Abstract
Salt stress can suppress the immune function of fish and other aquatic animals, but such an effect has not yet been examined in air-breathing vertebrates that frequently cope with waters (and prey) of contrasting salinities. We investigated the effects of seawater salinity on the strength and cost of mounting an immune response in the dunlin Calidris alpina, a long-distance migratory shorebird that shifts seasonally from freshwater environments during the breeding season to marine environments during migration and the winter period. Phytohaemagglutinin (PHA)-induced skin swelling, basal metabolic rate (BMR), body mass, fat stores, and plasma ions were measured in dunlins acclimated to either freshwater or seawater (salinity: 0.3 and 35.0 ‰, respectively). Seawater-acclimated dunlins mounted a PHA-induced swelling response that was up to 56 % weaker than those held under freshwater conditions, despite ad libitum access to food. Freshwater-acclimated dunlins significantly increased their relative BMR 48 h after PHA injection, whereas seawater-acclimated dunlins did not. However, this differential immune and metabolic response between freshwater- and seawater-acclimated dunlins was not associated with significant changes in body mass, fat stores or plasma ions. Our results indicate that the strength of the immune response of this small-sized migratory shorebird was negatively influenced by the salinity of marine habitats. Further, these findings suggest that the reduced immune response observed under saline conditions might not be caused by an energy or nutrient limitation, and raise questions about the role of osmoregulatory hormones in the modulation of the immune system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a key abiotic factor that can greatly affect the distribution, physiological performance and reproductive success of a wide range of organisms living in or around aquatic environments (Lei and Poulin 2011). Owing to their osmoregulatory physiology, most air-breathing vertebrates inhabiting marine and other saline environments face a special problem, since they often have to cope with high salt loads (reviewed by Schmidt-Nielsen 1997; Sabat 2000; McNab 2002; Willmer et al. 2005). To successfully maintain salt and water balance, these animals need to get rid of excess sodium chloride ingested with water and food by means of highly efficient excretory organs, such as the kidneys and/or a variety of cephalic saltglands (reviewed by Peaker and Linzell 1975; Skadhauge 1981; Bentley 2002; McNab 2002), which are assumed to impose significant energetic and physiological costs (e.g. Burger and Gochfeld 1984; Hildebrandt 1997; Ortiz 2001; Gutiérrez et al. 2011a).
In birds, it has recently been shown that the basal metabolic rate (BMR) and daily energy consumption of dunlins Calidris alpina, a long-distance migratory shorebird inhabiting both coastal and freshwater habitats, increased by 17 and 20 %, respectively, during seawater acclimation (Gutiérrez et al. 2011a). In addition to direct energetic costs, other experiments have shown that high dietary salt loads can result in reduced growth rates (e.g. Schmidt-Nielsen and Kim 1964; Johnston and Bildstein 1990; Doch 1997), loss of body mass (e.g. Purdue and Haines 1977; Klaassen and Ens 1990; Gutiérrez et al. 2011a) and dehydration (e.g. Purdue and Haines 1977; Bennett et al. 2003), which may ultimately affect individual fitness (e.g. Nyström and Pehrsson 1988; Tietje and Teer 1996; Doch 1997).
Immune function plays a significant role in an organism’s response to its environment and thus has the potential to shape the evolution of life-history strategies (Demas et al. 2011). It has been shown that changes in salinity exert immunosuppressive effects in fish (e.g. Fries 1986; Cuesta et al. 2005; Jiang et al. 2008) and shellfish (e.g. Joseph and Philip 2007; Matozzo et al. 2007; Bussel et al. 2008). Therefore, one might expect to see, despite their markedly different osmoregulatory physiologies, a similar effect of salinity in birds and other air-breathing vertebrates coping with osmotically challenging environments. To date, despite its importance in life-history evolution, the mechanisms underlying salinity effects on immune prospects are unknown in air-breathing vertebrates that exploit marine and other saline environments. Hence, understanding such a link could enhance our ability to predict how organisms will respond to changing environmental conditions (Poulin and Mouritsen 2006; Hasselquist 2007).
Many species of shorebirds (suborder Charadrii) and other waterbirds spend much of their annual cycle in marine/coastal habitats (Piersma et al. 1996; Frederick 2002; Goldstein 2002; Warnock et al. 2002), where they are confronted with osmotic and ionic problems (Klaassen and Ens 1990; Battley et al. 2003; Blakey et al. 2006; Gutiérrez et al. 2011a, 2012). Coping with salt may become particularly severe for shorebirds that return to temperate coastal (saline) wetlands from their (freshwater) tundra breeding grounds in late summer and have to switch from a diet of terrestrial invertebrates to a diet of marine invertebrates, which are generally isosmotic with the sea (see Piersma 2002). This dietary shift inevitably leads to substantial increases in salt load that should be counteracted by sophisticated and flexible (nasal) saltglands (Staaland 1967; Gutiérrez et al. 2012). However, as yet, we do not know how such birds are able to completely overcome the fundamental challenges posed by salty habitats. Among the shorebirds, the dunlin Calidris alpina is an apposite subject for studying osmoregulation and related physiological parameters. This small-sized (33–85 g) calidridine sandpiper can be considered an ‘osmotic generalist’ (sensu Blakey et al. 2006), since it occurs in habitats of widely disparate salinities, ranging from 0 to 150 ‰ (Masero 2002; Gutiérrez et al. 2011a).
In the present study, we investigated the effects of seawater salinity on the strength and cost of mounting an immune response in the dunlin. The parameters examined in dunlins acclimated to either freshwater or seawater conditions were (1) phytohaemagglutinin (PHA)-induced skin swelling response, (2) basal metabolic rate (BMR), (3) body mass, (4) fat stores, and (5) plasma ions (Na+, K+ and Cl−). Previous studies have shown that producing a PHA response elevates the BMR of both passerine (Martin et al. 2003, 2006a; Nilsson et al. 2007; but see Lee et al. 2005) and non-passerine species (Gutiérrez et al. 2011b). In this context, our aim was to specifically address the following two predictions. First, if the induced osmotic challenge is sufficient to force a trade-off with the immune response to PHA, we would expect see, to some extent, evidence of immunosuppression and/or impairment of the ability to maintain the osmotic balance (i.e. an increase in plasma ionic concentration). Second, since both the osmotic and immune challenges are thought to be energetically costly, one may expect doubly challenged individuals to resort to different metabolic strategies (e.g. energy savings through reductions in BMR and/or body mass).
Materials and methods
Animal capture and housing
Thirty-two overwintering dunlins (non-moulting adults) were mist-netted at their roosting sites in rice fields in the region of Extremadura in SW Spain (39°57′N, 5°44′W) during early February 2010. Upon capture, birds were measured, ringed and colour-banded and then transported to outdoor aviaries at the University of Extremadura (see Gutiérrez et al. 2011a, b for details), where they were provided with fly larvae Protophormia sp., commercial dry food pellets (Dibaq-Diproteg) and freshwater ad libitum. Ten days after capture, when birds were maintaining a stable body mass (50.53 ± 0.89 g) similar to their capture mass (50.04 ± 0.53 g) (paired t test: t 31 = 0.42, P = 0.67), we randomly assigned them to either freshwater (n = 16; salinity: 0.3 ± 0.0 ‰) or full-strength seawater (n = 16; salinity: 35.0 ± 0.9 ‰). To do so, birds were re-housed in four adjacent outdoor cages (5 m × 2.5 m × 2 m; eight birds per cage), each equipped with either freshwater or seawater ad libitum for drinking and bathing in a large pool (2.5 m × 2 m × 2 cm deep). Birds were observed daily for periods 1.5–2 h in the morning to ensure that all individuals drank water provided in the pools. Fly larvae and food pellets were provided daily ad libitum in separate plastic trays. Birds from the different groups experienced identical conditions, except salinity. The salinity of the water was measured daily with a portable multi-parameter instrument (WTW MultiLine P4 SET; Weilheim, Germany) and corrected when necessary by adding a corresponding amount of (dechlorinated) freshwater. Birds were allowed to acclimate to these conditions for 4 weeks before assessment of immune responsiveness (see below). Aviaries were cleaned twice a week, during which the condition of the birds was checked. The daily ambient temperature during the experiment ranged from 8.8 to 16.8 °C with the mean temperature being 12.3 °C. Once the experiment was completed, all birds were fed until they maintained a stable body mass (two more weeks), and were then released at the site of capture.
Immune responsiveness: PHA skin-swelling test
The birds’ pro-inflammatory potential (i.e. the ability to produce an inflammatory response) was assessed by the PHA skin-swelling test (Smits et al. 1999; Martin et al. 2006a, b; Tella et al. 2008; Vinkler et al. 2010). The response to PHA is related to immune cell activity, involving local infiltration of tissue by most types of immune cells (including lymphocytes, basophils, eosinophils, heterophils, macrophages and thrombocytes) at the site of injection, and is widely used as an index of immune responsiveness (Martin et al. 2006a, b; Vinkler et al. 2010; Vinkler and Albrecht 2011). We injected 60 μl of 1 mg ml−1 PHA-P (Sigma L-8754) in phosphate-buffered saline (PBS) intradermally in the left wing web (patagia) of each treatment dunlin (n = 8 for both freshwater and seawater regimes). Control dunlins (n = 8 for both freshwater and seawater regimes) were injected with the same volume of PBS alone. Then, to assess the time course of the PHA response, we measured the thickness of the injected wing web in triplicate with a pressure-sensitive spessimeter (Mitutoyo Absolute cod. 543-270BS, Japan), just prior to (0 h) and 24, 48, 72, and 96 h after injections. All measurements were performed by the same person (J.M.A.G.). As the repeatability of the measurements was high (r = 0.99, P < 0.001), we used the mean value for statistical analyses (Smits et al. 1999). PHA-induced swelling response was determined by subtracting the thickness of the wing web before injections from later thickness measures.
Respirometry
BMR was measured in terms of oxygen consumption using standard flow-through respirometry, as described in detail by Gutiérrez et al. (2011a, b). Briefly, birds were measured overnight under post-absorptive digestive conditions in dark metabolic chambers (3.6 l) at 27.0 °C (±0.3), i.e. within the dunlin’s thermoneutral zone (Kendeigh et al. 1977; Kelly 2000). Birds were weighed to the nearest 0.01 g when moved into and out of the metabolic chambers, and we used the mean of these values to indicate body mass. Before the start of the metabolic measurements, birds were scored for the extent of their subcutaneous fat stores using a semiquantitative scale for shorebirds (0–7, with 7 being the fattest), as described by Meissner (2009). Oxygen consumption was measured in 48-h intervals for each bird (at 0, 48, and 96 h) in order to allow time for the recovery of body mass lost during fasting and subsequent metabolic measurements. BMR was defined as the lowest 5-min average of oxygen consumption, which was calculated according to Hill (1972). The respiratory quotient used was 0.80 (Koteja 1996), and the metabolic rate was calculated assuming an energy equivalent of 20 kJ l−1 O2 (Gessaman and Nagy 1988).
Ion concentrations
A blood sample (~100–150 μl) was taken from the brachial vein of post-absorptive birds and collected into heparinised capillary tubes just prior to PHA or PBS injection (0 h) and 7 days post-injection. After collection, blood samples were immediately centrifuged for 10 min at 6,500 rpm to separate plasma from cells and stored at −20 °C within an hour until assay. Constant osmotic and ionic concentration of plasma is an accurate and reliable sign of successful osmoregulation (Skadhauge 1981). To evaluate the osmoregulatory condition of the birds, we determined plasma Na+, Cl− and K+ concentrations using an electrolyte analyzer with ion-specific electrodes (SPOTCHEM EL SE-1510; Menarini Diagnostics, Italy).
Statistical analyses
Means are given ± standard error. BMRs are expressed in kilojoules (kJ), in compliance with the international unit system. Analyses were performed using Statistica 7.0 (StatSoft, Tulsa, OK, USA) and SAS 9.0 (SAS Institute, Cary, NC, USA), and statistical significance was accepted at P < 0.05 for all tests.
As salinity potentially affects different physiological parameters of birds, including BMR, body mass and plasma osmotic concentration (Skadhauge 1981; Hughes 2003; Gutiérrez et al. 2011a), we first examined the response of freshwater and seawater groups separately. BMR, wing-web thickness, body mass, and plasma ions were analysed using general linear mixed models (GLMMs), with treatment (PHA or PBS) and time as fixed factors, and ‘individual’ as a random effect (nested within treatment). In the BMR and immune analyses, body mass and outdoor cage temperature (0.01 °C; measured 5 cm above the ground) were used as covariates. Fat score was evaluated through a GLIMMIX procedure in SAS with multinomial distribution error and log-link function (treatment, time and random effect as above). We performed the same type of analyses to compare the four experimental categories, but ‘treatment’ had four levels (PHA-freshwater, PBS-freshwater, PHA-seawater, and PBS-seawater). As BMRs varied extensively between individuals (CV = 17.9 %), we also tested the influence of PHA (or PBS) injection on the percent change in BMR at 48 and 96 h (using BMR of the evening prior to the challenge as a baseline; e.g. Martin et al. 2006a; Verhulst et al. 2005).
Comparisons among groups at each time point were performed using general linear models (ANOVA or ANCOVA, depending on the variable). Among the 32 birds, we failed to obtain a plateau of oxygen consumption at 48 h for 2 birds (1 control bird each from the freshwater and seawater groups). These birds were removed from further BMR analyses.
Results
PHA response
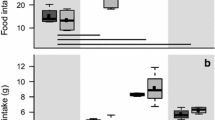
The thickness of the wing web did not differ between the experimental categories before injection (ANOVA: F 3,29 = 0.23, P = 0.87). Injection of PHA significantly increased wing-web thickness of challenged individuals relative to those injected with PBS alone (Table 1), peaking in both the freshwater and seawater group 24 h after injection (Fig. 1). After PHA injection, seawater birds always had significantly lower values of wing-web swelling than freshwater birds, with a difference in peak swelling response of 56.3 % (ANCOVA: F 1,15 = 5.16, P < 0.001; Fig. 1). Wing-web swelling response and time course induced by the PHA-injection differed as a function of the salinity (GLMM: treatment F 1,15 = 8.73, P = 0.008; treatment × time, F 4,55 = 35.71, P < 0.0001; Fig. 1). Wing-web swelling was not correlated with changes in body mass at any time, neither in the freshwater or seawater group (ANCOVA: P > 0.1 for all cases).
Basal metabolic rate
Before injection (0 h), the BMR of birds under seawater conditions was higher than under freshwater conditions, but the difference was not statistically significant (ANCOVA: F 3,27 = 1.61, P = 0.21). BMR values did not change significantly according to treatment, time, or the interaction treatment × time course, either in the freshwater or seawater dunlins (Table 1). Nor did we find any significant changes in BMR when comparing the four experimental categories together (GLMM: treatment, F 3,50 = 0.23, P = 0.87; time, F 2,50 = 1.09, P = 0.34; treatment × time, F 6,50 = 0.65, P = 0.69; Fig. 2). However, relative BMR varied significantly between PBS- and PHA-injected birds under the freshwater regime 48 h after injection, but not in seawater-acclimated dunlins (ANCOVA: F 1,12 = 5.14, P = 0.04; F 1,12 = 3.77, P = 0.08, respectively; Fig. 3). However, 96 h after injection, this difference between PHA- and PBS-injected birds under the freshwater regime turned out not to be statistically significant (F 1,12 = 0.25, P = 0.63; Fig. 3).
Body mass and fat scores
Body mass did not differ among the experimental categories before injection (ANOVA: F 3,28 = 2.29, P = 0.10), and did not change significantly over the time course, regardless of salinity (Table 1). Likewise, there were no differences in fat scores among the experimental categories before the experiment (χ 2 = 5.29, df = 3, P = 0.15), nor were there significant changes during the experiment according to treatment (GLIMMIX: treatment, F 3,28 = 0.81, P = 0.50; time, F 4,112 = 1.44, P = 0.22; treatment × time, F 12,112 = 1.78, P = 0.06).
Levels of plasma ions
At the beginning of the experiment, seawater-acclimated dunlins (PBS- and PHA-groups combined) had higher levels of plasma Na+ than freshwater-acclimated dunlins (t test: t 30 = −4.56, P = 0.02), whereas levels of plasma K+ and Cl− did not differ between environmental salinities (P = 0.47 and P = 0.78, respectively). Levels of plasma ions did not vary after the injection of either PHA or PBS, regardless of salinity (GLMM: P > 0.1 for all cases).
Discussion
The capacity to regulate plasma ions in the face of changing water (and prey) salinity is an obvious necessity for birds that rely on saline environments; however, it is not without costs. In the present study, we hypothesise that dunlins living under the osmotically challenging conditions of seawater face a trade-off between mounting an immune response and maintaining the osmotic homeostasis. Our results have shown that seawater salinity had a negative effect on the immune responsiveness of dunlins, even though they were allowed to eat at will. We have also shown that relative changes in BMR after PHA injection differed between freshwater- and seawater-acclimated dunlins. However, this differential immune and metabolic response to PHA injection between both treatments was not associated with significant changes in body mass, fat scores, or levels of plasma ions. Below, we discuss in detail several of the possible reasons that could explain the obtained results and try to provide an ecological interpretation.
In terms of immune responsiveness, we found that seawater-acclimated dunlins showed a significantly lower inflammatory response than freshwater-acclimated ones. This is consistent with the predicted trade-off between immune response and osmoregulation. Reduced immune responses are often associated with a reduction of body condition or body mass (e.g. Alonso-Alvarez and Tella 2001; Gutiérrez et al. 2011b); however, body condition (in its simplest forms, body mass and fat scores) did not differ among treatments, suggesting that salinity itself caused the observed decrease in immune responsiveness. An alternative interpretation of this result is that inflammatory responses might have been heightened in freshwater, rather than dampened in saltwater. However, this seems unlikely as there is considerable evidence that high salt loads can negatively affect distinct physiological parameters such as body mass, water balance, growth rates, or fitness (e.g. Schmidt-Nielsen and Kim 1964; Purdue and Haines 1977; Doch 1997; Bennett et al. 2003; Gutiérrez et al. 2011a).
In addition to the direct effects of salinity on organismal physiology, salinity is also considered a key factor in shaping parasite and pathogen distribution, thereby indirectly interacting with immune function. Previous studies have indicated that sea- and shorebirds living in marine/coastal saline (parasite-poor) habitats run lower risk of infection by blood parasites than those living in inland freshwater (parasite-rich) habitats (Bennett 1993; Piersma 1997; Figuerola 1999; Mendes et al. 2005; Quillfeldt et al. 2011), and it has been hypothesized that migratory shorebirds that rely on coastal saline habitats during the non-breeding season should invest less in immunity than those using freshwater habitats (Piersma 1997; Mendes et al. 2005). However, in a cross-species study of shorebirds, Mendes et al. (2006) found no difference in humoral or innate immune responses between four coastal wintering species and the ruff Philomachus pugnax (the only freshwater specialist in the study), suggesting that the relationships between immune response and infection are not likely to follow a broad general pattern. More recent studies on captive and free-living red knots Calidris canutus have provided evidence that constitutive immunity responds more strongly to differences in a bird’s immediate environment than to genetic differences (Buehler et al. 2008a, b, 2009a, b). Our results give additional, but different, support to this view, since in addition to factors such as loss of genetic variation for disease resistance (Piersma 1997), the ability of migratory shorebirds to mount an immune response might be constrained by the habitat salinity. Because of its potential impact on the immune system, salinity could affect interspecific interactions such as parasitism not only in birds but also in other secondarily air-breathing vertebrates (reptiles and mammals) that typically move between habitats with varying salinities.
From an energetic standpoint, it is noteworthy that seawater-acclimated dunlins exhibited higher BMR than freshwater-acclimated ones, although this difference failed to attain statistical significance. This result is in contrast with a previous study where seawater-acclimated dunlins had both higher BMR and lower body mass that those in a freshwater environment (Gutiérrez et al. 2011a). This discrepancy could be attributed to the NaCl content in the food dunlins consumed. In the experiment by Gutiérrez et al. (2011a), dunlins were fed with salt-loaded fly larvae, whereas here they were feeding ad libitum on live hyposmotic (with respect to seawater) prey, which could have alleviated salt stress considerably by diluting the ingested drinking water (Purdue and Haines 1975; Nyström and Pehrsson 1988; Johnston and Bildstein 1990). Nevertheless, the daily observations and the presence of nasal salt encrustations observed around the nares of our seawater dunlins indicate that they regularly drank seawater. As expected, freshwater dunlins challenged with PHA had a higher relative BMR at 48 h (presumed peak of the metabolic response; Martin et al. 2003; Gutiérrez et al. 2011b) compared to controls. This is consistent with previous studies that demonstrated that a PHA inflammatory response entails substantial energetic costs (Martin et al. 2003, 2006a; Nilsson et al. 2007; Gutiérrez et al. 2011b). However, there was no difference in relative BMR when comparing between treatment and control groups in seawater dunlins. It is known that the activation of acute phase responses following immune challenges can lead to behavioural (e.g. locomotor activity) and/or physiological changes (e.g. body temperature) (reviewed by Owen-Ashley and Wingfield 2007), which could have potentially masked a change in the BMR of our seawater-acclimated dunlins. Although we did not measure these variables in this experiment, this possibility seems unlikely, as PHA treatment in birds induces relatively mild acute phase responses with few relevant systemic effects (Klasing and Peng 1987; Merino et al. 1999). Owing to the fact that we only measured BMR at three time points after injection (0, 48, and 96 h), it is also possible that the potential rise in BMR occurred earlier or later. Nevertheless, we believe this is not the case, as other studies in birds have found that peak metabolic response typically occurs 48 h after PHA injection (Martin et al. 2003; Gutiérrez et al. 2011b; but see Martin et al. 2006a). Thus, the lack of significant differences in relative BMR between control and treatment birds under seawater conditions could have been simply a consequence of their weaker response to PHA, which was probably paralleled by a lower metabolic response (see Bonneaud et al. 2003).
From an osmoregulatory point of view, the injection of PHA did not translate into changes in plasma ion concentrations either in the freshwater or seawater dunlins, suggesting that birds successfully maintained the osmotic homeostasis throughout the experiment. One possible concern when interpreting this result is that we collected blood samples for plasma ion measurements 7 days after mitogen injection, that is, when inflammatory response was not near its peak. Therefore, the possibility that immune response was compromised by the need to maintain the osmotic balance (or vice versa) cannot be ruled out completely.
It is well established that adrenocortical hormones play a critical role in the acclimation/acclimatization of vertebrates to different environmental salinities (reviewed by Bentley 2002; McCormick and Bradshaw 2006). These hormones are essential for the functioning of the avian nasal saltglands (Peaker and Linzell 1975; Skadhauge 1981); for example, levels of plasma corticosterone, the major circulating glucocorticoid hormone in birds, are increased following salt loading in order to enhance both the rate and duration of the saltglands’ secretion (Peaker and Linzell 1975; Skadhauge 1981). At the same time, glucocorticoids in general, and corticosterone in particular, have a potential anti-inflammatory and immunosuppressive effect in birds (Sapolsky 1992; Martin et al. 2005). Prolonged exposure to corticosterone could then depress immune responses, especially cell-mediated immune responses (Sapolsky 1992; McEwen et al. 1997; Apanius 1998; Sapolsky et al. 2000; Martin et al. 2005), which would be adaptive to reduce the risk of immunopathology during stress (Råberg et al.1998). This might explain why dunlins coping with seawater exhibited a weaker inflammatory (and metabolic) response to PHA than dunlins under freshwater conditions. Investigating a possible hormonal basis for salt-stress-induced immunosuppression is therefore important.
To the best of our knowledge, this study represents the first attempt to assess the effect of salinity on the immune response of an air-breathing vertebrate. Although we detected marked differences in the magnitude of the PHA response between freshwater and seawater dunlins, it is important to note that the pro-inflammatory potential measured by the PHA test represents just one component of the immune system and cannot, alone, explain all the immunological patterns and processes (Martin et al. 2006a, b; see also Demas et al. 2011). Hence, to fully test the ideas presented here, studies on free-living birds using integrated immune measures will also be required. Whether variable conditions in the wild, such as weather conditions (especially ambient temperature), prey type and energy requirements that affect the birds’ osmoregulatory demands (Gutiérrez et al. 2012), may induce salt stress and enhance the risk of immunosuppression also remains to be studied. Furthermore, our results suggest that the relationships between salinity and immune responsiveness might not be based on energy trade-offs, but on a variety of mechanisms including modulation of the immune system by osmoregulatory hormones. This emphasises the need to study further the interrelationships between stress, osmoregulation, and immunity in order to understand the possible role played by hormones in the immune system of shorebirds and other air-breathing vertebrates that periodically exploit saline environments.
References
Alonso-Alvarez C, Tella JL (2001) Effects of experimental food restriction and body mass changes on the avian T-cell mediated immune response. Can J Zool 79:101–105. doi:10.1139/cjz-79-1-101
Apanius V (1998) Stress and immune defense. Adv Study Behav 27:133–153
Battley PF, Rogers DI, Piersma T, Koolhaas A (2003) Behavioural evidence for heat-load problems in great knots in tropical Australia fuelling for long-distance flight. Emu 103:97–103
Bennett DC, Gray DA, Hughes MR (2003) Effect of saline intake on water flux and osmotic homeostasis in Pekin ducks (Anas platyrhynchos). J Comp Physiol B 173:27–36. doi:10.1007/s00360-002-0306-8
Bennett GF (1993) Phylogenetic distribution and possible evolution of the avian species of the Haemoproteidae. Syst Parasitol 26:39–44
Bentley PJ (2002) Endocrines and osmoregulation: a comparative account in vertebrates, 2nd edn. Springer, Berlin
Blakey R, Zharikov Y, Skilleter GA (2006) Lack of an osmotic constraint on intake rate of the eastern curlew (Numenius madagascariensis). J Avian Biol 37:299–305. doi:10.1111/j.2006.0908-8857.03828.x
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379. doi:10.1086/346134
Buehler DM, Piersma T, Matson K, Tieleman BI (2008a) Seasonal redistribution of immune function in a migrant shorebird: annual-cycle effects override adjustments to thermal regime. Am Nat 172:783–796. doi:10.1086/592865
Buehler DM, Piersma T, Tieleman BI (2008b) Captive and free-living red knots exhibit differences in non-induced immunity suggesting different immune strategies in different environments. J Avian Biol 39:560–566. doi:10.1111/j.2008.0908-8857.04408.x
Buehler DM, Tieleman BI, Piersma T (2009a) Age and environment affect constitutive immune function in Red Knots (Calidris canutus). J Ornithol 150:815–825. doi:10.1007/s10336-009-0402-6
Buehler DM, Encinas-Viso F, Petit M, Vézina F, Tieleman BI, Piersma T (2009b) Limited access to food and physiological trade-offs in a long-distance migrant shorebird. II. Constitutive immune function and the acute-phase response. Physiol Biochem Zool 82:561–571. doi:10.1086/603635
Burger J, Gochfeld M (1984) Seasonal variation in size and function of the nasal salt gland of the Franklin’s Gull (Larus pipixcan). Comp Biochem Physiol 77:103–110
Bussell JA, Gidman EA, Causton DR, Gwynn-Jones D, Malham SK, Jones MLM, Reynolds B, Seed R (2008) Changes in the immune response and metabolic fingerprint of the mussel, Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress. J Exp Mar Biol Ecol 358:78–85. doi:10.1016/j.jembe.2008.01.018
Cuesta A, Laiz-Carrión R, Del Río MP, Meseguer J, Mancera JM, Esteban MA (2005) Salinity influences the humoral immune parameters of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 18:255–261. doi:10.1016/j.fsi.2004.07.009
Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80:710–730. doi:10.1111/j.1365-2656.2011.01813.x
Doch JJ (1997) Salt tolerance of nestling Laughing Gulls: an experimental field investigation. Col Waterbirds 20:449–457
Figuerola J (1999) Effects of salinity on rates of infestation of waterbirds by haematozoa. Ecography 22:681–685
Frederick PC (2002) Wading birds in the marine environment. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, New York, pp 617–655
Fries CR (1986) Effects of environmental stressors and immunosuppressants on immunity in Fundulus heteroclitus. Am Zool 26:271–282. doi:10.1093/icb/26.1.271
Gessaman JA, Nagy KA (1988) Energy-metabolism: errors in gas exchange conversion factors. Physiol Zool 61:507–513
Gutiérrez JS, Masero JA, Abad-Gómez JM, Villegas A, Sánchez-Guzmán JM (2011a) Understanding the energetic costs of living in saline environments: effects of salinity on basal metabolic rate, body mass and daily energy consumption of a long-distance migratory shorebird. J Exp Biol 214:829–835. doi:10.1242/jeb.048223
Gutiérrez JS, Masero JA, Abad-Gómez JM, Villegas A, Sánchez-Guzmán JM (2011b) Metabolic consequences of overlapping food restriction and cell-mediated immune response in a long-distance migratory shorebird, the little ringed plover Charadrius dubius. J Avian Biol 42:259–265. doi:10.1111/j.1600-048X.2011.05323.x
Gutiérrez JS, Dietz MW, Masero JA, Gill RE Jr, Dekinga A, Battley PF, Sánchez-Guzmán JM, Piersma T (2012) Functional ecology of saltglands in shorebirds: flexible responses to variable environmental conditions. Funct Ecol 26:236–244. doi:10.1111/j.1365-2435.2011.01929.x
Goldstein DL (2002) Water and salt balance in seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, New York, pp 467–483
Hasselquist D (2007) Comparative immunoecology in birds: hypotheses and tests. J Ornithol 148:S571–S582
Hildebrandt JP (1997) Changes in Na+/K+-ATPase expression during adaptive cell differentiation in avian nasal salt gland. J Exp Biol 200:1895–1904
Hill RW (1972) Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J Appl Physiol 33:261–263
Hughes MR (2003) Regulation of salt gland, gut and kidney interactions. Comp Biochem Physiol A 136:507–524. doi:10.1016/j.cbpb.2003.09.005
Jiang IF, Kumar VB, Lee DN, Weng CF (2008) Acute osmotic stress affects Tilapia (Oreochromis mossambicus) innate immune responses. Fish Shellfish Immunol 25:841–846. doi:10.1016/j.fsi.2008.09.006
Johnston JW, Bildstein KL (1990) Dietary salt as a physiological constraint in white ibis breeding in an estuary. Physiol Zool 63:190–207
Joseph A, Philip R (2007) Acute salinity stress alters the haemolymph metabolic profile of Penaeus monodon and reduces immunocompetence to white spot syndrome virus infection. Aquaculture 272:87–97. doi:10.1016/j.aquaculture.2007.08.047
Kelly JP (2000) Foraging distribution and energy balance in wintering Dunlin. PhD dissertation, University of California, Davis
Kendeigh SC, Dol’nik VR, Gavrilov VM (1977) Avian energetics. In: Pinowski J, Kendiegh SC (eds) Granivorous birds in ecosystems. Cambridge University Press, New York, pp 127–204
Klaassen M, Ens BJ (1990) Is salt stress a problem for waders wintering on the Banc d’Arguin, Mauritania? Ardea 78:67–74
Klasing KC, Peng RK (1987) Influence of cell sources, stimulating agents, and incubation conditions on release of interleukin-1 from chicken macrophages. Dev Comp Immunol 11:385–394. doi:10.1016/0145-305X(87)90082-6
Koteja P (1996) Measuring energy metabolism with open-flow respirometric systems: which design to choose? Funct Ecol 10:675–677
Lee KA, Martin LB, Wikelski MC (2005) Responding to inflammatory challenges is less costly for a successful avian invader, the house sparrow (Passer domesticus), than its less invasive congener. Oecologia 145:244–251. doi:10.1007/s00442-005-0113-5
Lei F, Poulin R (2011) Effects of salinity on multiplication and transmission of an intertidal trematode parasite. Mar Biol 158:995–1003. doi:10.1007/s00227-011-1625-7
Martin LB, Scheuerlein A, Wikelski M (2003) Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond B 270:153–158. doi:10.1098/rspb.2002.2185
Martin LB, Gilliam J, Han P, Lee KA, Wikelski M (2005) Corticosterone suppresses immune function in temperate but not tropical house sparrows, Passer domesticus. Gen Comp Endocrinol 140:126–135. doi:10.1016/j.ygcen.2004.10.010
Martin LB, Hasselquist D, Wikelski M (2006a) Immune investments are linked to pace of life in house sparrows. Oecologia 147:565–575. doi:10.1007/s00442-005-0314-y
Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M (2006b) Phytohaemagglutinin (PHA) induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20:290–300. doi:10.1111/j.1365-2435.2006.01094.x
Masero JA (2002) Why don’t Red Knots Calidris canutus feed extensively on the crustacean Artemia? Bird Study 49:304–306
Matozzo V, Monari M, Foschi J, Serrazanetti GP, Cattani O, Marin MG (2007) Effects of salinity on the clam Chamelea gallina. Part I: alterations in immune responses. Mar Biol 151:1051–1058. doi:10.1007/s00227-006-0543-6
McCormick SD, Bradshaw D (2006) Hormonal control of salt and water balance in vertebrates. Gen Comp Endocrinol 147:3–8. doi:10.1016/j.ygcen.2005.12.009
McEwen B, Biron C, Brunson K, Bulloch K, Chambers W, Dhabhar F, Goldfarb R, Kitson R, Miller A, Spencer R, Weiss J (1997) The role of adrenalcorticoids as modulators of immune function in health and disease: neural, endocrine, and immune interactions. Brain Res Rev 23:79–133
McNab BK (2002) The physiological ecology of vertebrates. A view from energetics. Cornell University Press, Ithaca
Meissner W (2009) A classification scheme for scoring subcutaneous fat depots of shorebirds. J Field Ornithol 80:289–296. doi:10.1111/j.1557-9263.2009.00232.x
Mendes L, Piersma T, Lecoq M, Spaans B, Ricklefs RE (2005) Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 109:396–404
Mendes L, Piersma T, Hasselquist D, Matson KD, Ricklefs RE (2006) Variation in the innate and acquired arms of the immune system among five shorebird species. J Exp Biol 209:284–291. doi:10.1242/jeb.02015
Merino S, Martínez J, Møller AP, Sanabria L, De Lope F, Pérez J, Rodríguez-Caabeiro F (1999) Phytohaemagglutinin injection assay and physiological stress in nestling house martins. Anim Behav 58:219–222
Nilsson JÅ, Granbom M, Råberg L (2007) Does the strength of an immune response reflect its energetic cost? J Avian Biol 38:488–494. doi:10.1111/j.2007.0908-8857.03919.x
Nyström KGK, Pehrsson O (1988) Salinity as a constraint affecting food and habitat choice of mussel-feeding diving ducks. Ibis 130:94–110. doi:10.1111/j.1474-919X.1988.tb00960.x
Ortiz RM (2001) Osmoregulation in marine mammals. J Exp Biol 204:1831–1844
Owen-Ashley NT, Wingfield JC (2007) Acute phase responses of passerine birds: characterization and seasonal variation. J Ornithol 148:S583–S591. doi:10.1007/s10336-007-0197-2
Peaker M, Linzell JL (1975) Salt glands in birds and reptiles. Cambridge University Press, Cambridge
Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631
Piersma T (2002) Energetic bottlenecks and other design constraints in avian annual cycles. Integr Comp Biol 42:51–67
Piersma T, van Gils J, Wiersma P (1996) Family Scolopacidae (sandpipers, snipes and phalaropes). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world, vol 3., Hoatzin to Auks. Lynx, Barcelona, pp 444–533
Poulin R, Mouritsen KN (2006) Climate change, parasitism and the structure of intertidal ecosystems. J Helminthol 80:183–191
Purdue JR, Haines H (1977) Salt water tolerance and water turnover in the Snowy Plover. Auk 94:248–255
Quillfeldt P, Arriero E, Martínez J, Masello JF, Merino S (2011) Prevalence of blood parasites in seabirds—a review. Front Zool 8:26
Råberg L, Grahn M, Hasselquist D, Svensson E (1998) On the adaptive significance of stress-induced immunosuppression. Proc R Soc Lond B 265:1637–1641. doi:10.1098/rspb.1998.0482
Sabat P (2000) Birds in marine and saline environments: living in dry habitats. Rev Chil Hist Nat 73:401–410. doi:10.1007/s00442-006-0377-4
Sapolsky RM (1992) Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D (eds) Behavioral endocrinology. MIT Press, Cambridge, pp 287–324
Sapolsky R, Romero L, Munck A (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. Cambridge University Press, New York
Schmidt-Nielsen K, Kim TY (1964) The effect of salt intake on the size and function of the salt gland of ducks. Auk 81:160–172
Skadhauge E (1981) Osmoregulation in birds. Springer, Berlin
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Staaland H (1967) Anatomical and physiological adaptations of the nasal glands in Charadriiformes birds. Comp Biochem Physiol 23:933–944
Tella JL, Lemus JA, Carrete M, Blanco G (2008) The PHA test reflects acquired T-cell mediated immunocompetence in birds. PLoS One 3:e3295. doi:10.1371/journal.pone.0003295
Tietje WD, Teer JG (1996) Winter feeding ecology of northern shovelers on freshwater and saline wetlands in south Texas. J Wildl Manag 60:843–855
Verhulst S, Riedstra B, Wiersma P (2005) Brood size and immunity costs in zebra finches Taeniopygia guttata. J Avian Biol 36:22–30. doi:10.1111/j.0908-8857.2005.03342.x
Vinkler M, Bainova H, Albrecht T (2010) Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol 24:1081–1086. doi:10.1111/j.1365-2435.2010.01711.x
Vinkler M, Albrecht T (2011) Handling ‘immunocompetence’ in ecological studies: do we operate with confused terms? J Avian Biol 42:490–493. doi:10.1111/j.1600-048X.2011.05499.x
Warnock N, Elphick C, Rubega MA (2002) Shorebirds in the marine environment. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, New York, pp 581–615
Willmer P, Stone G, Johnston I (2005) Environmental physiology of animals. Blackwell, Oxford
Acknowledgments
We thank Juan G. Navedo for assistance in the field. Birds were captured with permits from Junta de Extremadura (permit no. CN0001/10/AAN). Project CGL2011-27485 (Spanish Ministry of Science and Innovation) and a grant to J.S.G. from Junta of Extremadura provided financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver Love.
Rights and permissions
About this article
Cite this article
Gutiérrez, J.S., Abad-Gómez, J.M., Villegas, A. et al. Effects of salinity on the immune response of an ‘osmotic generalist’ bird. Oecologia 171, 61–69 (2013). https://doi.org/10.1007/s00442-012-2405-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2405-x