Abstract
We studied subspecies, age and environmental effects on constitutive immune function (natural antibody and complement titres, haptoglobin activity and leukocyte concentrations) in Red Knots (Calidris canutus). We compared C. c. islandica and C. c. canutus in the Wadden Sea and found no difference in immune function between subspecies. However, C. c. canutus on their wintering grounds in Banc d’Arguin had higher natural antibody and lower complement levels than C. c. canutus or C. c. islandica in the Wadden Sea. This suggests that immune function is determined more by the surrounding environment than by subspecies. We also compared age classes in the Wadden Sea and found that first year birds had significantly lower natural antibody levels than adults, but that second year birds no longer differed from adults. Finally, we examined the interaction of age and environment in Banc d’Arguin. We found that first year birds (but not adults) in a low quality habitat had higher leukocyte concentrations than first year birds or adults in a high quality habitat. Differences in available resources and defence needs between environments, and differences among individuals differentially distributed between sites, are likely important contributors to the variation in immune function we report. Future studies, which examine these factors on wild birds, will be important for our understanding of how animals function in their natural environment. (220).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals must survive in environments which differ in resources, resource demands and pathogen risk. These environments change over time, either because seasons change within an environment, or because animals migrate between environments. Furthermore, individuals may be differentially distributed among environments (i.e. on the basis of quality or age), and may differ in prior experience (i.e. recent migration) and future needs (i.e. continued migration or moult). All these factors may affect immune function or current infection status. Thus, spatial and temporal variation in immune function likely reflect differences in available resources and defence needs between environments, differences among individuals between sites and interactions between these factors.
Red Knots provide a good model system to examine spatial and temporal variation in immune function (Buehler and Piersma 2008). Their ecology is very well studied, their extensive migratory flyways cover a range of environments and catching efforts to study Knots are underway throughout many of these flyways over the annual cycle. In this study, we focus on the Calidris canutus islandica and C. c. canutus subspecies. C. c. islandica breed in northern Greenland and northeast arctic Canada and winter in western Europe, whereas C. c. canutus breed on the Taymyr Peninsula in central Siberia and winter in West Africa (Piersma 2007). The C. c. islandica and C. c. canutus flyways overlap in the Wadden Sea during southward migration.
This overlap in the use of the Wadden Sea provides a unique opportunity to compare different subspecies in a common environment, allowing us to examine the contributions of subspecies and environment to variation in immune function. C. c. islandica and C. c. canutus experience the same conditions in the Wadden Sea, and have bred in similar arctic areas and migrated similar distances from the breeding grounds before arrival (Piersma et al. 2005). After this period of overlap, however, C. c. canutus continue migration to wintering areas in Banc d’Arguin, while C. c. islandica remain in western Europe. These two environments differ in many respects including food quantity and quality (Piersma et al. 1993; Zwarts et al. 1990) and climate factors, such as temperature and salinity (Wolff and Smit 1990) which affect thermoregulatory costs (Wiersma and Piersma 1994) and may affect pathogen pressure. Thus, if the immediate environment is a dominating factor in the determination of immune function, we do not predict differences in immune indices between subspecies in the Wadden Sea; however, we do predict differences within C. c. canutus between the Wadden Sea and Banc d’Arguin.
The situation in the Wadden Sea also provides an opportunity to compare different age classes in a common environment. Knots of different ages have had different past experiences, and first year birds may still be developing their immune system. First year birds arrive in the Wadden Sea at about 2 months of age, having just completed their first migration (Piersma and Davidson 1992). Second year birds are 1 year old and have not migrated, but rather over-summer in the Wadden Sea (T. Piersma, personal observation). Adults are at least 2 years old and have just reproduced and migrated. If recent migration (or reproduction) has an effect on immune function, then we predict differences between second year birds and adults; and if immune system development is not yet complete at 2 months of age, then we predict differences between first year birds and the other age classes.
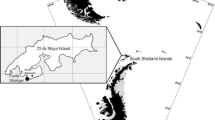
The Banc d’Arguin also provides an opportunity to look at age and environment interactions and their relationship with immune function. Within Banc d’Arguin, Knots of the C. c. canutus subspecies segregate into two roosting and feeding areas: Ebelk Aiznay and Baie d’Aouatif (see map in Fig. 3). Despite the close proximity of these sites, Knots show high fidelity to these areas (Leyrer et al. 2006a). The way that birds segregate between the sites, as well as survival data, indicate differences in habitat quality. Adults, which arrive in Banc d’Arguin earlier than first year birds, have first choice of habitat and make up a larger proportion of the birds at the Ebelk Aiznay site and a smaller proportion of the population at Baie d’Aouatif (Leyrer et al. 2006b). Furthermore, annual adult survival data from 2003 to 2008 indicate that survival in Ebelk Aiznay averaged 88 ± 1% (mean ± SE), which translates to an average lifespan of 7.9 years, whereas annual survival in Baie d’Aouatif was 77 ± 5% and lifespan was only 3.9 years (B. Spaans et al., unpublished data).
The immune system can be divided along a non-specific and a specific axis, and along a constitutive (non-induced) and induced axis (Schmid-Hempel and Ebert 2003). In this study, we focus on constitutive immune function as defined as immune function measured without inducing a response. Constitutive immunity represents a first line of defence which may be important for migrants encountering novel environments and for young birds who have not yet built up a repertoire of specific, induced responses. Furthermore, levels of constitutive immune function can be measured from a single capture making it ideal for studies on free-living birds. Specifically, we measured complement and natural antibody titres (Matson et al. 2005), haptoglobin activity (Matson 2006) and leukocyte concentrations (Campbell 1995). The complement cascade and natural antibodies provide a first line of defence against spreading infections via cell lysis, and natural antibodies link non-specific and specific immunity (Ochsenbein and Zinkernagel 2000). Haptoglobin is an acute phase protein that binds iron (haem) to keep it from providing nutrients to pathogens (Delers et al. 1988). Leukocyte concentrations provide information on circulating immune cells and can be used as an indicator of health (Campbell 1995).
In this study we examine subspecies, age and environmental effects on constitutive immune indices in two subspecies of Red Knot. Specifically we ask: How do constitutive immune indices differ between subspecies in the same environment, and within subspecies in different environments? Do first year, second year and adult C. c. islandica differ in immune function in the Wadden Sea? Does habitat quality affect immune function in C. c. canutus in Banc d’Arguin and does this effect differ between first year birds and adults?
Methods
Capture methods and samples
All birds in this study were captured at night using mist nets, and were ringed, weighed and aged on the basis of plumage characteristics (Prater et al. 1977) at capture. Sexes were determined using molecular techniques (Baker et al. 1999). In the Dutch Wadden Sea (53°15′N, 5°15′E), a total of 202 birds (31% male and 69% female) were caught during autumn migration (July–September) between July 2004 and July 2006. In Banc d’Arguin, Mauritania (19°54′N, 16°17′W) a total of 80 birds (41% male and 59% female) were caught between 13 and 21 December 2006.
Subspecies and environment comparisons in adults
We classified the subspecies C. c. islandica and C. c. canutus during overlap in the Wadden Sea using resightings in other parts of the flyway (B. Spaans, unpublished data), and based on wing moult and body mass criteria outlined in Nebel et al. (2000). To verify our classification, we also examined bill length. Validating our classification, C. c. islandica bills were significantly shorter than C. c. canutus bills in both the Wadden Sea and Banc d’Arguin (Fig. 1a, F1,109 = 10.9, P < 0.001; Nebel et al. 2000). Because C. c. canutus are refuelling in the Wadden Sea, we also examined body mass and found that C. c. canutus in the Wadden Sea were significantly heavier than C. c. islandica in the Wadden Sea or C. c. canutus in Banc d’Arguin (Fig. 1b, F 1,109 = 22.2, P < 0.001). However, body mass did not affect immune comparisons between these groups (all P’s > 0.4).
The relationship between constitutive immune indices for non-moulting adult Calidris canutus islandica and C. c. canutus in the Wadden Sea and C. c. canutus in Banc d’Aguin: bill length (a), body mass (b), agglutination (c), and lysis (d). Box plots show the median (thick line), interquartile range (boxes), range (whiskers), outliers (circles) and extremes (stars). Statistical significance determined by Tukey post-hoc tests is indicated by: ***P < 0.001, **P < 0.01, *P < 0.05. See Table 1 for full statistics
Both moulting and non-moulting C. c. islandica are found in the Wadden Sea; however, C. c. canutus use the area as a stopover on the way to wintering grounds and are not in wing moult. Because moult has been shown to affect immune function in captive Red Knots (Buehler et al. 2008b), we compared only non-moulting C. c. islandica adults (n = 27) with C. c. canutus adults (n = 21) to examine subspecies effects. To examine environmental effects, we compared C. c. canutus adults captured in Banc d’Arguin (n = 62) with both non-moulting C. c. islandica and C. c. canutus in the Wadden Sea. First year birds were not compared because we only had two C. c. canutus first year birds in the Wadden Sea.
Age comparisons in the Wadden Sea
We examined age effects in the Wadden Sea for C. c. islandica only. First year birds, second year birds and adults (older than 2 years) use the Wadden Sea during autumn migration, but they follow different schedules. Adults arrive in the area and begin prebasic and wing moult earlier than first year birds (Davidson and Wilson 1992). Second year birds generally do not travel to the breeding grounds and follow an “over-summering” annual cycle in which they are in pre-basic and wing moult when adults arrive on autumn migration (T. Piersma, personal observation). As a result, no first year bird, but all second year birds were moulting in our sample. Thus, to examine age effects in birds with similar moult status, we compared first year birds (n = 15,) with non-moulting adults (n = 27), and second year birds (n = 71) with moulting adults (n = 49).
Age and habitat quality comparisons in Banc d’Arguin
We captured first year and adult C. c. canutus at high tide roosts in Ebelk Aiznay (first year n = 3, adults n = 28) and in Baie d’Aouatif (first year n = 15, adults n = 34; see map in Fig. 3). We combined birds caught on the sandbank Zira with the sample from the high tide roost at Baie d’Aouatif. Resighting data indicated that 57% of birds resighted in a different location than where captured had moved between Zira and Baie d’Aouatif (or vice versa; J. Leyrer, unpublished data), while only 14% had moved between Zira and Ebelk Aiznay. A detailed description of the study area in Banc d’Arguin is given in Leyrer et al. (2006a).
Blood sampling
We collected 200–400 μl of blood into heparinised capillary tubes (Fisher Emergo) after sterilising the area around the brachial vein with 70% ethanol. Blood samples used for leukocyte count analysis were taken immediately after the birds were removed from the mist nets. We checked the nets every 5–25 min meaning that the absolute longest a bird could hang in a net was 25 min; thus, all samples were taken within 30 min of the bird hitting the net (mean ± SD = 14.2 ± 4.6 min). Time-series experiments show no change in leukocyte counts within 30 min of capture (Buehler et al. 2008a). Immediately after sampling, we made two blood smears and the remainder of the blood was stored in eppendorf tubes on ice and transported to the laboratory to be used in other assays. Blood samples not used for leukocyte concentrations were almost always taken within 2 h of capture (86.6 ± 54.8 min, range 3–240 min). Complement and natural antibody titres are insensitive to capture and handling times at least up to 2 h (Buehler et al. 2008a). Because the sensitivity of haptoglobin activity to capture and handling stress has not been tested, we included time between capture and sampling as a covariate in all statistics with haptoglobin as the response variable. Plasma was obtained by centrifuging blood samples for 10 min at 12,000g and was stored at −20°C.
Immune assays
Leukocyte concentrations
After staining (Giemsa Stain; Sigma-Aldrich, Germany) blood smears were examined at 1,000× magnification under oil immersion and the first 100 leukocytes were counted and classified as heterophils, eosinophils, lymphocytes or monocytes. Basophils were extremely rare (<0.5%) and were therefore not included in the counts. Eosinophils were included in the counts, but because they had a high proportion of zero values were excluded from further analysis. While counting the first 100 leukocytes, thrombocytes were also recorded as an estimate of the relative number of thrombocytes per leukocyte. Blood smears were randomised and counted blind to age and environment by a single observer (D.M. Buehler) using the criteria in Campbell (1995). Total leukocyte concentrations were obtained in combination with the blood smears using the indirect eosinophil Unopette method (Campbell 1995) following the manufacturer's instructions (No. 5877; Becton Dickinson). Sample sizes for total leukocyte concentrations are smaller than for other assays due to the need to sample birds within 30 min of capture.
Hemolysis-hemagglutination assay
We performed the assay on blood plasma as described by Matson et al. (2005). Complement action was assayed via the lysis of rabbit red blood cells and natural antibody action was assayed via the agglutination of rabbit red blood cells. Both lysis and agglutination were quantified by serial dilution. We placed 25 μl of plasma in the first and second rows of a 96-well plate and then from the 2nd to the 11th rows we performed ten 1:2 dilutions using Dulbecco’s PBS (Mauck et al. 2005). We then added 25 μl of 1% of rabbit red blood cell suspension to each well, and incubated the plates at 37°C for 90 min. After incubation, plates were tilted at a 45° angle and then digitally scanned (Epson Perfection 4990 scanner) for agglutination after 20 min and lysis after 90 min. The scans were randomised with respect to sample origin, plate, and location within the plate and were scored blindly by a single researcher (D.M. Buehler) using the criteria outlined in Matson et al. (2005).
Haptoglobin assay
Haptoglobin concentration in mg/ml was quantified from blood plasma following the ‘manual method’ instructions provided with a commercially available assay kit (#TP801; Tri-Delta Diagnostics, Morris Plains, NJ). Sample sizes for haptoglobin activity are smaller than for complement and natural antibodies because we did not have enough plasma to conduct the assay on every bird.
Statistics
We used general linear models to examine the main effects of subspecies (C. c. canutus or C. c. islandica), location (Wadden Sea or Banc d’Arguin), age (first year, second year or adult), and habitat quality (high or low in Banc d’Arguin). We included sex in our models as a co-factor, but since sex was never statistically significant and models including and excluding sex produced the same result, we present results from models excluding sex. Also, because condition may affect immune function, we ran all models with a condition index as a covariate (the unstandardised residuals from the regression of bill length on body mass). We also tested for an effect of body mass alone. Body mass was never statistically significant and condition was positively correlated with complement activity only in Banc d’Arguin (R 2 = 0.093, F = 6.34, P = 0.015, η 2P = 0.09). Models including and excluding these covariates produced the same result, therefore we present results from models excluding body mass and condition. For comparisons using data obtained from the Wadden sea in different years, we included year as a co-factor in our models. Finally, we included time between capture and blood sampling as a covariate for haptoglobin activity.
All data and residuals of parametric models were tested for normality using 1-sample Kolmogorov–Smirnov tests and histograms were examined visually. Leukocyte concentrations and haptoglobin activity were logarithmically (base 10) transformed. Agglutination was not normally distributed and transformation did not improve the situation, thus we present the results of both parametric and non-parametric models. Because we performed multiple comparisons, we present partial eta-squared values (η 2P ) as a measure of effect size. We do not use Bonferroni corrections because these may be problematic for ecological studies with small sample sizes (Nakagawa 2004). We used SPSS 14.0 for all statistical procedures.
Results
Subspecies and environment comparisons in adults
We found higher natural antibody mediated agglutination in C. c. canutus in Banc d’Arguin versus C. c. canutus and C. c. islandica in the Wadden Sea (Fig. 1c; F 1,109 = 4.96, P = 0.01, η 2P = 0.08; Kruskal–Wallis Test, Chi-square = 9.36, df = 2, P = 0.009). Conversely, we found lower complement mediated lysis in C. c. canutus in Banc d’Arguin versus C. c. canutus and C. c. islandica in the Wadden Sea (Fig. 1d; F 1,109 = 16.04, P < 0.001, η 2P = 0.23). Sample sizes were not large enough for comparisons of haptoglobin activity or total leukocyte concentrations (n = 1 for non-moulting adult C. c. islandica in the Wadden Sea).
Age comparisons in the Wadden Sea: C. c. islandica
Natural antibody mediated agglutination was lower in first year birds than in adults (Fig. 2a, Table 1; Mann–Whitney U = 82, Wilcoxon W = 202, z = −3.31, P = 0.001), but complement mediated lysis did not differ (Fig. 2b, Table 1). Second year birds and adults did not differ in natural antibody or complement titres, haptoglobin activity or leukocyte concentrations (Tables 1 and 2). Sample sizes were not large enough for comparisons of haptoglobin or total leukocyte concentrations between first year birds and adults (n = 1 for both first year birds and non-moulting adults).
The relationship between constitutive immune indices and age for C. c. islandica in the Wadden Sea: Agglutination (a) and lysis (b). Box plots show the median (thick line), interquartile range (boxes), range (whiskers), outliers (circles) and extremes (stars). Statistical significance at P < 0.01 is indicated by **. See Table 1 for full statistics
Age and habitat quality comparisons in Banc d’Arguin: C. c. canutus
There were no age or habitat main effects or interactions for natural antibody mediated agglutination, complement-mediated lysis or haptoglobin in Banc d’Arguin (Fig. 3a–c, Table 3). However, a significant age by habitat interaction indicated that first year birds had higher total leukocyte concentrations in Baie d’Aouatif than in Ebelk Aiznay, while adults did not differ (Fig. 3d, Table 3). This pattern was based mainly on heterophils and lymphocytes (Fig. 3e, f); whereas little effect was seen in monocytes or thrombocytes (Fig. 3g, h). First year birds showed a trend for higher monocytes irrespective of habitat (Table 3).
The relationship between constitutive immune indices, age and habitat for C. c. canutus in Banc d’Arguin. Agglutination (a) lysis (b), haptoglobin (c), total and differential leukocyte concentrations (d–h). Statistical significance at P < 0.05 is indicated by *. See Table 3 for full statistics. Box plots show the median (thick line), interquartile range (boxes), range (whiskers), outliers (circles) and extremes (stars). The dashed line in (a) indicates median values for C. c. islandica in the Wadden Sea. The map details the Banc d’Arguin study site and its location in Africa
Discussion
Subspecies and environment effects on immune indices
We found differences in immune indices between environments within C. c. canutus, but no differences between C. c. islandica and C. c. canutus while their flyways overlapped in the Wadden Sea (Fig. 1c, d). This result suggests that, at least at the within-species level, constitutive immunity responds more strongly to differences in a bird’s immediate environment than to genetic differences or prior events. This result is not surprising given that Knot subspecies are genetically similar (at least in terms of mitochondrial DNA; Buehler and Baker 2005) and that C. c. islandica and C. c. canutus breed and migrate in very similar environments prior to arrival in the Wadden Sea (Piersma et al. 2005). However, after the period of overlap in the Wadden Sea, C. c. islandica begin prebasic and wing moult, while C. c. canutus continue migration. Thus, our results also suggest that C. c. canutus do not adjust immune indices in anticipation of future challenges (i.e. migration or different pathogen pressures in West Africa) while still in the Wadden Sea.
Comparing between environments, we found higher natural antibody and lower complement titres in Banc d’Arguin than in the Wadden Sea. Differences in natural antibody levels may represent differences among individuals sampled rather than adjustments within individuals to the environment. It is now thought that birds using the Wadden Sea are only a subset of the total C. c. canutus population wintering in Banc d’Arguin (T. Piersma et al., unpublished data). Furthermore, natural antibodies are unique among other indices measured in this study in that they are not flexible over the annual cycle (Buehler et al. 2008b), and their levels cannot be tied to current infection status (Matson et al. 2005). Natural antibodies differ from specific antibodies in that they are present in the absence of exogenous antigenic stimulation (Ochsenbein et al. 1999), they have broad specificity but low affinity to antigens (can bind to more than one antigen, but only weakly; Baumgarth et al. 2005), and they appear to confer little or no immunological memory (Janeway et al. 2004). Taken together this suggests that, after initial development, a natural antibody repertoire likely characterises an individual and would not be expected to change between environments.
In contrast, complement activity is flexible over the annual cycle (Buehler et al. 2008b), thus the lower levels of complement activity we found in Banc d’Arguin may be the result of a combination of among-individual differences and within individual adjustments to the environment (see below). From an environmental standpoint, resource quality may be lower in Banc d’Arguin (van Gils et al. 2005), whereas resource demands may be higher due to higher predation pressure (more energy and time spent avoiding predators; T. Piersma, unpublished data). Thus, birds in Banc d’Arguin may have fewer resources available for investment in immune defence. In terms of pathogen pressure, differences in climate and salinity between Banc d’Arguin and the Wadden Sea may lead to lower overall pathogen threat in Banc d’Arguin, or more likely, different pathogen communities requiring different strategies of immune defence between the two environments. However, these ideas remain to be tested (see below).
It is also important to note that the differences seen within C. c. canutus may be affected by a combination of both environmental and seasonal differences since C. c. canutus in the Wadden Sea are on migratory stopover, whereas C. c. canutus in Banc d’Arguin are wintering. The fact that migrating C. c. canutus did not differ from non-migrating C. c. islandica in the Wadden Sea supports the notion that the differences we report are based on environmental influences. This is further strengthened by the fact that we did not find differences between moulting and non-moulting C. c. islandica within the Wadden Sea for natural antibodies (Fig. 2a; F 1,75 = 0.03, P = 0.86; Mann–Whitney U = 160.5, Wilcoxon W = 538.5, z = −0.047, P = 0.964), complement (Fig. 2b; F 1,75 = 0.30, P = 0.59) or haptoglobin (F 1,18 = 0.06, P = 0.81). However, immune indices which we were not able to measure or we did not have adequate sample sizes to look at in this study will need to be examined in the future. In a year-long study on captive C. c. islandica, natural antibody and complement titres did not vary with moult; however, moult did seem to affect other indices of immune function. Heterophils and some measures of microbial killing were lower during peak moult, while lymphocyte and monocyte concentrations increased. Therefore, to definitively tease apart environmental and seasonal influences, we suggest future sampling of leukocyte concentrations and microbial killing during different annual cycle stages within the same environment (e.g. moulting and non-moulting C. c. islandica in the Wadden Sea, and moulting and non-moulting C. c. canutus in Banc d’Arguin).
Age effects on immune indices in C. c. islandica in the Wadden Sea
First year birds had significantly lower natural antibody levels than adults (Fig. 2a). Like acquired antibody repertoires, natural antibody repertoires are developed early in life (Baumgarth et al. 2005; Janeway et al. 2004). Our results suggest that first year birds, which are only about 2 months old, are still developing their natural antibody repertoire. In chickens, natural antibody levels increase rapidly between 20 days and 12 weeks of age (Matson et al. 2005; Seto and Henderson 1968). We also found that second year birds no longer differed from adults (Fig. 2a), which suggests that in Red Knots natural antibody development is completed within the first year of life. Indeed, natural antibody repertoire may be developed by about 6 months in Red Knots, since C. c. canutus first year birds did not differ from adults in Banc d’Arguin and their titres were higher than those of 2-month-old C. c. islandica, but similar to those of adult C. c. islandica (Fig. 3a). In contrast, complement requires no early repertoire development (Janeway et al. 2004); therefore, it is not surprising that we did not find differences complement between first year birds and adults.
The lack of differences in immune indices between second year birds, which over-summer in the Wadden Sea, and adults, which have just migrated, indicates that recent migratory flights may have little effect on immune function, at least after the birds have landed and refuelled.
Habitat quality and age effects on immune indices in C. c. canutus in Banc d’Arguin
Age and environment had interactive effects in Banc d’Arguin. First year birds (but not adults) in Baie d’Aouatif had higher leukocyte concentrations than first year birds or adults in Ebelk Aiznay (Fig. 3d). Ebelk Aiznay and Baie d’Aouatif differ in many respects that might explain a difference in immune function between sites (see below). But why were only juveniles affected by these differences? High leukocyte counts may indicate current infection, thus juveniles in Baie d’Aouatif may be fighting disease while adults are not. C. c. canutus spend 6 months of the year in Banc d’Arguin, where they presumably encounter similar pathogens year after year, and thus adults might possess immunity (i.e. specific antibodies) against pathogens that juveniles are encountering for the first time. To test this hypothesis, we would need more information about specific pathogens infecting knots in Banc d’Arguin, antibody assays for those specific antigens and records of infection history.
Contributors to spatial variation in immune indices at the environmental and individual level
This study found significant spatial variation in immune indices (i.e. between the Wadden Sea and Banc d’Arguin, and between high and low quality habitats within Banc d’Arguin). The sites at which we sampled differ along two main lines that could contribute to the patterns we describe: features of the environment itself, and differences among individuals differentially distributed between sites.
Environments at our sampling sites differ in resource availability, resource demands and possibly pathogen pressure, all of which could contribute to a bird’s immune profile (Piersma 2006). Food quantity and quality have differed historically in both the Wadden Sea and Banc d’Arguin (Piersma et al. 1993; Zwarts et al. 1990) and today food is likely more abundant (T. Piersma, unpublished data), but of lower quality in Banc d’Arguin (van Gils et al. 2005). Ambient temperatures also differ between the sites, affecting thermoregulatory costs, with birds in Banc d’Arguin spending less on thermoregulation (Wiersma and Piersma1994). In terms of predation risk, there appear to be more predators in Banc d’Arguin (though the situation may be changing; T. Piersma, unpublished data) leading to higher time and energy expenditure on predator avoidance. Finally, temperature and salinity differences between the Wadden Sea and Banc d’Arguin (Wolff and Smit 1990) may affect pathogen pressure between the sites; however, this has not yet been studied. Within Banc d’Arguin, food quality appears to be lower in Baie d’Aouatif (J.A. van Gils, unpublished data), predation risk appears to be higher in Baie d’Aouatif (P.J. van den Hout and J.A. van Gils, unpublished data) and pathogen pressure may also differ, though that, too, has yet to be studied.
Resource availability in terms of food quantity and quality, and resource demand in terms of thermoregulation and predation pressure (energy and time spent avoiding predators), affect the amount of resources a bird has to invest in their immune system. Furthermore, survival in environments with differing pathogen pressures likely requires differing investments in immune protection. However, these environmental influences can affect immune investment in complex and sometimes conflicting ways. For example, higher temperatures in Africa mean lower thermoregulatory costs, but this may be overruled by higher energy and time expenditure on predator avoidance. Furthermore, higher food abundance may be negated by lower food quality. In terms of pathogen pressure, some pathogens may be more prevalent in the tropics (i.e. malaria), but others may do better in the temperate zone (i.e. influenza). Additionally, climate and salinity differences between temperate estuarine mudflats and tropical desert mudflats may affect pathogen pressure in different ways. Higher temperature in the tropics may argue for higher overall pathogen pressure; however, higher salinity and lower humidity in desert mudflats may argue for lower overall pathogen pressure. Therefore, we suggest future studies which quantify these environmental influences directly in order to test which environmental variables underlie the variability we present.
The second major factor affecting immune function between sites is the fact that individuals of differing quality (or age) are differentially distributed between sites. For example, birds sampled in the Wadden Sea may represent only a subset of the total C. c. canutus population in Banc d’Arguin. Thus, the differences we find may be affected by the fact that birds sampled in the different locations represent different individuals. Within Banc d’Arguin, lower quality individuals are likely found in the lower quality habitat at Baie d’Aouatif. Birds appear to select their roost site upon arrival in Banc d’Arguin and lower quality birds, which arrive late, likely get the last choice. The age and sex segregation of individuals between the two sites (more females and more adults at Ebelk Aiznay; Leyrer et al. 2006b) supports this idea: females arrive before males, and adults arrive before juveniles (Piersma et al. 1992). Additionally, more aggression between individuals (especially juveniles) occurs in Ebelk Aiznay (P.J. van den Hout, unpublished data), supporting the idea that individuals must be of high quality both to obtain and to retain their position in the higher quality habitat. Therefore, the differences we find in immune function may be caused by individual differences, in addition to, and likely interacting with, environmental differences between sites.
As discussed above, it is beyond the scope of this study to say which environmental and individual level factors explain the patterns in our data. Indeed, interactions between all of these factors likely contribute to overall investment in immune function or current infection status in wild birds. What is clear from this study is the importance of age and environment on immune function. Future studies, on wild birds, that examine questions of how resource availability, resource demands, predation, pathogen pressure and individual quality affect immune function will be important for our understanding of how animals function in their natural environment.
Zusammenfassung
Alter und Umweltfaktoren beeinflussen Paratemeter des Konstitutiven Immunsystems beim Knutt (Calidris canutus)
Am Knutt haben wir die Einflüsse von Unterart-Zugehörigkeit, Alter und Umweltfaktoren auf die Konstitutive Immunabwehr (Titer von natürlichen Antikörpern und Komplementsystem, Haptoglobin-Aktivität und Leukozyten-Konzentrationen) untersucht.
Beim Vergleich von C. c. islandica und C. c. canutus im Wattenmeer fanden wir keinen Unterschied in der Immunfunktion zwischen den Unterarten. In den Überwinterungsgebieten an der Banc d’Arguin hatte C. c. canutus jedoch höhere Titer natürlicher Antikörper und niedrigere Komplementsystemwerte als C. c. canutus oder C. c. islandica im Wattenmeer. Das legt nahe, dass die Immunfunktion stärker von Umweltfaktoren, als von der Unterartzugehörigkeit bestimmt wird. Im Wattenmeer verglichen wir außerdem Altersklassen und fanden heraus, dass erstjährige Vögel deutlich niedrigere Level natürlicher Antikörper hatten, als adulte Vögel, dass sich Vögel im zweiten Jahr aber nicht mehr von adulten unterschieden. Schließlich untersuchten wir die Interaktion zwischen Alter der Knutts und Umweltfaktoren an der Banc d’Arguin. Wir fanden heraus, dass erstjährige (nicht jedoch adulte) Vögel in einem Habitat geringer Qualität höhere Leukozyten-Konzentrationen aufwiesen, als erstjährige oder adulte Vögel in einem qualitativ hochwertigen Habitat. Unterschiede in der Ressourcenverfügbarkeit und in der Notwendigkeit einer Immunabwehr und Unterschiede zwischen Individuen, die sich unterschiedlich auf die Flächen verteilen tragen wahrscheinlich bedeutend zur nachgewiesenen Variation in der Immunfunktion bei. Zukünftige Arbeiten, die diese Faktoren an Wildvögeln untersuchen, sind für unser Verständnis darüber, wie Tiere in ihrer natürlicher Umwelt zurechtkommen, wichtig.
References
Baker AJ, Piersma T, Greenslade AD (1999) Molecular vs. phenotypic sexing in red knots. Condor 101:887–893
Baumgarth N, Tung JW, Herzenberg LA (2005) Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Semin Immunopathol 26:347–362
Buehler DM, Baker AJ (2005) Population divergence times and historical demography in red knots and dunlins. Condor 107:497–513
Buehler DM, Piersma T (2008) Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Philos Trans R Soc Lond B 363:247–266
Buehler DM, Bhola N, Barjaktarov D, Goymann W, Schwabl I, Tieleman BI, Piersma T (2008a) Constitutive immune function responds more slowly to handling stress than corticosterone in a shorebird. Physiol Biochem Zool 81:673–681
Buehler DM, Piersma T, Matson K, Tieleman BI (2008b) Seasonal redistribution of immune function in a shorebird: annual cycle effects override adjustments to thermal regime. Am Nat 172:783–796
Campbell TW (1995) Avian hematology and cytology, 2nd edn. Iowa State University Press, Ames Iowa
Davidson NC, Wilson JR (1992) The migration system of European-wintering knots Calidris canutus islandica. Wader Study Group Bull 64(Supplement):39–51
Delers F, Strecker G, Engler R (1988) Glycosylation of chicken haptoglobin: isolation and characterization of three molecular variants and studies of their distribution in hen plasma before and after turpentine-induced inflammation. Biochem Cell Biol 66:208–217
Janeway CA, Travers P, Walport M, Shlomchik M (2004) Immunobiology: the immune system in health and disease, 6th edn. Garland, New York
Leyrer J, Spaans B, Camara M, Piersma T (2006a) Small home ranges and high site fidelity in red knots (Calidris c. canutus) wintering on the Banc d’Arguin, Mauritania. J Ornithol 147:376–384
Leyrer J, Spaans B, Piersma T (2006b) Sex, age and survival differences between adjacent functional units of tropical wintering habitat in a flocking long-distance migrant shorebird. J Ornithol 147 (Supplement 1 Abstracts of the 24th International Ornithological Congress, Hamburg, Germany):202
Matson KD (2006) Are there differences in immune function between continental and insular birds? Proc R Soc Lond B 273:2267–2274
Matson KD, Ricklefs RE, Klasing KC (2005) A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 29:275–286
Mauck RA, Matson KD, Philipsborn J, Ricklefs RE (2005) Increase in the constitutive innate humoral immune system in Leach’s storm-petrel (Oceanodroma leucorhoa) chicks is negatively correlated with growth rate. Funct Ecol 19:1001–1007
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Nebel S, Piersma T, van Gils JA, Dekinga A, Spaans B (2000) Length of stopover, fuel storage and a sex-bias in the occurrence of red knots Calidris canutus canutus and C.c. islandica in the Wadden Sea during southward migration. Ardea 88:165–176
Ochsenbein AF, Zinkernagel RM (2000) Natural antibodies and complement link innate and acquired immunity. Immunol Today 21:624–630
Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM (1999) Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156–2159
Piersma T (2006) Understanding the numbers and distributions of waders and other animals in a changing world: habitat choice as the lock and the key. Stilt 50:3–14
Piersma T (2007) Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J Ornithol 148(Suppl 1):S45–S59
Piersma T, Davidson NC (1992) The migrations and annual cycles of five subspecies of knots in perspective. Wader Study Group Bull 64(Suppl):187–197
Piersma T, Prokosch P, Bredin D (1992) The migration system of Afro-Siberian knots Calidris canutus canutus. Wader Study Group Bull 64(Suppl):52–63
Piersma T, de Goeij TP, Tulp I (1993) An evaluation of intertidal feeding habitats from a shorebird perspective: towards relevant comparisons between temperate and tropical mudflats. Netherlands J Sea Res 31:503–512
Piersma T, Rogers DI, González PM, Zwarts L, Niles LJ, de Lima Serrano do Nascimento I, Minton CDT, Baker AJ (2005) Fuel storage rates before northward flights in red knots worldwide: facing the severest constraint in tropical intertidal environments? In: Greenberg R, Marra PP (eds) Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press, Baltimore, pp 262–273
Prater AJ, Marchant JH, Vuorinen J (1977) Guide to the identification and aging of Holarctic waders. BTO Guide, Tring, UK
Schmid-Hempel P, Ebert D (2003) On the evolutionary ecology of specific immune defence. Trends Ecol Evol 18:27–32
Seto F, Henderson WG (1968) Natural and immune hemagglutinin forming capacity of immature chickens. J Exp Zool 169:501–511
van Gils JA, Battley PF, Piersma T, Drent R (2005) Reinterpretation of gizzard sizes of red knots world-wide, emphasizes overriding importance of prey quality at migratory stopover sites. Proc R Soc Lond B 272:2609–2618
Wiersma P, Piersma T (1994) Effects of microhabitat, flocking, climate and migratory goal on energy expenditure in the annual cycle of knots. Condor 96:257–279
Wolff WJ, Smit CJ (1990) The Banc d’Arguin, Mauritania, as an environment for coastal birds. Ardea 78:17–38
Zwarts L, Blomert A-M, Ens BJ, Hupkes R, van Spanje TM (1990) Why do waders reach high feeding densities on the intertidal flats of the Banc d’Arguin, Mauritania? Ardea 78:39–52
Acknowledgments
We thank Alberto Castillo for help in the field and laboratory; Nina Bhola, Daliborka Barjaktarov, François Vézina, Magali Petit, Anne Dekinga, Bernard Spaans and the crew of the H. M. Navicula for support in the Wadden Sea; Bernard Spaans, Jutta Leyrer, Jeroen Reneerkens, Edward Koomson, H. ould M. El Hacen and M. Camara for support in Mauritania; Bernard Spaans and Jutta Leyrer for help identifying C. c. islandica and C. c. canutus from resight/recapture data; Anneke Bol for help with molecular sexing and Dick Visser for finalising the figures. Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) PGSB-267701-2003, a University of Groningen Ubbo Emmius Scholarship, a Netherlands Organization for Scientific Research (NWO) travel grant, and Schure-Beijerinck-Popping Fonds to D.M.B., N.W.O. and the University of Groningen to B.I.T. and T.P., operational grants from the Royal Netherlands Institute for Sea Research (NIOZ) to T.P. and a MAVA grant to T.P. and J. Leyrer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
Buehler, D.M., Irene Tieleman, B. & Piersma, T. Age and environment affect constitutive immune function in Red Knots (Calidris canutus). J Ornithol 150, 815–825 (2009). https://doi.org/10.1007/s10336-009-0402-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-009-0402-6