Abstract
Migratory shorebirds inhabit environments that may yield contrasting salinity-temperature regimes—with widely varying osmoregulatory demands, even within a given species—and the question is: by which physiological means and at which organisational level do they show adjustments with respect to these demands? Red knots Calidris canutus winter in coastal areas over a range of latitudes. The nominal subspecies winters in salty areas in the tropics, whereas the subspecies Calidris canutus islandica winters in north-temperate regions of comparatively lower salinities and temperatures. In this study, both subspecies of red knot were acclimated to different salinity (28/40 ‰)–temperature (5/35 °C) combinations for 2-week periods. We then measured food/salt intakes, basal metabolic rate (BMR), body mass and temperature, fat and salt gland scores, gizzard mass, heat-shock proteins, heterophils/lymphocytes (H/L) ratio and plasma Na+ to assess the responses of each taxon to osmoregulatory challenges. High salinity (HS)-warm-acclimated birds reduced food/salt intake, BMR, body mass, fat score and gizzard mass, showing that salt/heat loads constrained energy acquisition rates. Higher salt gland scores in saltier treatments indicated that its size was adjusted to higher osmoregulatory demands. Elevated plasma Na+ and H/L ratio in high-salinity-warm-acclimated birds indicated that salt/heat loads might have a direct effect on the water-salt balance and stress responses of red knots. Subspecies had little or no effect on most measured parameters, suggesting that most adjustments reflect phenotypic flexibility rather than subspecific adaptations. Our results demonstrate how salinity and temperature affect various phenotypic traits in a migrant shorebird, highlighting the importance of considering these factors jointly when evaluating the environmental tolerances of air-breathing marine taxa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osmotic homeostasis, i.e. the maintenance of a relatively constant salt and water balance in the body fluids, is one of the best-defended physiological states in vertebrates (Evans 2009; Bradley 2009). Amphibians, reptiles, birds, and mammals typically have blood concentrations of around 250–300 mOsm to ensure proper functioning of cells (Bradley 2009). The problem of maintaining the internal environment within fairly well-defined limits varies with the degree of gain or loss of salts and water as a result of environmental pressures (Phillips and Ensor 1972). Therefore, the ability to identify conditions when osmotic homeostasis is not achieved, or is compromised in the face of conflicting ecological demands, could be a means to identify periods of environmental stress (Gutiérrez 2014).
In marine and other saline environments, organisms may face problems of water conservation because of high salt concentration. Moreover, these problems could be compounded by factors such as temperature, which additionally demands physiological and behavioural adjustments to maintain homeostasis in terms of salt and water balance (e.g. Phillips and Ensor 1972; Skadhauge 1981; Battley et al. 2003; Gutiérrez et al. 2012). In nature, salinity and temperature often vary in concert. In considering the potential impacts of climate-induced changes in these (and other) abiotic factors, it is important to understand their influence on the physiological performance of organisms and, ultimately, on fitness (e.g. Pörtner 2002; Bozinovic et al. 2011). Nevertheless, the combined effects of these abiotic factors have been largely overlooked in aquatic air-breathing vertebrates. By using large-scale biogeographical analyses and environmental tolerances, Brischoux et al. (2012, 2013a) showed that salinity and temperature influence the distribution and dispersal of marine snakes (Laticauda spp.), with the species with the more efficient salt glands and lower dehydration rates exploiting more saline oceanic areas.
This may also apply to shorebirds (Charadriiformes, suborders Charadrii and Scolopaci) undertaking seasonal migrations between the far north and further south (e.g. van de Kam et al. 2004), during which they are subject to large changes in the salinity and temperature of their experienced environments. Outside the breeding season, the species that opt to winter in coastal habitats (Piersma 1997) feed on marine invertebrates [typically isosmotic to surrounding seawater (Prusch 1983)] and have limited or no access to freshwater (Gutiérrez 2014). To cope with salt loads, shorebirds—like marine birds—use renal and extrarenal mechanisms which jointly ensure the excretion of excess salt without a net loss of water (Peaker and Linzell 1975; Goldstein and Skadhauge 2000; Gutiérrez 2014). The phenotypic flexibility in osmoregulatory structures (e.g. supraorbital salt glands) is critically important in allowing migratory shorebirds to successfully overcome the osmoregulatory challenges faced in the course of their annual cycles (Gutiérrez et al. 2012). Nevertheless, little is known about the physiological and ecological consequences of living in saline environments and the situations in which osmotic homeostasis is compromised (Gutiérrez 2014).
For example, it has been shown that two shorebird species (red knots Calidris canutus and sanderlings Calidris alba) wintering at Banc d’Arguin in Mauritania, a coastal wetland on the boundary between the Sahara and the Atlantic Ocean, reduced their food intake when switched from fresh to seawater under experimental conditions, suggesting that salinity may constrain food intake and thus energy acquisition (Klaassen and Ens 1990). Indeed, recent work on red knots has shown that individuals maintained on seawater have lower food and water consumption rates than those having freshwater available (Oudman et al. 2014). In addition, there is evidence that an increase in salinity substantially increases the basal metabolic rate (BMR) of dunlins Calidris alpina, which demonstrates that osmoregulation entails significant energetic costs (Gutiérrez et al. 2011; see also Nelhs 1996 and Peña-Villalobos et al. 2013). Other studies have also shown that shorebirds can suffer adverse effects when living in saline environments (e.g. Purdue and Haines 1977; Hannam et al. 2003; Gutiérrez et al. 2013). However, salinity is not the only factor challenging osmotic homeostasis. By affecting physiological routes of heat/water loss, temperature also exerts a considerable influence on the osmoregulatory performance of birds (e.g. Phillips and Ensor 1972; Skadhauge 1981; Verboven and Piersma 1995; Gutiérrez et al. 2012). At similar salinities, the salt gland mass of red knots increases at both low and high temperatures, which probably reflects increased energy demands at low temperatures (i.e. higher food/salt intake) and elevated water loss at high temperatures (Gutiérrez et al. 2012). Taken together, these results reveal that changes in both salinity and temperature can have significant effects on shorebirds’ physiological state and performance.
Long-distance migrant shorebirds breeding in the high Arctic and wintering along tropical coasts may encounter a greater range of conditions (e.g. in terms of salinity and temperature) compared with temperate-wintering birds. An interesting contrast in this respect is provided by two subspecies of red knots: Calidris canutus islandica breeds in high arctic Canada and winters in temperate coasts of western Europe, whereas Calidris canutus canutus breeds in high arctic Siberia and winters in hot and salty (sub)tropical coasts in West and South Africa (Piersma et al. 2005; Piersma 2007; Piersma and van Gils 2011). In the western Wadden Sea, the main wintering site for C. canutus islandica, the average salinity is typically about 28 ‰ (based on daily water salinity recorded throughout this experiment) and winter mean air temperature during the coldest month (February) is about 3 °C [based on monthly ambient temperature recorded over 29 years (see Vézina et al. 2006)]. At the Banc d’Arguin, the main wintering site for C. canutus canutus, the average salinity is about 40 ‰ [with values over 50 ‰ in some areas where strong evaporation occurs (Wolff and Smit 1990; Lavaud et al. 2013)] and the average air temperature is around 25 °C [with values over 40 °C during the daylight period (Wolff and Smit 1990)]. Because their osmoregulatory and thermoregulatory demands during the winter must differ widely, birds of these subspecies might either show ecophysiological adaptions at the level of the subspecies, or ‘just’ show general flexible responses to environmental conditions (Piersma et al. 1996).
In this study, we exposed these two red knot subspecies, under controlled captive conditions, to different salinity–temperature combinations: high salinity (HS)–warm (40 ‰, 35 °C); HS–cold (40 ‰, 5 °C); LS–warm (28 ‰, 35 °C); and LS–cold (28 ‰, 5 °C). Such combinations can be encountered in the wild and should elicit different osmoregulatory (e.g. Gutiérrez et al. 2011, 2012) and thermoregulatory (e.g. Vézina et al. 2006) responses. To capture a broad picture of the physiological state and performance, we measured a suite of physiological and morphological parameters:
-
1.
Food intake has profound implications for the relative intakes of water and salts, and thus, for an animal’s osmoregulatory responses (reviewed by Gutiérrez 2014).
-
2.
The sizes of the gizzard and the salt glands are indicators of relative food intake (van Gils et al. 2003, 2005a, c) and concentrating ability (Staaland 1967; Gutiérrez et al. 2012), respectively.
-
3.
Data on BMR, fat stores, body mass and temperature provide valuable information on an animal’s nutritional and energetic state (Butler and Woakes 2001; McNab 2002); also, changes in BMR may be linked to organ size because organs have high metabolic activities relative to other body structures (Hammond and Diamond 1997; Piersma et al. 2004).
-
4.
Blood parameters such as levels of heat-shock proteins (HSPs; also known as ‘stress proteins’), leukocyte profiles [specifically the heterophil to lymphocyte (H/L) ratio], and natremia (plasma Na+ concentration) are recognized as good indicators of stress.
HSPs are molecular chaperones that play key roles in the maintenance of cellular homeostasis under variable environmental conditions (reviewed by Sørensen et al. 2003). In particular HSP70 is considered to be the major HSP family in vertebrates and has been used as an index of a range of stressors including low/high temperatures and salinities (Sørensen et al. 2003). HSPs are not biased by handling stress and, therefore, they are an appropriate measure of short- and long-term stress (Herring and Gawlik 2007). The H/L ratio is positively related to the magnitude of the stressor and to the circulating glucocorticoids (reviewed by Davis et al. 2008; see also Müller et al. 2011). Natremia, an indicator of osmotic and ionic balance, should reflect the outcome of the osmotic challenges faced during the different salinity-temperature regimes (see Brischoux et al. 2013b, 2014).
We hypothesized that red knots will adjust physiological and morphological parameters as a function of their osmoregulatory and thermoregulatory demands, and that those adjustments might vary within subspecies. On the basis of previous studies, we predict that adjustments (e.g. in BMR, food intake and body composition) would be more pronounced under more osmotically challenging conditions (i.e. HS-warm treatment). We also predict higher BMRs at HS (Gutiérrez et al. 2011) and cold (Vézina et al. 2006); reduced food intake, body mass and gizzard size (Vézina et al. 2006; Oudman et al. 2014), but enlarged salt glands (Gutiérrez et al. 2012), in birds exposed to HS–warm conditions. Second, if the physiological adjustments are partially fixed at the subspecies level, we would predict that tropically wintering C. canutus canutus deal with HS–warm better than temperate-wintering C. canutus islandica, i.e. they would regulate their natremia more precisely and show a weaker stress response (lower H/L ratio and HSP70 levels). Lastly, we would also predict that C. canutus islandica would show a weaker stress response under cold exposure than C. canutus canutus.
Materials and methods
Birds and housing
Twenty-one adult (non-moulting) red knots were used in this experiment. Of these, 11 individuals (five males, six females) belonged to the subspecies C. canutus islandica and ten individuals (seven males, three females) belonged to the subspecies C. canutus canutus. All birds were captured with mist nets in the western Dutch Wadden Sea (53°31′N, 6°23′E) in August–September 2013. Birds were aged according to plumage characteristics and sexed using molecular sexing (Baker et al. 1999). They were also assigned to one of the two subspecies co-occurring in the Wadden Sea during southward migration following Nebel et al. (2000). Afterwards, birds were brought into captivity at the Royal Netherlands Institute for Sea Research (NIOZ), Texel, the Netherlands, where they were housed in outdoor cages (4.5-m × 1.5-m surface × 2.5-m height) with unlimited access to water and trout pellets (see Vézina et al. 2006 for details). Each cage held four to six same-subspecies individuals throughout the experiment.
In January 2014, birds were randomly assigned to four indoor identical cages with the same dimensions as the outdoor cages. Then, the birds were fed a diet composed exclusively of 2 to 4 mm mud snails Peringia ulvae (formerly Hydrobia ulvae) collected by dredging in the Wadden Sea (as in Vézina et al. 2006). Mud snails were presented in two trays (60-cm × 40-cm surface × 5-cm height) with running seawater. This gastropod species is one of the main prey for red knots wintering in the Wadden Sea (van Gils et al. 2003, 2005b) and staging along southern European coasts during migration (Moreira 1994). Because this prey is isosmotic to seawater (Todd 1964), red knots inevitably consume salt when they ingest them whole. After 2 weeks of acclimation to mud snails, which ensured that red knots had enough time to adjust to a diet of hard-shelled mollusc prey (Piersma et al. 1993), the four groups were exposed to four salinity-temperature combinations: HS–warm (40 ‰, 35 °C), HS–cold (40 ‰, 5 °C), LS–warm (28 ‰, 35 °C), and LS–cold (40 ‰, 5 °C). LS seawater was pumped directly from the sea, whereas HS levels were achieved by adding pure (99.9 %) refined salt (NaCl) to LS seawater. Each treatment lasted 2 weeks. Throughout, birds were held under a 12-h light:12-h dark photoperiod with no access to freshwater.
Experimental protocol
We employed a staggered design because it was logistically impossible to measure all the physiological parameters in all groups and individuals simultaneously. The choice of a 2-day time lag among groups (i.e. 6-day time lag between the first and the last group) allowed us to do so. On day 12, starting at 1000 hours, we began daily food-intake measurements, which were carried out over 2 consecutive days. On the last day of each treatment (days 14/15 starting at 1000 hours) we collected two to three birds from one cage for blood sampling, salt gland scoring and ultrasound measurements (see below). We then placed the birds in individual holding cages (0.60-m × 0.40-m surface × 0.30-m height) with access to saltwater (salinity of the corresponding treatment) but no food for about 5 h to create a post-absorptive condition prior the onset of the BMR measurements (see below). Prior to metabolic measurements, birds were weighed (to the nearest 0.1 g) and scored for the extent of their subcutaneous fat stores using a semiquantitative scale for shorebirds [0–7, with 7 being the fattest (Meissner 2009)]. We then measured BMR over a period lasting 17 h starting at 1600 hours. At the end of the metabolic trials temperature was monitored with an electronic temperature recorder (Omega 450 ATT; Stanford, CT) attached to a copper constantan thermocouple that was inserted 1.5 cm into the bird’s cloaca. Afterwards, we measured body mass for the second time and moved the birds back to the cages to start the following treatment. A random treatment sequence was used. Water salinity and temperature were measured daily with a portable multi-parameter instrument (HD2156.1; Delta Ohm, Benelux). The experimental timeline is shown in Table 1.
Food and salt intake
We measured overall food intake in all the groups over 2 consecutive days in each treatment (as in Vézina et al. 2006). Every morning we sieved freshly thawed mud snails to remove all visible water and then took three subsamples (ca. 30 g) of food from this stock. Then we gave a pre-weighed amount of food from the same stock to the birds in a tray containing running saltwater (salinity depending on the treatment) at precisely 1000 hours. The following day, the food trays were removed from the cages at 1000 hours and the remaining food was carefully sieved to remove the water, and weighed again. The subsamples and leftover food were then dried for several days to constant mass in an oven at 60 °C. Following this, the dried samples were burned at 560 °C in a furnace for 5 h to obtain ash mass. Data are presented as the ash-free dry mass (AFDM) of mud snails consumed per bird over 24 h.
We additionally estimated salt (NaCl) concentration of dead mud snails provided as food in both LS and HS treatments using a modified protocol of Mahoney and Jehl (1985) and Blakey et al. (2006). To do this, we repeatedly blotted the surface water from duplicate samples weighing about 1 g each until we felt we could get no more water out without crushing the mud snails. Each sample was then mashed using a mortar, mixed with 0.4 ml Na+-free water, and centrifuged for 10 min at 4000g to separate the supernatant solution. A subsample of 30 µl was digested in 30-ml Teflon vials containing 1 ml of concentrated ultrapure HNO3 and heated at 125 °C in a Savillex hot block during 48 h. After digestion, 3 ml of ultrapure water (MilliQ) was added and 1 ml of subsample was diluted with 9 ml of 0.1 M ultrapure HNO3. An additional 10× dilution was necessary for determining Na+ concentration by inductively coupled plasma mass spectrometry (ICP–MS; Thermo Scientific iCAP Q ICP-MS; Thermo Fisher Scientific, Bremen). Na+ was then converted to salt intake assuming the 1:1.81 weight ratio of Na+ and Cl− in seawater and osmoconforming marine invertebrates (e.g. Blakey et al. 2006). Salt intake rate (SIR; expressed as grams of salt consumed per bird over 24 h) was estimated for both LS and HS treatments by:
where WMm is the wet mass of consumed mud snails (in grams), BWm is the body water fraction of wet mud snail samples (32.50 %), S m is the salinity of mud snails (25.26 and 37.95 g kg−1 in LS and HS, respectively), ADm is the adherent water fraction on wet mud snail samples (26.91 %), and S t is the salinity of each treatment (27.04 and 39.80 g kg−1 in LS and HS, respectively). Note that this is a conservative estimate of salt intake since we did not consider salt ingested by drinking. However, daily focal observations made from behind a one-way mirror suggest that salt intake by drinking was negligible.
Blood sampling
Blood samples for leukocyte profiles, stress proteins and ions analyses were obtained by puncturing the alar vein using a 26-gauge needle and collecting blood (≈200 µl) into heparinized capillary tubes. For each individual and treatment, blood smears were obtained by transferring a small drop of blood from the capillary tube to a clean air-dried glass slide. The remaining blood was transferred to Eppendorf tubes which were centrifuged for 10 min at 4000g to separate plasma from cells and then stored at −20 °C until assay.
Leukocyte profiles
Blood smears were sent to the European Veterinary Laboratory, Woerden, the Netherlands, and examined by one person who was blind to the treatments. The slides were air-dried, fixed in absolute methanol and then stained with Wright Giemsa. The first 200 white blood cells per slide were identified and counted as lymphocytes, heterophils, basophils, monocytes or eosinophils. We then calculated the H/L ratio. A subsample of smears (n = 20) were scanned twice, and repeatability analyses (details below) showed that there was moderately good agreement between these two scans within individuals [heterophils, R = 0.67, 95 % credible intervals (CI) 0.40–0.93, P < 0.001; lymphocytes, R = 0.56, 95 % CI 0.24–0.89, P < 0.001; H/L ratio, R = 0.61, 95 % CI 0.31–0.91, P < 0.001].
Heat-shock proteins
We determined HSP70 in blood cells as in Herring et al. (2011) with some modifications. Red blood cells were washed three times with phosphate-buffered saline and after centrifugation the supernatant was aspirated from the final wash and mixed with ×1 extraction reagent and protease inhibitor cocktail (Sigma Aldrich, Spain). Samples were sonicated for 1 min and incubated on ice for 30 min. with occasional mixing. Then, samples were centrifuged again (10 min, 21,000g, 4 °C) and the supernatant was removed and used in posterior analyses. Cell lysates total protein concentration was determined by the Bradford method using bovine serum albumin as the standard. Concentrations of HSP70 were determined from the cell lysates by means of an enzyme-linked immunosorbent assay kit specific for inducible HSP70 (HSP70 EIA kit; Enzo Life Sciences, Lausen, Switzerland). All samples were run in duplicate and the means of duplicates were used in all analyses. All final HSP70 values were standardized by dividing HSP70 concentration by total protein to facilitate comparisons. Based on internal standards run in duplicate, the mean intra- and inter-assay coefficients of variation were 4.1 and 6.9 %, respectively.
Plasma Na+ concentrations
Plasma Na+ concentrations were determined using an electrolyte analyser with ion-specific electrodes (SPOTCHEM EL SE-1510; Menarini, Milan). Briefly, we allowed stored plasma samples and exclusive E-plates to reach room temperature (15–20 °C), stirred the samples without foaming, and then distributed subsamples (22 µl) on the E-plates before starting the automated measurement.
Basal metabolic rate
Measurements of basal rates of O2 consumption and CO2 production were taken from each individual at the end of each treatment using open-flow respirometry. For details about the respirometry set-up and BMR measurement protocol, see Piersma et al. (2004). Briefly, birds were measured overnight under post-absorptive digestive conditions in dark metabolic chambers at 21.0 °C (±0.1), i.e. within the red knot’s thermoneutral zone (Piersma et al. 1995). BMR was defined as the lowest 10-min average of O2 consumption, which was calculated with the appropriate formulas for our set-up (Piersma et al. 2004).
Salt gland scores
We did not use ultrasonography for estimating salt gland size since the head is a uniformly heavily feathered area and the air in the feathers limits the use of such a technique (Dietz et al. 1999). Instead, we estimated salt gland size for each individual using a novel non-invasive approach (Gutiérrez 2014). We used sensory evaluation to score the thickness of the salt glands at the postorbital ridge. This consisted of sliding a finger across a smooth polyvinylchloride plate prepared with five increasing thicknesses (0–0.8 mm; artificially created by overlapping 0.16-mm-thick tape portions) at regular distances from each other, and then comparing these thicknesses with those of the postorbital salt gland ridge. Two trained observers (J. S. G. and A. S.-R.) blind to the treatments independently scored the salt gland ridge on an 11-point ordinal scale (0–5, including half-points). Repeatability analyses (details below) showed that there was good agreement between observers (R = 0.71–0.78, always P < 0.001), so we used the mean of the two scores for the subsequent analyses. Glandular tissue can vary in height and width but rarely in length (Staaland 1967; Siegel-Causey 1990); then, the thickness (i.e. height) of the saltglands at the postorbital ridge might be used a proxy of saltgland size.
Ultrasonography
Measurements of the gizzard dimensions (height and width) were done in duplicate by one observer (A. D.) with a Pie 200 ultrasound instrument with a 7.5-MHz linear probe (Pie Medical Benelux, BBV, Maastricht, the Netherlands) as described by Dietz et al. (1999). Prior to the experiment, calibration curves were made for the observer using 20 dead red knots with a wide range of gizzard sizes. As the repeatabilities between the first and the second measurements were high (gizzard height, R = 0.90–0.96, always P < 0.001; gizzard width, R = 0.73–0.95, always P < 0.001), we used the mean values for statistical analyses. The gizzard dimensions were converted to gizzard masses as follows:
Statistical analyses
Analyses were performed using R version 3.1.0 statistical software (R Development Core Team). Throughout, we used P < 0.05 as the level of significance. Data are presented as mean ± SEM.
We fitted linear mixed-effects models [package nlme (Pinheiro et al. 2014)] with each parameter (food and salt intake, BMR, body mass, body temperature, fat and salt gland scores, gizzard mass, HSP70, H/L ratio and Na+) as the response variable; salinity, temperature and subspecies as fixed factors; and group and individual nested within group as random effects (except for food and salt intake where group values are reported). Low sample sizes did not allow a comparison by sex; however, earlier studies did not find sex-dependent differences in various physiological traits, including total body mass and fat stores (Piersma et al. 1999), stress levels (Reneerkens et al. 2002) and immune function (Buehler et al. 2009a, b). We always started with the full model and simplified it using backwards elimination based on ANOVA with P < 0.05 as the selection criterion until reaching the minimal adequate model. Model assumptions were checked using the residuals of the final model. For those traits measured in duplicate for each salinity-temperature regime, we calculated ANOVA-based repeatabilities (the intraclass correlation coefficient) using the package rptR [rpt.aov function (Nakagawa and Schielzeth 2010)]. Three individuals (two C. canutus canutus and one C. canutus islandica) did not adjust to the combination of HS and high temperature (body mass below the critical threshold of 100 g) so they were returned to outdoor aviaries with unlimited access to freshwater and trout pellets and excluded from further analysis. The sample size for HSP70 differs from that for other response variables because the HSP70 concentration in 12 samples was below the detection threshold, independently of the treatment and subspecies. Three extreme outliers (>3 SD) for this response variable were considered as missing values.
Results
Baseline values, measured prior to the experimental conditions, did not differ significantly between subspecies in any of the 11 parameters measured (always P > 0.05). Therefore, actual values for each subspecies were used in all analyses. Because BMR was significantly correlated with body mass (linear regression, F 1,74 = 50.44, P < 0.001), we also used residuals of the body mass regressions for this variable in subsequent analyses.
Responses in food and salt intake
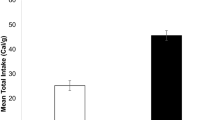
Temperature affected food intake rate with birds acclimated to the cold eating on average 35 % more food (32.29 ± 1.14 g AFDM bird−1 day−1) than individuals from the warm treatments (20.89 ± 2.11 g AFDM bird−1 day−1), independently of salinity and subspecies (Table 2; Fig. 1a). We found no significant effect of salinity (Table 2), although birds of both subspecies substantially reduced food intake rates under HS–warm conditions (Fig. 1a). As a result, the interaction term salinity × temperature, but not subspecies, was highly significant (Table 2). Interestingly, the food intake rate of warm-acclimated birds was on average 55 % lower when they fed at elevated salinity (14.27 ± 1.45 g AFDM bird−1 day−1 for HS–warm and 27.50 ± 2.10 g AFDM bird−1 day−1 for LS–warm).
Food (a) and salt (b) intakes in two red knot subspecies acclimated to different salinity-temperature combinations. Box-and-whisker plots give means estimated by the full model (large filled square), medians (horizontal line within plot), interquartile ranges (box), and ranges (bars). Shaded areas depict wintering salinity-temperature conditions potentially experienced by red knots in West Africa (Calidris canutus canutus) and Western Europe (Calidris canutus islandica), respectively. Group means that are statistically significantly different from each other (at the 5 % confidence level as indicated by post hoc Tukey tests) are connected by horizontal bars (subspecies did not differ within treatments in any case, so we did not include it in the tests). Note that these two variables represent group-specific measurements. HS High salinity, LS low salinity
Salinity, temperature and their interaction significantly affected SIR (Table 2). Birds exposed to HS–warm showed the lowest SIRs (3.16 ± 0.24 g NaCl bird−1 day−1), whereas those exposed to HS–cold showed the highest SIRs (8.67 ± 0.59 g NaCl bird−1 day−1) (Fig. 1b). Regardless of the treatment, both subspecies exhibited similar SIRs (subspecies, P = 0.704; Fig. 1b).
Responses in fat stores and digestive organs
Fat score and body mass were similarly affected by explanatory variables (Figs. 2a, 3a; Tables 3, 4, respectively), since they were highly correlated with each other (linear regression, F 1,74 = 451.5, P < 0.001, subspecies and treatments combined). For fat score, salinity and the three-way interaction (salinity × temperature × subspecies) were significant (Table 3). HS–acclimated birds had lower fat score, especially under warm conditions (Fig. 2a). At HS–cold, C. canutus canutus exhibited lower fat scores than C. canutus islandica (Fig. 2a).
Fat score (a), salt-gland score (b), and gizzard mass (c) in two red knot subspecies acclimated to different salinity-temperature combinations. See Fig. 1 for more details and abbreviations
Body mass (a), basal metabolic rate (BMR) (b), residual BMR (c), and body temperature (d) in two red knot subspecies acclimated to different salinity-temperature combinations. See Fig. 1 for more details and other abbreviations
Salt gland score (an indicator of concentrating ability) was affected by salinity only (Table 2). Salt gland scores were higher under the HS conditions, regardless of temperature and subspecies (Fig. 2b; Table 3). Overall, salt gland scores were positively correlated with mass-corrected (i.e. residual) BMR (linear regression, F 1,74 = 5.58, P = 0.021) and negatively correlated with body mass (linear regression, F 1,74 = 11.87, P < 0.001). The variation in gizzard mass (an indicator of relative food intake) was affected by salinity and temperature, but not subspecies (Table 3). HS–warm-acclimated birds showed the smallest gizzards (5.51 ± 0.26 g), whereas LS–cold-acclimated birds showed the largest gizzards (7.20 ± 0.32 g) (Fig. 2c). Gizzard mass positively correlated with whole-organism BMR (linear regression, F 1,74 = 14.02, P<0.001), but not with mass-corrected BMR (linear regression, F 1,74 = 0.08, P = 0.776). We found no correlation between salt gland scores and gizzard mass either when data were pooled (linear regression, F 1,74 = 3.14, P = 0.081) or when data were analysed for each of the treatments separately (always P > 0.123).
Responses in body mass, basal metabolism and temperature
Body mass decreased with salinity and temperature in both subspecies (Fig. 3a). Regardless of the salinity and temperature, C. canutus islandica overall exhibited higher body masses than C. canutus canutus (Fig. 3a; Table 4).
Both whole-organism and mass-corrected BMR were affected by salinity and temperature (Table 4). Overall, BMR tended to be higher in cold treatments (Table 4; Fig. 3b, c). The salinity × subspecies interaction was also significant for both whole-organism and residual BMR (Table 4), indicating that C. canutus canutus had higher levels under low salinities.
None of the explanatory variables significantly affected body temperature (Table 4; Fig. 3d). We found no relationship between body temperature and body mass or fat scores (linear regressions, F 1,74 = 1.73, P = 0.19; F 1,74 = 0.67, P = 0.41, respectively). However, body temperature positively correlated with both whole-organism (linear regression, F 1,74 = 10.39, P = 0.002) and mass-corrected BMR (linear regression, F 1,74 = 9.074, P = 0.004).
Responses in blood parameters
We found no direct effect of temperature, salinity and subspecies on HSP70 (Table 5; Fig. 4a), although HS-exposed birds had somewhat higher levels of HSP70 (0.39 ± 0.09 ng mg−1) than LS-exposed ones (0.35 ± 0.06 ng mg−1). There was a significant interaction between salinity and subspecies such that C. canutus islandica had higher HSP70 levels than C. canutus canutus in HS but lower levels in LS (Table 5; Fig. 4a). The variation in H/L ratios was affected by salinity with HS-exposed knots having higher ratios than LS-exposed ones (1.12 ± 0.13 vs. 0.93 ± 0.10) (Table 5; Fig. 4b). The significant interaction between salinity and subspecies indicated that C. canutus canutus had higher ratios in HS treatments (Table 5; Fig. 4b). H/L ratio and HSP70 levels were not significant correlated (linear regression, F 1,62 = 2.43, P = 0.123).
Heat-shock protein 70 (HSP70) (a), heterophils/lymphocytes (H/L) ratio (b), and plasma Na+ (c) in two red knot subspecies acclimated to different salinity-temperature combinations. See Fig. 1 legend for more details and other abbreviations
Salinity and its interaction with subspecies affected plasma Na+ levels (Table 5). Plasma Na+ increased with salinity, with HS–warm-acclimated birds showing the highest levels. Under such conditions, C. canutus canutus exhibited higher Na+ levels (173.25 ± 4.74 mmol L−1) than C. canutus islandica knots (168.64 ± 2.90 mmol L−1) (Fig. 4c). Na+ level was not correlated with H/L ratio (linear regression, F 1,74 = 2.43, P = 0.123) or HSP70 (linear regression, F 1,74 = 0.04, P = 0.85).
Discussion
In the present study we investigated the physiological adjustments to salinity and temperature in two red knot subspecies with contrasting non-breeding osmoregulatory demands. We found that the simultaneous demands of osmoregulation and thermoregulation yielded changes in energy acquisition and allocation as reflected in phenotypic flexibility of food and salt intake, basal metabolism, body composition and stress responses. Importantly, our results demonstrate that, regardless of the subspecies, the combination of relatively high salinities and temperatures (naturally encountered by shorebirds and other migratory waterbirds) limited the rate of food (energy) acquisition with direct and indirect consequences for the size of metabolically active organs. As a consequence, birds coping with HS–warm conditions showed several significantly reduced physiological and condition-related traits (body mass, fat stores, gizzard mass and BMR) and augmented stress-related traits (HSP70, H/L ratio and plasma Na+).
It is well established that salt loads can have a direct negative effect on the physiology of birds that inhabit saline environments (Sabat 2000; Gutiérrez 2014). Previous studies have shown that salt intake can constrain food intake in captive red knots (Klaassen and Ens 1990; Oudman et al. 2014). In this study we found that red knots feeding on HS mud snails under warm conditions extensively reduced food and energy intake rate. Assuming that 40 % of Peringia AFDM constitutes indigestible ballast material (Quaintenne et al. 2011), an energy density of 22 kJ g−1 AFDM (Zwarts and Wanink 1993) and an assimilation efficiency of 80 % (Kersten and Piersma 1987), the metabolisable energy intake rate of HS-warm-exposed red knots was about 136 kJ day−1, in contrast with the 290 kJ day−1 consumed by LS-warm-exposed ones. These crude estimates represent only a quarter to a half of the upper limit of daily metabolizable energy for a red knot feeding for 12 h day−1 [544 kJ day−1 (van Gils et al. 2005a)], which suggests that managing salt and heat loads may pose a major osmoregulatory challenge for tropical-wintering shorebirds feeding on poor-quality molluscs. This could partly explain why red knots at tropical intertidal sites have lower fuelling rates than birds at higher latitudes (Piersma et al. 2005). Daily food intake, and hence fuelling rates, may be constrained by heat production due to external heat loads in the tropics (Verboven and Piersma 1995; Battley et al. 2003), but also by additional salt loads (Klaassen and Ens 1990; Verboven and Piersma 1995). Our results clearly show that fuelling rates are likely to be constrained by the combination thereof.
Likewise SIR is reduced in warm-acclimated birds, even at relatively low salinities. This suggests that in hyperosmotic environments food/salt intake is probably limited by evaporative water loss rather than by the total amount of ingested salt. That is, water loss could exceed salt gain resulting in water imbalance. Surprisingly, the direct salt intake in captive knots reached up to 9 g day−1 leading to a mass-specific salt intake of 71 mg g−1 body mass. This mass-specific salt intake is much higher than reported for other marine birds, including that of wintering common eiders Somateria mollissima feeding on whole-shelled bivalves [24 mg g−1 (Nelhs 1996)]. Assuming the overall cost of salt turnover at 1.3 kJ g−1 NaCl (Nelhs 1996), HS–cold-acclimated red knots would demand ca. 11 kJ day−1 to eliminate excess salt, which represents approximately 14 % of their BMR. Such a figure is comparable to the energetic cost of osmoregulation and other physiological demands in waterbirds and probably sufficient to trade-off with other physiological demands (Gutiérrez 2014).
Arguably the experimental conditions described here do not always very closely resemble conditions in the field. However, these results can be used as a proxy for comparative examinations of salt tolerance under different thermal environments. Although our experimental birds were acclimated to the different treatments over relatively short time periods, the fact that three individuals did not adjust to the HS–warm treatment and that they all continued to lose mass under such conditions suggests that we may have come close to their maximum salt tolerance. It is worthwhile to note that birds were exposed to a constant elevated temperature under windless aviary conditions which did not allow them to benefit from the lower night temperatures and the sea breeze they normally experience at tropical intertidal sites. In this sense, it is possible that shorebirds fuelling at the tropical sites avoid heat stress by feeding at night (Zwarts et al. 1990a), and by means of evaporative cooling and cutaneous evaporation (Battley et al. 2003).
The decrease in food intake was accompanied by a reduction in BMR when red knots were simultaneously exposed to HS and heat. This supports earlier findings that red knots downregulate BMR in response to heat acclimation/acclimatization over short periods (e.g. Piersma et al. 1995; Kersten and Piersma 1987; Vézina et al. 2006). However, BMR did not increase with salinity as previously shown in a closely related species (Gutiérrez et al. 2011), possibly because salinity and temperature generated opposite responses in basal metabolism (e.g. Vézina et al. 2006; Gutiérrez et al. 2011), making it impossible to disentangle the effects of the two factors. Nevertheless, birds with higher salt gland scores showed higher BMR after accounting for differences in body mass, indicating that when exposed to saltier conditions birds do pay an additional energetic cost for osmoregulation.
We found no substantial changes in body temperature throughout the experiment. A slight daily or seasonal decrease in body temperature (facultative hypothermia) is shown in many avian species in response to stressful and energy-limiting conditions [e.g. breeding, migratory fuelling, low food supply and low temperature (Butler and Woakes 2001; McKechnie and Lovegrove 2002)]. Although birds exposed to warmth and HS tended to have lower body temperature, it did not differ significantly among the four treatments. Nevertheless, this should be interpreted cautiously since our birds were measured at the end of the metabolic trials, during which they were resting overnight under thermally neutral conditions and thus were not directly exposed to the experimental conditions in the acclimatised aviaries. Overall, body temperature was positively correlated with BMR, indicating that individuals modulated these two traits in parallel. The decline of BMR and body temperature during prolonged heat exposure could have prevented the birds from overheating (Kersten and Piersma 1987; Klaassen 1990).
Based on increased HSP70 levels and H/L ratio, it seems that experimental red knots experienced the combination of HS and heat as stressful, resulting in an energy-saving strategy that reduced their food intake rate and BMR. Such metabolic downregulation was also parallelled by a reduction in gizzard mass, suggesting that differences in the size of several digestive organs of red knots could be correlated with differences in BMR (Piersma et al. 1996). In contrast, salt glands measurably increased in size with higher salinity at both high and low temperatures, which suggests that red knots require large salt glands to meet increased osmoregulatory demands (Gutiérrez et al. 2012), despite low food intake. Because salt gland hypertrophy in saline-acclimated birds can occur over short time scales (Peaker and Linzell 1975), it is likely that the size of the salt glands had reached a maximum after 2 weeks of HS acclimation and thus we could not detect differences associated with ambient temperature (see Gutiérrez et al. 2012).
It is interesting to note that red knots, especially those of the subspecies C. canutus canutus, exposed to HS showed elevated plasma Na+ (up to 197 mmol L−1). One might interpret this as an indication of either osmotic stress or a certain tolerance to hypernatremia over short time periods. The lack of correlation between Na+ and stress indicators (HSP70 and H/L ratio) supports the latter interpretation. Indeed, the elevated natremia we recorded in captive red knots is within the range of values reported for other bird species with salt glands [135–216 mmol L−1 (Skadhauge 1981)]. Recent studies that reported elevated natremia in sea snakes (Brischoux et al. 2013b, 2014) suggested that greater tolerance to hypernatremia would be advantageous since active salt excretion would occur only when plasma Na+ dangerously exceeds an upper threshold and this, in turn, would substantially decrease energetic costs associated with salt gland functioning. Whether C. canutus canutus could have developed a certain degree of tolerance to hypernatremia remains to be further explored. Moreover, the fact that C. canutus canutus had somewhat greater H/L ratios and lower body masses and fat scores when exposed to HS provides an interesting avenue for exploring possible differences at the subspecies level. For instance, C. canutus islandica held in captivity maintained the schedules of moult and body mass changes of their counterparts in the wild, whereas C. canutus canutus showed deviations from normal annual rhythms (Piersma et al. 1996; Piersma and Ramenofsky 1998). Experimental and observational evidence (Piersma et al. 1996; Piersma and Ramenofsky 1998) from red knots that have been kept for research purposes under long-term captive conditions at research facilities at northern temperate latitudes suggests that C. canutus canutus are more susceptible to stress than C. canutus islandica. One is then tempted to pose the question: do C. canutus canutus ‘wintering’ in such an aviary environment somehow feel that they are at the ‘wrong’ latitude? Birds from different latitudes and with different migratory habits could, for example, have different photosensitive phases (e.g. Berthold 1974; Gwinner and Scheuerlein 1999). Further experiments under photoperiod and temperature regimes resembling those of the red knots’ natural environments are necessary to identify the reason(s) for such subspecific differences.
In addition to the physiological mechanisms examined here, behavioural strategies leading to a decrease in salt intake are likely crucial in maintaining the osmotic balance (Gutiérrez 2014). Selective feeding on low-salt-load prey is a viable osmoregulatory strategy to avoid salt stress in saline environments (e.g. Purdue and Haines 1977; Nyström and Pehrsson 1988). For example, red knots staging on intertidal areas during the spring migration do not feed extensively on the crustacean brine shrimp Artemia spp. at supratidal salinas (100–150 ‰), an observation that has been explained by the avoidance of salt stress (Masero 2002). Instead, the mud snail (the prey used in this study) is the main prey for red knots staging on the mudflats of southern European coasts (Moreira 1994; Masero 2002). Feeding on less salty mud snails—despite their relatively low energy content (van Gils et al. 2005a)—might enable red knots to overcome osmotic stress.
A better understanding of avian adaptations and tolerances to saline environments is important to both basic science and conservation (reviewed in Gutiérrez 2014). In view of the fact that rather inactive red knots exposed to HS and temperature in captivity appeared challenged to even maintain body mass, it remains a big puzzle how hard-working birds in the field that have to commute between feeding and resting areas and may be forced into a lot of evasive flying by aerial predators (see Leyrer et al. 2012; van den Hout et al. 2014) can ever deposit the stores for onward flight in a place like Banc d’Arguin (Zwarts et al. 1990b; Piersma et al. 2005). Such knowledge is rather relevant at a time when fluctuating temperatures and salinity conditions predicted under various climate change scenarios (reviewed in Gutiérrez 2014) will affect the options open to birds and other aquatic air-breathing vertebrates such as sea turtles, snakes, and mammals.
Author contribution statement
This study was conceived and designed by J. S. G., A. D., J. A. M. and T. P. The experiment was performed by J. S. G., A. S.-R. and A. D. HSP were measured by A. V. Data analyses were performed by J. S. G. and A. S.-R. The manuscript was written by J. S. G., J. A. M., A. V. and T. P.
References
Baker AJ, Piersma T, Greenslade AD (1999) Molecular vs. phenotypic sexing in red knots. Condor 101:887–893
Battley PF, Rogers DI, Piersma T, Koolhaas A (2003) Behavioural evidence for heat-load problems in great knots in tropical Australia fuelling for long-distance flight. Emu 103:97–103
Berthold P (1974) Circannual Clocks. In: Pengelley ET (ed) Circannual rhythms in birds with different migratory habits. Academic Press, New York, pp 55–94
Blakey R, Zharikov Y, Skilleter GA (2006) Lack of an osmotic constraint on intake rate of the eastern curlew (Numenius madagascariensis). J Avian Biol 37:299–305
Bozinovic F, Calosi P, Spicer JI (2011) Physiological correlates of geographic range in animals. Annu Rev Ecol Evol Syst 42:155–179
Bradley TJ (2009) Animal osmoregulation. Oxford University Press, NewYork
Brischoux F, Kornilev YV (2014) Hypernatremia in dice snakes (Natrix tessellata) from a coastal population: implications for osmoregulation in marine snake prototypes. PLoS One 9(3):e92617
Brischoux F, Tingley R, Shine R, Lillywhite HB (2012) Salinity influences the distribution of marine snakes: implications for evolutionary transitions to marine life. Ecography 35:994–1003
Brischoux F, Tingley R, Shine R, Lillywhite HB (2013a) Behavioral and physiological correlates of the geographic distributions of amphibious sea kraits (Laticauda spp.). J Sea Res 76:1–4
Brischoux F, Briand MJ, Billy G, Bonnet X (2013b) Variations of natremia in sea kraits (Laticauda spp.) kept in seawater and fresh water. Comp Biochem Physiol A 166:333–337
Buehler DM, Tieleman BI, Piersma T (2009a) Age and environment affect constitutive immune function in red knots (Calidris canutus). J Ornithol 150:815–825
Buehler DM, Tieleman BI, Piersma T (2009b) Indices of immune function are lower in red knots (Calidris canutus) recovering protein than in those storing fat during stopover in Delaware Bay. Auk 127(2):394–401
Butler PJ, Woakes AJ (2001) Seasonal hypothermia in a large migrating bird: saving energy for fat deposition? J Exp Biol 204:1361–1367
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72(1):28–37
Evans DH (2009) Osmotic and ionic regulation: cells and animals. CRC, Boca Raton
Goldstein DL, Skadhauge E (2000) Renal and extrarenal regulation of body fluid composition. In: Whittow GC (ed) Sturkey’s avian physiology. Academic Press, New York, pp 265–297
Gutiérrez JS (2014) Living in environments with contrasting salinities: a review of physiological and behavioural responses in waterbirds. Ardeola 61(2):233–256
Gutiérrez JS, Masero JA, Abad-Gómez JM, Villegas A, Sánchez-Guzmán JM (2011) Understanding the energetic costs of living in saline environments: effects of salinity on basal metabolic rate, body mass and daily energy consumption of a long-distance migratory shorebird. J Exp Biol 214:829–835
Gutiérrez JS, Dietz MW, Masero JA, Gill RE Jr, Dekinga A, Battley PF, Sánchez Guzmán JM, Piersma T (2012) Functional ecology of salt-glands in shorebirds: flexible responses to variable environmental conditions. Funct Ecol 26:236–244
Gutiérrez JS, Abad-Gómez JM, Villegas A, Sánchez-Guzmán JM, Masero JA (2013) Effects of salinity on the immune response of an ‘osmotic generalist’ bird. Oecologia 171:61–69
Gwinner E, Scheuerlein A (1999) Photoperiodic responsiveness of equatorial and temperate-zone stonechats. Condor 101:347–359
Hammond KA, Diamond J (1997) Maximum sustained energy budgets in humans and animals. Nature 386:457–462
Hannam MK, Oring lW, Herzog MP (2003) Impacts of salinity on growth and behavior of American avocet chicks. Waterbirds 26:119–125
Herring G, Gawlik DE (2007) The role of stress proteins in the study of allostatic overload in birds: use and applicability to current studies in avian ecology. Sci World J 7:1596–1602
Herring G, Cook MI, Gawlik DE, Call EM (2011) Food availability is expressed through physiological stress indicators in nestling white ibis: a food supplementation experiment. Funct Ecol 25:682–690
Kersten M, Piersma T (1987) High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea 75:175–187
Klaassen M (1990) Short note on the possible occurrence of heat stress in roosting waders on the Banc d’Arguin, Mauritania. Ardea 78:63–65
Klaassen M, Ens BJ (1990) Is salt stress a problem for waders wintering on the Banc d’Arguin, Mauritania? Ardea 78:67–74
Lavaud R, Thébault J, Lorrain A, van der Geest M, Chauvaud L (2013) Senilia senilis (Linnaeus 1758), a biogenic archive of environmental conditions on the Banc d’Arguin (Mauritania). J Sea Res 76:61–72
Leyrer J, Lok T, Brugge M, Dekinga A, van Gils B, Spaans JA, Sandercock BK, Piersma T (2012) Small-scale demographic structure suggests preemptive behavior in a flocking shorebird. Behav Ecol 23:1226–1233
Masero JA (2002) Why don’t red knots Calidris canutus feed extensively on the crustacean Artemia? Bird Study 49:304–306
McKechnie AE, Lovegrove BG (2002) Avian facultative hypothermic responses: a review. Condor 104:705–724
McNab BK (2002) The physiological ecology of vertebrates. A view from energetics. Cornell University Press, Ithaca
Meissner W (2009) A classification scheme for scoring subcutaneous fat depots of shorebirds. J Field Ornithol 80:289–296
Moreira F (1994) Diet and feeding rates of knots Calidris canutus in the Tagus estuary (Portugal). Ardea 82:133–135
Müller C, Jenni-Eiermann S, Jenni L (2011) Heterophils/lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct Ecol 25:566–576
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Nebel S, Piersma T, van Gils J, Dekinga A, Spaans B (2000) Length of stopover, fuel storage and a sex-bias in the occurrence of two subspecies of red knots Calidris c. canutus and C. c. islandica in the Dutch Wadden Sea during southward migration. Ardea 88:165–176
Nelhs G (1996) Low costs of salt turnover in common eiders Somateria mollissima. Ardea 84:23–30
Nyström KGK, Pehrsson O (1988) Salinity as a constraint affecting food and habitat choice of mussel-feeding diving ducks. Ibis 130:94–110
Oudman T, Onrust J, de Fouw J, Spaans B, Piersma T, van Gils JA (2014) Digestive capacity and toxicity cause mixed diets in red knots that maximize energy intake rate. Am Nat 183:650–659
Peaker M, Linzell JL (1975) Salt glands in birds and reptiles. Cambridge University Press, Cambridge
Peña-Villalobos I, Valdés-Ferranty F, Sabat P (2013) Osmoregulatory and metabolic costs of salt excretion in the Rufous-collared sparrow Zonotrichia capensis. Comp Biochem Physiol A 164:314–318
Phillips JG, Ensor DM (1972) The significance of environmental factors in the hormone mediated changes of nasal (salt) gland activity in birds. Gen Comp Endocrinol 3:393–404
Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631
Piersma T (2007) Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J Ornithol 148(Suppl 1):S45–S59
Piersma T, Ramenofsky M (1998) Long-term decreases of corticosterone in captive migrant shorebirds that maintain seasonal mass and moult cycles. J Avian Biol 29:97–104
Piersma T, Van Gils JA (2011) The flexible phenotype. A body-centred integration of ecology, physiology, and behaviour. Oxford University Press, Oxford
Piersma T, Koolhaas A, Dekinga A (1993) Interactions between stomach structure and diet choice in shorebirds. Auk 110:552–564
Piersma T, Cadée N, Daan S (1995) Seasonality in basal metabolic rate and thermal conductance in a long-distance migrant shorebird, the knot (Calidris canutus). J Comp Physiol 165:37–45
Piersma T, Bruinzeel L, Drent R, Kersten M, van der Meer J, Wiersma P (1996) Variability in basal metabolic rate of a long-distance migrant shorebird (red knot, Calidris canutus) reflects shifts in organ sizes. Physiol Zool 69:191–217
Piersma T, Gudmundsson GA, Lilliendahl K (1999) Rapid changes in the size of different functional organ and muscle groups during refuelling in a long-distance migrating shorebird. Physiol Biochem Zool 72:405–415
Piersma T, Gessaman JA, Dekinga A, Visser GH (2004) Gizzard and other lean mass components increase, yet basal metabolic rates decrease, when red knots Calidris canutus are shifted from soft to hard-shelled food. J Avian Biol 35:99–104
Piersma T, Rogers DI, González PM, Zwarts L, Niles LJ, de Lima S, do Nascimento I, Minton CDT, Baker AJ (2005) Fuel storage rates before northward flights in red knots worldwide: facing the severest ecological constraint in tropical intertidal environments? In: Greenberg R, Marra PP (eds) Birds of two worlds: the ecology and evolution of temperate tropical migration systems. Johns Hopkins University Press, Baltimore, pp 262–273
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1-118. http://CRAN.R-project.org/package=nlme
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol 132:739–761
Prusch RD (1983) Evolution of invertebrate homeostasis: osmotic and ionic regulation. Comp Biochem Physiol 76:753–761
Purdue JR, Haines H (1977) Salt water tolerance and water turnover in the snowy plover. Auk 94:248–255
Quaintenne G, van Gils JA, Bocher P, Dekinga A, Piersma T (2011) Scaling up ideals to freedom: are densities of red knots across western Europe consistent with ideal free distribution? Proc R Soc 278:2728–2736
Reneerkens J, Morrison RIG, Ramenofsky M, Piersma T, Wingfield JC (2002) Baseline and stress-induced levels of corticosterone during different life-cycle sub-stages in a shorebird on the high arctic breeding grounds. Physiol Biochem Zool 75:200–208
Sabat P (2000) Birds in marine and saline environments: living in dry habitats. Rev Chil Hist Nat 73:401–410
Siegel-Causey D (1990) Phylogenetic patters of size and shape of the nasal gland depression in Phalacrocoracidae. Auk 107:107–118
Skadhauge E (1981) Osmoregulation in birds. Springer, Berlin
Staaland H (1967) Anatomical and physiological adaptations of the nasal glands in Charadriiformes birds. Comp Biochem Physiol 23:933–944
Todd ME (1964) Osmotic balance in Hydrobia ulvae and Potamopyrgus jenkinsi (Gastropoda: Hydrobiidae). J Exp Biol 41:665–677
van de Kam J, Ens BJ, Piersma T, Zwarts L (2004) Shorebirds. An illustrated behavioural ecology. KNNV, Utrecht
van den Hout PJ, van Gils JA, Robin F, van der Geest M, Dekinga A, Piersma T (2014) Interference from adults forces young red knots to forage for longer and in dangerous places. Anim Behav 88:137–146
van Gils JA, Piersma T, Dekinga A, Dietz MW (2003) Cost–benefit analysis of mollusc-eating in a shorebird. II. Optimizing gizzard size in the face of seasonal demands. J Exp Biol 206:3369–3380
van Gils JA, Battley PF, Piersma T, Drent R (2005a) Reinterpretation of gizzard sizes of Red Knots world-wide emphasises overriding importance of prey quality at migratory stopover sites. Proc Biol Sci 272:2609–2618
van Gils JA, Dekinga A, Spaans B, Vahl WK, Piersma T (2005b) Digestive bottleneck affects foraging decisions in red knots Calidris canutus. II. Patch choice and length of working day. J Anim Ecol 74:120–130
van Gils JA, de Rooij SR, van Belle J, van der Meer J, Dekinga A, Piersma T, Drent R (2005c) Digestive bottleneck affects foraging decisions in red knots Calidris canutus. I. Prey choice. J Anim Ecol 74:105–119
Verboven N, Piersma T (1995) Is the evaporative water-loss of knot Calidris canutus higher in tropical than in temperate climates? Ibis 137:308–316
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Wolff WJ, Smit CJ (1990) The Banc d’Arguin, Mauritania, as an environment for coastal birds. Ardea 78(1):17–38
Zwarts L, Wanink JH (1993) How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body weight, biomass, burying depth and behaviour of tidal-flat invertebrates. Neth J Sea Res 31:441–476
Zwarts L, Blomert A-M, Hupkes R (1990a) Increase of feeding time in waders preparing their spring migration from the Banc d’ Arguin, Mauritania. Ardea 78:237–256
Zwarts L, Ens BJ, Kersten M, Piersma T (1990b) Moult, mass and flight range of waders ready to take off for long-distance migrations. Ardea 78:339–364
Acknowledgments
We are grateful to the fellow members of the Department of Marine Ecology of NIOZ for helpful suggestions and comments during the course of the experiment. We thank Maarten Brugge for technical support; Ewout Adriaans, Sander Holthuijsen and Job ten Horn for help collecting mud snails; Wim Boer for analysing Na+ concentrations in samples of mud snails; Allert I. Bijleveld, Thomas Oudman and Eldar N. Rakhimberdiev for advice on statistical analyses; and David Chivall for improving the English. Indrikis Krams, Hannu J. Ylonen and two anonymous reviewers made helpful comments that considerably improved the manuscript. Bird handling and experimental protocols were carried out under a permit from the animal experiment committee of the Royal Netherlands Academy of Sciences (KNAW DEC; protocol NIOZ. 13.02). This research was supported by a NIOZ operating grant to T. P., by a postdoctoral grant from the Government of Extremadura (PO12025) to J. S. G. and by the project CGL2011-27485 (Spanish Ministry of Science and Innovation).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Indrikis Krams.
Rights and permissions
About this article
Cite this article
Gutiérrez, J.S., Soriano-Redondo, A., Dekinga, A. et al. How salinity and temperature combine to affect physiological state and performance in red knots with contrasting non-breeding environments. Oecologia 178, 1077–1091 (2015). https://doi.org/10.1007/s00442-015-3308-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3308-4