Abstract
It is essential to evaluate the effects of operating conditions in submerged cultures of filamentous microorganisms. In particular, the impeller type influences the flow pattern, power consumption, and energy dissipation, leading to differences in the hydrodynamic environment that affect the morphology of the microorganism. This work investigated the effect of different impeller types, namely the Rushton turbine (RT-RT) and Elephant Ear impellers in up-pumping (EEUP) and down-pumping (EEDP) modes, on cellular morphology and clavulanic acid (CA) production by Streptomyces clavuligerus in a stirred-tank bioreactor. At 800 rpm and 0.5 vvm, the cultivations performed using RT-RT and EEUP impellers provided higher shear conditions and oxygen transfer rates than those observed with EEDP. These conditions resulted in higher clavulanic acid production using RT-RT (380.7 mg/L) and EEUP (453.3 mg/L) impellers, compared to EEDP (196.6 mg/L). Although the maximum CA concentration exhibited the same order of magnitude for RT-RT and EEUP impellers, the latter presented 40% of the specific power consumption (4.9 kW/m3) compared to the classical RT-RT (12.0 kW/m3). The specific energy for CA production (\({E}_{CA}\)), defined as the energy cost to produce 1 mg of CA, was 3.5 times lower using the EEUP impeller (1.91 kJ/mgCA) when compared to RT-RT (5.91 kJ/mgCA). Besides, the specific energy for O2 transfer (\({E}_{{O}_{2}}\)), the energy required to transfer 1 mmol of O2, was 2.3 times lower comparing the EEUP impeller (3.28 kJ/mmolO2) to RT-RT (7.65 kJ/mmolO2). The results demonstrated the importance of choosing the most suitable impeller configuration in conventional bioreactors to manufacture bioproducts.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many biotechnological products in the pharmaceutical and food industries are produced by submerged cultivation of strictly aerobic filamentous microorganisms in stirred-tank bioreactors. Streptomyces clavuligerus is an important producer of secondary metabolites, among them medical products such as cephamycin C and clavulanic acid (CA) [1,2,3]. The clinical importance of CA has resulted in extensive research focused on increasing its production using a variety of strategies involving aspects such as operating conditions [1, 2, 4], medium composition [5,6,7], fed-batch operation mode [8, 9], extractive fermentation [10], new microbial strains [11], and temperature-shift techniques [12, 13].
In submerged culture, S. clavuligerus presents variable patterns of growth, with its morphology including compact pellets, clumps, and branched and unbranched free filaments, which influence the rheology of the culture broth. The shapes and sizes of the filamentous growth forms are affected by many factors, including medium composition, dissolved oxygen concentration, reactor model, and power input [14], consequently influencing the production of the bioproducts of interest. Therefore, the investigation of bioprocessing strategies can greatly contribute to further improvements in S. clavuligerus submerged cultivations.
Although the effects of cell morphology on process productivity are widely studied for some submerged cultures of filamentous microorganisms, Aspergillus niger e.g. [14,15,16,17], few studies have evaluated the relationships among S. clavuligerus morphology, bioreactor hydrodynamics, and clavulanic acid production [4, 18]. S. clavuligerus submerged cultivation presents a complex rheology, with high broth viscosity [19], requiring a suitable agitation level to provide adequate mixing, oxygen mass transfer [1], and bioproduct formation. Although bioreactor operating conditions and impeller type determine oxygen transfer in stirred tanks, high shear conditions and hydrodynamic stress can affect microorganism growth and production in certain situations [20]. Therefore, the effect of operating conditions (agitation and aeration) in submerged culture must be carefully evaluated, since the impeller type selected will determine the hydrodynamic forces affecting the morphology of the microorganism [21, 22]. The hydrodynamic conditions define the flow pattern and are related to power consumption, energy dissipation, broth rheology, and bioreactor/impeller geometric parameters [20, 23]. Hence, the correct choice of impeller can improve the cultivation performance in terms of cell growth, and morphology, and biosynthesis of the product of interest.
The Rushton turbine (RT) is the most common impeller type used in microbial cultivations. Despite its advantages such as elevated oxygen transfer and good mixing, the Rushton turbine has high energy consumption [24, 25]. In this sense, it becomes interesting to evaluate the behavior of other impeller geometries, such as the so-called Elephant ear (EE) design, whose inclined blades promote a mixed flow pattern (axial/radial) in the culture broth, being indicated as suitable for the cultivation of shear-sensitive cells [26].
Bustamante et al. [27, 28] evaluated the in vitro performance of Rushton turbine (RT) and Elephant Ear (EE) impellers, with the latter in down-pumping (EEDP) and up-pumping (EEUP) configurations, in a conventional bench-scale stirred-tank bioreactor. It was observed that the EEDP impellers generated lower average shear rate (\({\dot{\upgamma }}_{{\mathrm{av}}}\)) values, while RT-RT and EEUP impellers exhibited similar orders of magnitude for shear conditions. Also, the EEUP impeller combined good oxygen mass transfer and adequate shear conditions.
Considering the earlier discoveries, the current study sought to assess how different impeller types (RT, EEDP, and EEUP), and their respective shear rate and oxygen transfer conditions, influence both cellular morphology and clavulanic acid production by Streptomyces clavuligerus in a conventional (stirred and aerated) tank bioreactor.
Materials and methods
Bioreactor and impellers
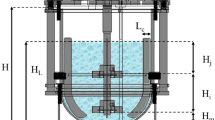
Cultivations were performed in a commercial stirred-tank bioreactor (Bioflo IIC fermenter, New Brunswick Scientific, USA) with a 4-L working volume, internal diameter (Dt) of 0.17 m, and four baffles. The dissolved oxygen concentration was measured using an amperometric probe (model InPro 6800, Mettler-Toledo). The bioreactor was equipped with pH and temperature control systems and was fitted with a stainless-steel ring gas sparger with 4 holes of 1 mm diameter.
Three impeller configurations were used in this study. The first consisted of two conventional six-flat-blade Rushton turbines (RT-RT), spaced one impeller diameter (di = 0.076 m) apart, with impeller blade width (W) of 0.016 m, length (L) of 0.019 m, disk diameter (d0) of 0.063 m, and thickness (T) of 0.0015 m (Fig. 1a). The second and third impeller configurations consisted of a single three-blade Elephant Ear (EE) impeller (New Brunswick Scientific, USA) with a diameter of 0.08 m and blade width (W) of 0.073 m. The second configuration was operated in the “up-pumping” mode (EEUP, Fig. 1b) while the third was in the “down-pumping” mode (EEDP, Fig. 1c).
Microorganism and culture media
The microorganism used in this work was Streptomyces clavuligerus ATCC 27064, stored as vegetative cells (5 g/L dry weight) at -70 ºC in 4 mL cryotubes containing glycerol (10% v.v−1).
The seed medium, proposed by Rosa et al. [1], had the following composition (g.L−1): glycerol (15.0); bacto peptone (10.0); malt extract (1.0); K2HPO4 (0.8); MgSO4.7H2O (0.75); MnCl2.4H2O (0.0001); FeSO4.7H2O (0.001); ZnSO4.7H2O (0.001); and 3-(N-morpholino) propane sulfonic acid (MOPS) buffer (21.0) at pH 6.8. The inoculum medium was based on that used by Teodoro et al. [9] and contained (g.L−1): glycerol (15.0); soybean protein isolate (25.0); K2HPO4 (0.8); MgSO4.7H2O (0.75); MnCl2.4H2O (0.0001); FeSO4.7H2O (0.001); ZnSO4.7H2O (0.001); and 3-(N-morpholino) propane sulfonic acid buffer (21.0) at pH 6.8. The production medium had the same composition as the inoculum medium, except that no MOPS buffer was used. All the media were autoclaved at 121 ºC for 15 min.
Batch cultivations
Vegetative cell suspensions were transferred from the cryotubes to 500 mL Erlenmeyer flasks containing 50 mL of seed medium, followed by incubation for 24 h in a rotary shaker at 250 rpm and 30 ºC. Erlenmeyer flasks (500 mL) containing 45 mL of the inoculum medium were inoculated with 5 mL of cultivated seed broth and incubated for 24 h at 250 rpm and 30 ºC.
For the production stage, 400 mL of the inoculum suspension were transferred to the bioreactor containing the production medium, resulting in a 4-L working volume. Three batch cultivations, each with a different impeller, were performed in duplicate, at 800 rpm and 0.50 vvm. The operating conditions were kept constant to assess only the impact of impeller design in the cultivations. The temperature was kept at 30 ºC and the pH was controlled at 6.8±0.1 by the addition of 1 M NaOH and 2 M HCl solutions. An antifoaming agent (30% m.m−1, Dow Corning Co.) was used to avoid foam formation. Samples (25 mL) were withdrawn every 6 h for analysis of cell morphological parameters, cell growth, and the concentrations of clavulanic acid (CA) and glycerol.
Analytical methods
Cell growth was evaluated indirectly by measuring the broth consistency index (K) of the power law model (Eq. 1) which relates the rheological variables shear stress (\(\tau\)) and shear rate (\(\dot{\gamma }\)). In cultivations using filamentous microorganisms, K is mainly affected by biomass concentration, growth rate, and cellular morphology [2, 29, 30].
where \(\tau\) is the shear stress (Pa), \(K\) is the broth consistency index (Pa.sn), \(\dot{\gamma }\) is the shear rate (s−1), and \(n\) is the broth flow index (-).
The broth rheological parameters \(K\) and \(n\) were determined from rheograms (\(\tau\) vs \(\dot{\gamma }\)) of samples of the cultivation broths using a digital concentric-cylinders rheometer (model LV-DVIII + , Brookfield Engineering Laboratories Inc., USA).
The concentrations of clavulanic acid and glycerol were determined in the supernatants of broth samples centrifuged at 17,500·g for 5 min, at 4 ºC, and filtered through a 0.22 µm membrane.
The clavulanic acid concentrations (CCA) were determined by UV spectrophotometric analysis [31], which consisted of reading at the 311 nm wavelength of the product of the derivation of clavulanic acid with the imidazole reagent. The standard used was CA contained in the pharmaceutical product Clavulin® (Glaxo-SmithKline Farmacêutica, Rio de Janeiro, Brazil).
The glycerol concentrations (CGly) were determined by an enzymatic method, using a triglycerides GPO-PAP test kit (Laborlab, Brazil).
Image analysis
The cultivation broth samples were diluted using distilled water, fixed, and stained with methylene blue, as described by Pamboukian et al. [32]. The dilution resulted in a suitable cell concentration for observation using an optical microscope. The cell morphology parameters were measured using Image-Pro Plus 6.0 software and an optical microscope (Olympus BX-50) operated at magnifications of 10 × and 20x [32]. A total of 50 images with approximately 200 and 300 elements were captured for each sample, including pellets (P), clumps (C), branched hyphae (BH), and unbranched hyphae (UH). The images were recorded in JPG format, with a resolution of 2048 × 1536 pixels.
Volumetric oxygen transfer coefficient (\({{\mathrm{k}}}_{{\mathrm{L}}}{\mathrm{a}})\) and average shear rate \(\left({\dot{\upgamma }}_{{\mathrm{av}}}\right)\)
The \({{\mathrm{k}}}_{{\mathrm{L}}}{\mathrm{a}}\) and \({\dot{\gamma }}_{av}\) values for the stirred-tank bioreactor equipped with different impellers (RT-RT, EEDP, and EEUP) were determined using the correlations proposed by Bustamante et al. [27, 28]:
where N is the impeller speed (N = 800 rpm) and ϕair is the specific air flow rate (ϕair = 0.50 vvm).
Knowing the profiles for kLa and the dissolved oxygen concentration in the broth (\({C}_{{O}_{2}}\)) along the S. clavuligerus cultivations, the oxygen transfer rate (OTR) could be calculated using Eq. 8:
where, \({C}_{{O}_{2}}^{*}\) and \({C}_{{O}_{2}}\) are the oxygen solubility in the medium (mol.L−1) and the oxygen concentration in the medium (mol.L−1), respectively.
Gassed power consumption (Pg)
The gassed power consumption (Pg, in W), at 800 rpm and 0.50 vvm, was calculated as the product of the torque required for the agitation of the gas-liquid dispersion (T, in N.m) and the angular velocity of the agitator shaft (\(\upomega =2\cdot\uppi \cdot {\mathrm{N}}\), in rad.s−1). The torque was calculated as the product of the force (F, in N) and the length of the arm (b = 0.175 m) connecting to the motor shaft (\({\mathrm{T}}=F\cdot {\mathrm{b}}\)) [33]. The gassed power consumption was then calculated using Eq. (9):
The motor was placed over a vertical ball bearing allowing torque measurement. The force (F) was measured in triplicate using a digital dynamometer (FG 6005SD, Lutron, accuracy of 0.4% full scale) attached to the arm coupled to the motor shaft.
The gassed power consumption was measured using a solution of glycerol (67% v.v−1) as a model fluid. This solution was chosen because it had a viscosity (0.0015 Pa.s) similar to the average apparent viscosity observed for the culture broths during the S. clavuligerus cultivations (data not shown).
Results and discussion
Effect of impeller type on clavulanic acid production
The influence of the impeller configuration on clavulanic acid production was evaluated by performing three cultivations at 800 rpm and 0.50 vvm in the stirred-tank bioreactor.
Figure 2 shows the experimental values of the clavulanic acid concentration (CCA), broth consistency index (K), and dissolved oxygen in the broth (DO) during the cultivations. In the cultivation performed with the RT-RT impeller (Fig. 2a), the clavulanic acid concentration reached a maximum value (380.7 mg.L−1) at 54 h. The cultivation performed with the EEUP impeller (Fig. 2b) exhibited the highest CCA value among the three assays (453.3 mg.L−1), at 45 h of cultivation. In these two assays (RT-RT and EEUP), no oxygen limitation was observed, since the dissolved oxygen levels were higher than 42% of air saturation throughout the cultivations. The EEUP cultivation exhibited higher and faster clavulanic acid production, compared to the RT-RT cultivation, which also indicated higher clavulanic acid productivity (Table 1).
Clavulanic acid concentration (CCA), broth consistency index (K), and dissolved oxygen (DO) during the S. clavuligerus cultivations at 800 rpm and 0.50 vvm in the stirred-tank bioreactor equipped with different impellers: (a) Rushton turbines (RT-RT), (b) Elephant Ear in “up-pumping” operating mode (EEUP), and (c) Elephant Ear in “down-pumping” operating mode (EEDP)
In the cultivation performed with the EEDP impeller, dissolved oxygen reached a critical value of about 10% at 21 h (Fig. 2c), which probably explained the low clavulanic acid production, with a maximum value of 196.6 mg.L−1 at 45 h. Low clavulanic acid productivity was also observed for this impeller configuration (Table 1). Rosa et al. [1], who performed S. clavuligerus cultivations at low agitation speeds (300 and 600 rpm) and 0.50 vvm, also observed that oxygen limitation led to low (or no) clavulanic acid production.
Based on the maximum clavulanic acid production values (\({C}_{CA}^{max}\)) and the time required to achieve that condition, the clavulanic acid productivity (\({P}_{CA}\), in mg.L−1.h−1) was calculated for the three cultivations (RT-RT, EEUP, and EEDP) and compared to literature data, as shown in Table 1. All the values were obtained for the same operating conditions (800 rpm and 0.50 vvm). For the RT-RT and EEUP configurations, clavulanic acid production and productivity were of similar orders of magnitude as values reported previously.
It is important to highlight that the only difference among the cultivations in the present study was in the utilization of different impeller configurations (RT-RT, EEUP, and EEDP), which led to differences in flow patterns and oxygen transfer (Fig. 3) [26, 34].
Expected fluid flow fields in the 4-L stirred-tank bioreactor equipped with different impellers: (a) Rushton turbines (RT-RT), (b) Elephant Ear in “up-pumping” operating mode (EEUP), and (c) Elephant Ear in “down-pumping” operating mode (EEDP).
Effect of impeller type on S. clavuligerus morphology
In submerged cultures, the mycelial cell morphology of the microorganism can significantly affect cultivation performance [35]. The effect of impeller configuration on S. clavuligerus morphology was investigated in three cultivations using the RT-RT, EEUP, and EEDP impeller configurations. Four morphological classes, namely pellets (P), clumps (C), branched hyphae (BH), and unbranched hyphae (UH), were characterized and quantified by image analysis. These morphologies are illustrated in Fig. 4.
Based on the quantification of each morphological class (P, C, BH, and UH) and its contribution to the total quantity (sum of all the classes), a morphological class distribution (in %) was obtained during each cultivation (Fig. 5). The initial morphological class distribution was similar for the three cultivations, consisting of about 12.3% of pellets, 56.2% of clumps, and 31.5% of branched hyphae. No unbranched hyphae were observed at the beginning of the cultivations.
Distribution of morphological classes (in %) during the S. clavuligerus cultivations at 800 rpm and 0.50 vvm in a 4-L working volume stirred-tank bioreactor equipped with different impellers: (a) Rushton turbines (RT-RT), (b) Elephant Ear in “up-pumping” operating mode (EEUP), and (c) Elephant Ear in “down-pumping” operating mode (EEDP). Legend: pellets (P), clumps (C), branched hyphae (BH), and unbranched hyphae (UH)
The behavior observed for the variation of the morphological classes, shown in Fig. 5, could be summarized as follows:
-
The number of pellets decreased rapidly, with no pellets observed at 12, 3, and 15 h in the cultivations using the RT-RT, EEUP, and EEDP impellers, respectively.
-
The number of clumps decreased until reaching stable values around 7.3% (at 15 h), 12.0% (at 39 h), and 19.2% (at 21 h) in the cultivations using the RT-RT, EEUP, and EEDP impellers, respectively. These dynamics resulted from a complex relationship among pellet fragmentation (leading to increased clumps), hyphae formation (leading to decreased clumps), and cell multiplication.
-
The branched and unbranched hyphae distributions increased in all three cultivations, exhibiting different dynamics and reaching fairly stable values for the different impellers.
Despite the complex relationship among morphology, broth rheology, and clavulanic acid production, the cultivation broth exhibited higher shear rates in the cultivations using the RT-RT and EEUP impellers, compared to the EEDP cultivations. This was supported by the following observations:
-
Higher clavulanic acid production was obtained in the RT-RT and EEUP cultivations, compared to the EEDP cultivation. This could be expected since clavulanic acid production is positively affected by the shear rate [4]. As reported by Rosa et al. [1], a high shear rate leads to greater cell wall disruption, releasing clavulanic acid from the periplasmic space between the membrane and the cell wall, consequently increasing the CA concentration in the medium.
-
The stable value for clumps in the EEDP cultivation, of around 19.2%, was higher than in the other cultivations (RT-RT and EEUP).
-
The hyphae distribution (branched and unbranched) in the EEDP cultivation, of about 80.8%, was lower than in the RT-RT (92.7%) and EEUP (88.0%) cultivations.
Finally, the dynamics of the number of hyphae (branched and unbranched, BH + UH) were similar in the RT-RT and EEUP cultivations, with the hyphae numbers becoming almost constant after a certain cultivation time (Fig. 6). The BH + UH values became almost stable at 24 h and 39 h in the RT-RT and EEUP cultivations, respectively.
Branched and unbranched hyphae (BH + UH) distributions and broth consistency index (K) values during the S. clavuligerus cultivations conducted at 800 rpm and 0.50 vvm in a stirred-tank bioreactor with a working volume of 4-L, using Rushton turbines (RT-RT) and Elephant Ear “up-pumping” operating mode (EEUP) impeller. The solid lines indicate the general trends for variation of the BH + UH values
A comparison of the BH + UH profiles with the corresponding broth consistency index (K) profiles showed that there was a direct relationship between the stabilization of the hyphae distribution and the maximum consistency index value (Fig. 6). This behavior could explain the higher clavulanic acid production observed in the EEUP cultivation (453.3 mg.L−1), compared to the RT-RT cultivation (380.7 mg.L−1). After stabilization, both EEUP and RT-RT achieved similar BH + UH values (88.0 and 92.7%, respectively). However, distinct dynamics were observed. While RT-RT provided faster fragmentation kinetics (stable BH + UH values at 24 h), EEUP exhibited a slower fragmentation with stabilization of BH + UH values at 39 h. This behavior allowed reaching a higher cell concentration in EEUP cultivation, indicated by the higher K values, when compared to RT-RT, resulting in higher CA production. After the maximum value of the consistency index (K) is reached, a decrease in cultivation occurred due to cell lysis as reported by Cerri and Badino (2012) [2].
Therefore, it could be concluded that the use of different impellers led to differences in the morphological and rheological behaviors, which affected the production of clavulanic acid.
Effect of oxygen transfer and power consumption on clavulanic acid production
The utilization of different impellers (RT-RT, EEUP, and EEDP) led to differences in clavulanic acid production and productivity, as a result of different oxygen transfer rates and shear levels (Table 2). Ribeiro et al. [4] performed S. clavuligerus cultivations for evaluation of the isolated effects of average shear rate (\({\dot{\gamma }}_{av}\)) and initial oxygen transfer rate (OTRinitial) on CA productivity (\({P}_{CA}\)), using a stirred-tank bioreactor equipped with RT-RT impellers. It was observed that both \({\dot{\gamma }}_{av}\) and OTRinitial had a positive effect on \({P}_{CA}\), with higher initial OTR values resulting in faster clavulanic acid production, while higher shear rates increased the maximum CA concentration. It was found that \({\dot{\gamma }}_{av}\) had the greatest influence on \({P}_{CA}\).
The shear and oxygen transfer rate values were similar in the cultivations performed with the RT-RT and EEUP impellers, while lower values were obtained in the cultivation using the EEDP impeller. These findings hold particular significance given the variation in the number of impellers installed in the stirred tank bioreactor. While RT-RT consisted of a dual-impeller system, EEUP and EEDP were operated in a single impeller mode. Despite of this, RT-RT and EEUP promoted quite similar variations in the morphological classes and shear rates. Buffo et al. [15] employed A. niger pellets to assess shear conditions in a stirred tank bioreactor equipped with different dual-impeller associations: a two Rushton turbines system (RT-RT) and a two Elephant Ear system (EEDP-EEUP). Through pellet fragmentation assays, the authors observed no significant differences between the evaluated configurations.
Waldherr et al. [36] assessed the fragmentation of A. niger pellets under non-growing conditions in a 3-L stirred tank bioreactor equipped with different impellers, including the Rushton turbine (RT), propeller (Prop), and wave-ribbon (WRI). By conducting the fragmentation assay at a specific power input equal to 0.59 W/kg, the authors verified the following trend in pellet size reduction: WRI > Prop > RT. This outcome highlights the Rushton turbines as the least shearing impeller among those examined.
Buffo et al. [37] employed morphology engineering to boost cellulolytic enzyme production by A. niger. By manipulating parameters like spore concentration and pH, diverse initial inoculum morphologies were achieved, ranging from pellets of various sizes to fully dispersed inoculum. Subsequent cultivations took place in a 4-L stirred tank bioreactor equipped with two impeller configurations: RT-RT or EEDP-EEUP. Irrespective of the inoculum morphology and the operating conditions, cultivations with the Elephant Ear impeller association consistently exhibited higher endoglucanase activity.
Oliveira et al. [38] examined the impact of impeller type utilizing the same impeller associations as employed by Buffo et al. [15] (two Rushton turbines (RT-RT) and two Elephant Ear impellers (EEDP-EEUP)) on the production of red colorant by Talaromyces amestolkiae in a 4-L stirred-tank bioreactor. The authors assessed the oxygen transfer coefficient using a non-growing medium composed of monosodium glutamate-glucose (without fungi). At 100 rpm and a specific airflow rate ranging from 0.5 to 2.0 vvm, the EEDP-EEUP association demonstrated higher kLa values. Following this, cultivations were carried out at 100 rpm and 2.0 vvm and the Elephant Ear association promoted the highest production of red colorant. The morphology of T. amestolkiae also exhibited variation based on the impeller association: dense and spherical pellets were observed with RT-RT, whereas the EEDP-EEUP association facilitated the formation of hairy pellets, predominantly characterized by an increased number of long hyphae.
The higher \({\dot{\gamma }}_{av}\) values for the RT-RT and EEUP cultivations resulted in higher CA production and productivity (Table 2), with the EEUP cultivation presenting the highest \({C}_{CA}^{max}\) and \({P}_{CA}^{max}\). This result by itself showed the suitability of using EEUP impellers for clavulanic acid production by S. clavuligerus. The results achieved using the EEUP configuration became even more impressive when the energy requirement for the agitation provided by the impellers was taken into consideration. The average power consumption (\({{\mathrm{P}}}_{{\mathrm{g}},{\mathrm{av}}}\)) during the S. clavuligerus cultivations was highest for RT-RT, with a value around 2.5 and 4 times higher than for EEUP and EEDP, respectively (Table 2). The lower power input for Elephant Ear impellers compared to the Rushton turbines was consistent with previous studies [39, 40].
The relation between power consumption and clavulanic acid productivity, defined by the specific energy for CA production (\({{E}_{CA}=P}_{g,av}/{(P}_{CA}^{max}\cdot V\))), expresses the energy cost to produce 1 mg of clavulanic acid. Here, the EEUP configuration required 3.5 times less energy, compared to the use of the RT-RT impeller (Fig. 7).
Values of specific energy for clavulanic acid production (E_CA) and O2 transfer (E_(O_2)) in the S. clavuligerus cultivations at 800 rpm and 0.50 vvm in a stirred-tank bioreactor with 4-L working volume, equipped with different impellers. Legend: RT-RT: Rushton turbines; EEUP: Elephant Ear in “up-pumping” operating mode; EEDP: Elephant Ear in “down-pumping” operating mode
Regarding oxygen transfer, the relation between power consumption and the average oxygen transfer rate, defined by the specific energy for O2 transfer (\({{E}_{{O}_{2}}=P}_{g,av}/{(OTR}_{av}\cdot V)\)), expresses the energy cost to transfer 1 mmol of O2 from the gas phase to the liquid phase. The RT-RT and EEUP configurations presented similar average oxygen transfer rates during the cultivations (Table 2), with the RT-RT impeller requiring more energy to transfer 1 mmol of oxygen to the culture media, compared to the EEUP impeller (Fig. 7).
The findings collectively suggest that employing the EEUP impeller yields superior performance in clavulanic acid production and energy efficiency, particularly when contrasted with the RT-RT impeller at 800 rpm and 0.5 vvm.
Conclusions
Clavulanic acid production was higher using the RT-RT (380.7 mg.L−1) and EEUP (453.3 mg.L−1) impellers, compared to the EEDP impeller (196.6 mg.L−1), showing that a change in impeller configuration, on its own, can result in a different level of clavulanic acid production. The branched and unbranched hyphae distribution exhibited a direct relationship with broth consistency index variation during the cultivations using the RT-RT and EEUP configurations, demonstrating that the use of different impellers modified the morphological and rheological behaviors, consequently affecting clavulanic acid production. The single EEUP impeller required 3.5 times less energy to produce clavulanic acid, as well as half the energy to transfer oxygen, when compared to the dual RT-RT impeller, and exhibited the best relation between clavulanic acid production and energy cost, when compared to the RT-RT and EEDP impellers, at 800 rpm and 0.5 vvm. The combined clavulanic acid production and energy requirements criteria provide an important parameter for the proper impeller selection and will impact the scale-up strategy.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
Rosa JC, Neto AB, Hokka CO, Badino AC (2005) Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst Eng 27(2):99–104
Cerri MO, Badino AC (2012) Shear conditions in clavulanic acid production by Streptomyces clavuligerus in stirred tank and airlift bioreactors. Bioprocess Biosyst Eng 35(6):977–984
Mayer AF, Deckwer WD (1996) Simultaneous production and decomposition of clavulanic acid during Streptomyces clavuligerus cultivations. Appl Microbiol Biotechnol 45(1–2):41–46
Ribeiro R, Esperanca MN, Sousa APA, Neto AB, Cerri MO (2021) Individual effect of shear rate and oxygen transfer on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst Eng 44(8):1721–1732
Maranesi GL, Baptista-Neto A, Hokka CO, Badino AC (2005) Utilization of vegetable oil in the production of clavulanic acid by Streptomyces clavuligerus ATCC 27064. World J Microbiol Biotechnol 21(4):509–514
Ortiz SCA, Hokka CO, Badino AC (2007) Utilization of soybean derivatives on clavulanic acid production by Streptomyces clavuligerus. Enzyme Microb Technol 40(5):1071–1077
Feng T, Zhao J, Chu J, Wang YH, Zhuang YP (2021) Statistical optimizing of medium for clavulanic acid production by streptomyces clavuligerus using response surface methodology. Appl Biochem Biotechnol 193(12):3936–3948
Teodoro JC, Baptista-Neto A, Cruz-Hernandez IL, Hokka CO, Badino AC (2006) Influence of feeding conditions on clavulanic acid production in fed-batch cultivation with medium containing glycerol. Appl Microbiol Biotechnol 72(3):450–455
Teodoro JC, Baptista-Neto A, Araujo M, Hokka CO, Badino AC (2010) Influence of glycerol and ornithine feeding on clavulanic acid production by Streptomyces clavuligerus. Braz J Chem Eng 27(4):499–506
Costa CLL, Badino AC (2015) Overproduction of clavulanic acid by extractive fermentation. Electron J Biotechnol 18(3):154–160
Li RF, Townsend CA (2006) Rational strain improvement for enhanced clavulanic acid production by genetic engineering of the glycolytic pathway in Streptomyces clavuligerus. Metab Eng 8(3):240–252
Rodrigues KCS, Costa CLL, Badino AC, Pedrolli DB, Pereira JFB, Cerri MO (2019) Application of acid and cold stresses to enhance the production of clavulanic acid by streptomyces clavuligerus. Appl Biochem Biotechnol 188(3):706–719
Feng T, Zhao J, Bai YF, Chu J, Wang YH, Zhuang YP (2021) Effect of temperature on synthesis of clavulanic acid and impurity substance G during fermentation by Streptomyces clavuligerus. Prep Biochem Biotechnol 52(8):937–941
El-Enshasy H, Hellmuth K, Rinas U (1999) Fungal morphology in submerged cultures and its relation to glucose oxidase excretion by recombinant Aspergillus niger. Appl Biochem Biotechnol 81(1):1–11
Buffo MM, Esperança MN, Farinas CS, Badino AC (2020) Relation between pellet fragmentation kinetics and cellulolytic enzymes production by Aspergillus niger in conventional bioreactor with different impellers. Enzyme Microbial Technol 139:109587
Bocking SP, Wiebe MG, Robson GD, Hansen K, Christiansen LH, Trinci APJ (1999) Effect of branch frequency in Aspergillus oryzae on protein secretion and culture viscosity. Biotechnol Bioeng 65(6):638–648
Papagianni M, Moo-Young M (2002) Protease secretion in glucoamylase producer Aspergillus niger cultures: fungal morphology and inoculum effects. Process Biochem 37(11):1271–1278
Yepes-García J, Caicedo-Montoya C, Pinilla L, Toro LF, Ríos-Estepa R (2020) morphological differentiation of streptomyces clavuligerus exposed to diverse environmental conditions and its relationship with clavulanic acid biosynthesis. Processes 8(9):1038
Olmos E, Mehmood N, Husein LH, Goergen JL, Fick M, Delaunay S (2013) Effects of bioreactor hydrodynamics on the physiology of Streptomyces. Bioprocess Biosyst Eng 36(3):259–272
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27(2):153–176
Justen P, Paul GC, Nienow AW, Thomas CR (1996) Dependence of mycelial morphology on impeller type and agitation intensity. Biotechnol Bioeng 52(6):672–684
Chisti Y (2001) Hydrodynamic damage to animal cells. Crit Rev Biotechnol 21(2):67–110
Kumaresan T, Joshi JB (2006) Effect of impeller design on the flow pattern and mixing in stirred tanks. Chem Eng J 115(3):173–193
Okabe M, Kuwajima T, Satoh M, Kimura K, Okamura K, Okamoto R (1992) Preferential and high-yield production of a Cephamycin-C by dissolved-oxygen controlled fermentation. J Ferment Bioeng 73(4):292–296
McFarlane CM, Nienow AW (1995) Studies of high solidity ratio hydrofoil impellers for aerated bioreactors. 1. Review. Biotechnol Progress 11(6):601–607
Zhu H, Nienow AW, Bujalski W, Simmons MJH (2009) Mixing studies in a model aerated bioreactor equipped with an up- or a down-pumping “Elephant Ear” agitator: Power, hold-up and aerated flow field measurements. Chem Eng Res Des 87(3A):307–317
Bustamante MCC, Cerri MO, Badino AC (2013) Comparison between average shear rates in conventional bioreactor with Rushton and Elephant ear impellers. Chem Eng Sci 90:92–100
Bustamante MCC, Cerri MO, Badino AC (2014) Comparison between average shear rates in conventional bioreactor with Rushton and Elephant ear impellers (vol 90, pg 92, 2013). Chem Eng Sci 107:328–328
Allen DG, Robinson CW (1990) Measurement of rheological properties of filamentous fermentation broths. Chem Eng Sci 45(1):37–48
Olsvik E, Kristiansen B (1994) Rheology of filamentous fermentations. Biotechnol Adv 12(1):1–39
Bird AE, Bellis JM, Gasson BC (1982) Spectrophotometric assay of clavulanic acid by reaction with imidazole. Analyst 107(1279):1241–1245
Pamboukian CRD, Guimaraes LM, Facciotti MCR (2002) Applications of image analysis in the characterization of Streptomyces olindensis in submerged culture. Braz J Microbiol 33(1):17–21
Badino AC, Barboza M, Hokka CO (1994) Power input and oxygen transfer in fed-batch penicillin production process. In: Galindo E, Ramírez OT (eds) Advances in bioprocess engineering. Springer, pp 157–162
Doran PM (2013) Chapter 8. Mixing. In: Doran PM (ed) Bioprocess engineering principles, 2nd edn. Elsevier, pp 261–281
Kim HJ, Kim JH, Oh HJ, Shin CS (2002) Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochem 38(5):649–655
Waldherr P, Bliatsiou C, Böhm L, Kraume M (2023) Fragmentation of Aspergillus niger pellets in stirred tank bioreactors due to hydrodynamic stress. Chem Eng Res Des 195:116–131
Buffo MM, Ferreira ALZ, Almeida RMRG, Farinas CS, Badino AC, Ximenes EA, Ladisch MR (2021) Cellulolytic enzymes production guided by morphology engineering. Enzyme Microb Technol 149:109833
Oliveira F, Zapata-Boada S, Silva SS, Cuéllar-Franca RM, Santos-Ebinuma VC (2022) Improving the environmental sustainability of polyketides colorants production by Talaromyces strain through better hydrodynamic design in bioreactors. ACS Sustain Chem Eng 10:14136–14150
Wang H, Duan X, Feng X, Mao Z-H, Yang C (2022) Effect of impeller type and scale-up on spatial distribution of shear rate in a stirred tank. Chin J Chem Eng 42:351–363
Botlagunta M, Vinay R, Pardhasaradhi M (2022) Oxygen mass transfer coefficient and power consumption in a conventional stirred-tank bioreactor using different impellers in a non-newtonian fluid: an experimental approach. Iran J Chem Chem Eng 41(2):533–543
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES, Finance Code 001), the National Council for Scientific and Technological Development (CNPq, grant 454295/2009-5), and the São Paulo State Research Foundation (FAPESP, grant 2011/23807-1).
Author information
Authors and Affiliations
Contributions
Maritza C. C. Bustamante: Conceptualization; Data curation; Formal analysis; Investigation; Methodology. Cecília L. L. Costa: Conceptualization; Data curation; Formal analysis; Investigation; Methodology. Mateus N. Esperança: Data Curation; Formal analysis; Investigation; Visualization; Roles/Writing—Original Draft; Writing—Review & Editing. Marcel O. Cerri: Data Curation; Formal analysis; Investigation; Visualization; Roles/Writing—Original Draft; Writing—Review & Editing. Vitor T. Mazziero: Visualization; Roles/Writing—Original Draft. Alberto C. Badino: Conceptualization; Formal analysis; Funding acquisition; Project administration; Resources; Supervision; Validation; Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Luis Henrique Souza Guimaraes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bustamante, M.C.C., Costa, C.L.L., Esperança, M.N. et al. Effect of impeller type on cellular morphology and production of clavulanic acid by Streptomyces clavuligerus. Braz J Microbiol 55, 1167–1177 (2024). https://doi.org/10.1007/s42770-024-01306-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01306-0