturation did not succeed in improving CA production, or even cell growth, as indicated by the K values found. The DO control was performed by varying the impeller frequency (BC6). The maximum productivity achieved in BC6 was ca. 5.0 mgAC L−1 h−1, i.e., 50% of that achieved in batches BC3, BC4 and BC5. The impeller speed (N) was low during the first 12–15 h of cultivations, reaching values above 500 rpm only after the 15th hour. Near the end of the cultivation it declined to values within 300 and 400 rpm. The impeller tip speed (v tip) varied from 1.0 to 3.2 m s−1 . These results indicate that, if DO is maintained above a critical value, production is not limited by oxygen level, since further improvement depends on other variables, such as shear stress. Indeed, the highest productivity, 11.4 mgAC L−1 h−1, was attained in batch BC4 (Table 2), where DOmin was 28% and v tip = 4.0 m s−1.
In Fig. 2 are plotted the experimental values of maximum CA concentration (C CAmax) and maximum productivity (P CAmax), as well as DOmin, observed in cultivations utilizing only air \((y_{{\text{O}}_2 }^{\text{i}} = 0.21),\) against the speed of the impeller, i.e., v tip of 1.2, 2.4, 3.2, 4.0 m s−1, corresponding to the runs BC1, BC2, BC3 and BC4, respectively. These results show that, within the range of stirrer speed (N) studied, the CA production is directly related to the shear conditions, represented by v tip.
The highest CA production was obtained in BC4, performed with specific air flow rate of 0.5 vvm \((y_{{\text{O}}_2 }^{\text{i}} = 0.21)\) and under extreme shear conditions, i.e., 1,000 rpm (v tip=4.0 m s−1). These results contradict data presented in the literature. Tarbuck et al. [9] found that CA concentration decreased with increasing tip speed (v tip) in a 10 L fermentor. Belmar-Beiny and Thomas [2] reported that the CA production rate shows little dependence on stirrer speed (N). Large et al. [11] found that the highest CA production occurred at an optimal intermediate tip speed (2.4 m s−1) in a 5 L batch cultivation of S. clavuligerus, utilizing lipid as carbon source. These authors suggest that above 2.4 m s−1, CA production is limited by hyphal fragmentation and decrease in lipase activity; while below this value it is probably limited by mass transfer.
However, the culture media and experimental conditions used by Tarbuck et al. [9] and by Belmar-Beiny and Thomas [2] were different from those in the present study. The authors employed as the production medium the seed medium proposed originally by Reading and Cole [10]. Additionally, the production medium was inoculated directly with a spore suspension, which is not appropriate for a real production process. Perhaps, these conditions led to the low CA concentrations found by Tarbuck et al. [9] and the small range of CA production obtained by Belmar-Beiny and Thomas [2] in 120 h (between 180 and 210 mg L−1) at various stirrer speeds. Indeed, Roubos et al. [13] state that the effects of shear conditions on fermentation performance strongly depend on the strain, medium and pre-culture conditions. Therefore, it seems clear that the experimental conditions employed here have generated different results from those found in the literature, with regard to effects of shear conditions. It should be noted that in the present study the production medium employed is an inexpensive complex medium suitable for industrial production.
It is known from the literature that during the biosynthesis of β-lactam antibiotics, such as penicillins, intermediates such as 6-aminopenicillanic acid (6-APA) are synthesized in the Golgi complex, and transferred to the periplasmic space between the membrane and cell wall, remaining there until secretion [18]. CA is a β-lactam and, as such, the fact that its production is directly related to shear conditions may be explained by the higher stress upon the cell wall promoting CA liberation into the medium. Also, improved mass transfer could account for the enhancement of CA production, as higher shear would reduce the diffusion film outside the cell. It is stated that glycerol provides the carbon skeleton of the β-lactam ring, without any intermediate rearrangement of the three carbons [19], and glycerol transport into the cell may be promoted by higher shear, improving CA production.
The small influence of the DO concentration on CA production is more evident when the cultivations BC3 and BC5, performed under the same shear conditions but with different oxygen flow rates, are compared. The results in Table 2 show that the performance of these batches, in terms of CA concentration and volumetric productivity, was similar and rather lower than that obtained in run BC4, although the DOmin of BC5 (43%) is twice that of run BC3 (21%). Furthermore, the CA production obtained in run BC6 (DOmin=50%) was the lowest, indicating that as long as the DO be kept above a critical level, the shear conditions are determinant in optimizing CA production. Indeed, except for the run BC1, where oxygen limitation was evident (DOmin=0%), the results confirm that, in the absence of oxygen limitation, shear conditions strongly affect CA production.
Comparing BC5 and BC3 runs, however, the same CA production was observed, meaning higher specific CA production rate in BC5 than in BC3 run. Probably, the stress caused by the high level of DO at the beginning of the BC5 run may have affected positively the secondary metabolism. The results obtained by Pinto et al. [20] support this hypothesis. Indeed, their results show that higher tip speed, in 0.5 m3 fermentor, leads to larger hyphal fragmentation than in 5 m3 fermentor. However, this fact did not seem to affect the production profile. The lower biomass obtained due to high shear stress in the smaller fermentor was counterbalanced by the high productivity of these stressed cells.
3.1 Conclusion
CA production by S. clavuligerus in complex media containing glycerol and soybean derivative as the main nutrients is strongly affected by shear conditions, once sufficient oxygen is supplied to attain a DO concentration above a critical value, approximately 20% air saturation. Increasing the DO level, by using oxygen enriched air does not lead to better performance in terms of the CA production. High impeller speeds, producing high shear rate and stress, improve CA titer and productivity, probably by increasing the rates of transfer of glycerol into the cells and CA out of the cells.
References
Mayer AF, Deckwer W-D (1996) Simultaneous production and decomposition of clavulanic acid during Streptomyces clavuligerus cultivations. Appl Microbiol Biotechnol 45:41–46
Belmar-Beiny MT, Thomas CR (1991) Morphology and clavulanic acid production of Streptomyces clavuligerus: effect of stirrer speed in batch fermentations. Biotechnol Bioeng 37:456–462
Gavrilescu M, Roman RV, Efimov V (1993) The volumetric oxygen mass transfer coefficient in antibiotic biosynthesis liquids. Acta Biotechnol 13:59–70
Smith JJ, Lilly MD, Fox RI (1990) The effect of agitation on the morphology and penicillin production of Penicillium chrysogenum. Biotechnol Bioeng 35:1011–1023
Yegneswaran PK, Gray MR, Westlake DWS (1988) Effects of reduced oxygen on growth and antibiotic production in Streptomyces clavuligerus. Biotechnol Lett 10:479–484
Rollins MJ, Jensen SE, Westlake DWS (1988) Effect of aeration on antibiotic production by Streptomyces clavuligerus. J Ind Microbiol 3:357–364
Rollins MJ, Jensen SE, Westlake DWS (1991) Effect of dissolved oxygen level on the ACV synthetase synthesis and activity during growth of Streptomyces clavuligerus. Appl Microbiol Biotechnol 35:83–88
Yegneswaran PK, Gray MR, Thompson BG (1991) Experimental simulation of dissolved fluctuations in large fermentors: effect on Streptomyces clavuligerus. Biotechnol Bioeng 38:1203–1209
Tarbuck LA, Ng MH, Leigh JR, Tampion J (1985) Estimation of the progress of Streptomyces clavuligerus fermentations for improved on-line control of antibiotic production. In: Johnson A (ed) Modelling and control of biotechnological process. Pergamon, Oxford, pp 171–178
Reading C, Cole M (1977) Clavulanic acid: a β-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother 11:852–857
Large KP, Ison AP, Williams DJ (1998) The effect of agitation rate on lipid utilisation and clavulanic acid production in Streptomyces clavuligerus. J Biotechnol 63:111–119
Sebastine IM, Stocks SM, Cox PW, Thomas CR (1999) Characterisation of percentage viability of Streptomyces clavuligerus using image analysis. Biotechnol Tech 13:419–423
Roubos JA, Krabben P, Luiten RGM, Verbruggen HB, Heijnen JJ (2001) A quantitative approach to characterizing cell lysis caused by agitation of Streptomyces clavuligeurs. Biotechnol Prog 17:336–347
Foulstone M, Reading C (1982) Assay of amoxicillin and clavulanic acid, the components of Augmentin, in biological fluids with HPLC. Antimicrob Agents Chemother 22:753–762
Neves AA, Pereira DA, Vieira LM, Menezes JC (2000) Real time monitoring biomass concentration in Streptomyces clavuligerus cultivations with industrial media using a capacitance probe. J Biotechnol 84:45–52
Badino AC, Facciotti MCR, Schmidell W (2001) Volumetric oxygen transfer coefficients (kLa) in batch cultivations involving non-newtonian broths. Biochem Eng J 8:111–119
Baptista Neto A, Gouveia ER, Badino AC, Hokka CO (2000) Phenomenological model of the clavulanic acid production process utilizing Streptomyces clavuligerus. Braz J Chem Eng 17:809–818
Hersbach GJM, Van der Beek LP, Van Dijck PWM (1984) The penicillins: properties, biosynthesis and fermentation. In: Vandamme EJ (ed) Biotechnology of industrial antibiotics. Marcel Dekker Inc, New York, pp 45–140
Pitlik J, Townsend CA (1997) The fate [2,3,3−2 H3, 1,2−13 C2]-d,l-glycerate in clavulanic acid biosynthesis. Chem Commun 225–226. DOI 10.1039/a607078g
Pinto LS, Vieira LM, Pons MN, Fonseca MMR, Menezes JC (2004) Morphology and viability analysis of Streptomyces clavuligerus in industrial cultivation systems. Bioprocess Biosyst Eng 26:177–184
Acknowledgements
This work was supported by FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (Grant Proc. 00/01383-0 and Grant Proc. 03/11722-5).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosa, J.C., Neto, A.B., Hokka, C.O. et al. Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus . Bioprocess Biosyst Eng 27, 99–104 (2005). https://doi.org/10.1007/s00449-004-0386-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-004-0386-9