Abstract
Epididymosomes (apocrine secreted epididymal vesicles) are assumed to play a crucial role in sperm maturation. Our aim has been to analyze the fusogenic properties of bovine epididymosomes and their involvement in the transfer of membrane components (lipids, proteins, plasma membrane Ca2+-ATPase 4 [PMCA4]) into bovine sperm. The fusogenic properties of epididymosomes with spermatozoa were investigated in vitro by using octadecyl rhodamine-B (R18)-labeled epididymosomes. Spermatozoa isolated from the epididymal caput showed a higher fusion rate than those taken from the cauda. The fusion rate was dependent on pH and time. Furthermore, the lipid and protein content in spermatozoa changed during epididymal transit and after in vitro fusion with epididymosomes. Following the in vitro fusion of caput spermatozoa with epididymosomes, the cholesterol/total phospholipid ratio of the sperm plasma membrane decreased. The effect was comparable with the cholesterol/total phospholipid ratio of native cauda spermatozoa. Co-incubation experiments of spermatozoa with biotinylated epididymosomes additionally revealed that proteins were transferred from epididymosomes to sperm. To examine the potential transfer of epididymis-derived PMCA4 to spermatozoa, immunofluorescence analysis and Ca2+-ATPase activity assays were performed. In caput spermatozoa, the PMCA4 fluorescence signal was slightly raised and Ca2+-ATPase activity increased after in vitro fusion. Thus, our experiments indicate significant changes in the lipid and protein composition of epididymal sperm following interaction with epididymosomes. Moreover, our results substantiate the presumption that PMCA4 is transferred to spermatozoa via epididymosomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spermatozoa acquire their fertilization capacity during their passage through the epididymis (Cooper 1998, 1999). Thereby, in addition to alterations in the phospholipid and cholesterol composition of the sperm plasma membrane, surface proteins are modified, eliminated, or added. The mechanism by which epididymal proteins are transferred onto the surface of the maturing spermatozoa remain unknown (for reviews, see Caballero et al. 2011; Cooper 1998, 1999). The epididymal epithelium has been shown to release secretory proteins via both a merocrine and an apocrine pathway (Hermo and Jacks 2002). The apocrine secretion mode has been analyzed in detail for the rat coagulating gland by our group. Apocrine-secreted proteins are synthesized on polyribosomes and are released through blebs (“aposomes”) formed by the apical plasma membrane. Resident cytoplasmic proteins, such as lactate dehydrogenase (LDH), are however excluded from the aposomes during the apocrine secretion process (Aumüller et al. 1999; Groos et al. 1999; Wilhelm et al. 1998, 1999). The macrophage migration inhibitory factor (MIF) is secreted by epithelial cells of the rat epididymis via such an apocrine secretion mode (Eickhoff et al. 2001). This factor (Eickhoff et al. 2001) and other proteins are assumed to be transferred to spermatozoa via a cell-to-cell transfer from apocrine secreted vesicles of the epididymal epithelium onto the epididymal sperm (Caballero et al. 2011; Frenette et al. 2002; Frenette and Sullivan 2001; Kirchhoff and Hale 1996; Sullivan et al. 2005; Wandernoth et al. 2010).
Previous work of our group has shown that Ca2+-ATPase activity in bovine spermatozoa is higher in spermatozoa isolated from the epididymal cauda relative to spermatozoa isolated from the epididymal caput (Sanchez-Luengo et al. 2004). In further studies, we have been able to establish that variable quantities of the two splice variants of the plasma membrane Ca2+-ATPase (PMCA) isoform 4 (4a and 4b) are present in epididymal bovine spermatozoa, with a clear shift of the 4b to the 4a calcium pump from caput to cauda spermatozoa. As PMCA4a is exclusively detectable in the apical membranes of epithelial cells of the epididymal cauda, we have hypothesized that PMCA4a is transferred onto bovine sperm in the cauda epididymidis via aposomes (epididymosomes; Brandenburger et al. 2011). PMCA4 seems to be an important epididymal protein necessary for spermatozoa function, as homozygous PMCA4 knockout mice are known to be infertile (Okunade et al. 2004; Schuh et al. 2004). In mouse spermatozoa, PMCA has been shown to be the essential pump for achieving a low resting Ca2+ concentration (Wennemuth et al. 2003).
In the present study, we have examined the in vitro fusion of epididymosomes with spermatozoa by using the fluorescence self-quenching method (Arienti et al. 1997). Furthermore, the influence of epididymosomes on lipid composition, on Ca2+-ATPase activity and on protein localization in general and for PMCA4 in particular has been analyzed by in vitro experiments on purified epididymosomes and sperm taken from the epididymal caput. Taken together, our results strongly indicate that epididymosomes are capable of fusing with spermatozoa and thereby are involved in changes of the lipid and protein composition of the membrane of epididymal sperm rendering them functionally mature.
Materials and methods
Materials
Chemicals and reagents were obtained from Acros Organics (Geel, Belgien), Merck (Darmstadt, Germany), Roth (Mannheim, Germany), Sigma Aldrich (Deisenhofen, Germany), and Serva (Heidelberg, Germany), unless otherwise specified.
Preparation of bull tissue and isolation of spermatozoa and epididymosomes from bovine epididymis
Bovine epididymides were obtained from the local slaughterhouse immediately after slaughter. To release sperm and epididymosomes from the caput and cauda epididymidis, the tissue was cut several times with a sharp razor blade and was swirled in TRIS-buffered saline (TBS, pH 6.5). Sperm were pelleted at 700g for 10 min at 4 °C, re-suspended and washed in TBS (pH 6.5). The supernatant contained epididymosomes and cell debris. To eliminate cell debris, the supernatant was centrifuged at 12,000g for 20 min at 4 °C. The resulting supernatant was centrifuged at 100,000g for 60 min at 4 °C. The pellet, which contained epididymosomes and amorphous material, was re-suspended in TBS. Epididymosomes were purified by chromatography on a Sephadex G-200 column that had been equilibrated with TBS (pH 6.5), sedimented at 100,000g for 1 h at 4 °C and re-suspended in fusion buffer (0.32 mol/l sucrose in 20 mmol/l MES or 2 mmol/l HEPES) at the required pH 5–8.
Microsomal membranes of bovine epididymidis were prepared as previously described (Sikdar et al. 1991). The epididymal tissue was homogenized in homogenization buffer (25 mM imidazole pH 7.5, 1 mM EDTA, 1 mM dithiothreitole, 250 mM sucrose, 1 tablet/50 ml protease inhibitor Complete [Roche Diagnostics, Mannheim, Germany]) by using a tissue homogenizer. The homogenate was centrifuged at 600g for 10 min at 4 °C. The pellet was re-suspended in homogenization buffer and centrifuged again. The pooled supernatants were collected. These steps were repeated and the pooled supernatants were spun at 12,000g for 15 min at 4 °C. The microsomal membranes were sedimented at 100,000g for 1 h at 4 °C. The pellet was re-suspended in homogenization buffer. Protein concentrations were determined as previously described (Bradford 1976).
Labeling of epididymosomes with octadecyl rhodamine-B or biotin
The insertion of octadecyl rhodamine-B (R18; Pierce, Brockville, ON, Canada) into epididymosomes (epididymosomal membrane) was performed as previously described (Hoekstra et al. 1984). R18 was dissolved in 1 ml ethanol (1 mg/ml) and 50 μl of this solution was added to 1 ml epididymosome suspension. The mixture was kept in the dark for 1 h at room temperature. Subsequently, the epididymosomes were chromatographed on a Sephadex G-50 column to eliminate unbound R18 by using TBS (pH 7.5) as running buffer.
The biotinylation of epididymosomes (epididymosomal proteins) was performed as described by Frenette et al. (2002). Purified epididymosomes (500 μg) were re-suspended in 250 μl phopshate-buffered saline (PBS, pH 7.4) containing 0.7 mg sulfo-NHS-LC-biotin (Pierce, Thermo Fisher Scientific, Bonn, Germany) at room temperature for 30 min. The biotin-epididymosome suspension was diluted with 3750 μl 0.15 M NaCl and was centrifuged at 100,000g for 1 h at 4 °C. The pellet was re-suspended in a small volume of 0.15 M NaCl.
In vitro fusion of epididymosomes and spermatozoa
Assay with R18-labeled epididymosomes
The in vitro fusion of R18-loaded epididymosomes with unlabeled epididymal spermatozoa was performed as previously described (Arienti et al. 1997; Palmerini et al. 1999). The fusion assay was carried out in a cuvette containing fusion buffer (0.32 mol/l sucrose in 20 mmol/l MES (pH 5) or 0.32 mol/l sucrose in 2 mmol/l HEPES pH 7–8) and 50 μg R18-loaded epididymosomes. The fusion process was started by adding unlabeled sperm (100 μg) to the epididymosomes. The increments of fluorescence intensity at the emission wavelength of 580 nm (excitation 560 nm) were monitored by using a Delta Scan Illumination System for 10 min at 37 °C. The calibration of the assay was performed by taking the fluorescence produced after the addition of 0.1% Triton X-100 as 100%. No increase of fluorescence was detected before the addition of sperm. The following relationship (Corazzi et al. 1991) was used to calculate the extent of fusion from the percentage of fluorescence self-quenching relief: F = PD (1 + r/s), where F was the fusion, PD was the percentage of fluorescence dequenching, r was the amount of R18-loaded epididymosomes (μg), and s was the amount of unloaded sperm (μg). Fluorescence microscopy on sperm smears was also performed to verify the transfer of membrane components at selected pH values (pH 5, pH 6.5, pH 7). Sperm smears were prepared as described below.
Assay with biotinylated or unlabeled epididymosomes
The in vitro fusion of biotinylated or unlabeled epididymosomes (50 μg protein) with unlabeled epididymal spermatozoa (107) was performed in a Falcon tube containing fusion buffer (0.32 mol/l sucrose in 20 mmol/l MES pH 6.5, plus 1 mM ZnCl2) at 37 °C for 3 h as previously described (Frenette et al. 2002). At the end of the fusion process, the epididymal sperm were washed three times in 1 ml 0.15 M NaCl and centrifuged at 700g for 5 min at 4 °C. The supernatant with the epididymosomes was discarded and the pellet with sperm was used for further analysis (immunocytochemistry, lipid analyses, Ca2+-ATPase assays) as described below.
SDS-polyacrylamide gel electrophoresis and Western blotting
Protein concentrations of epididymosomes and of epididymal homogenate and cytosol were determined as previously described (Bradford 1976). Samples containing 15 μg (epididymosomes) or 30 μg protein (cytosol, homogenate) were separated on 4%–12% SDS-polyacrylamide gels (NuPAGE Noves; Bis-Tris Mini Gels, Invitrogen, Karlsruhe, Germany). Proteins were transferred onto 0.45-μm nitrocellulose membranes. Five percent skimmed milk powder in TBS was used as the blocking buffer. Thereafter, the nitrocellulose membranes were incubated with the isoform-specific PMCA4 antibody (1:1000 JA9 monoclonal; Affinity Bioreagents, Golden, Colo., USA), the splice variant specific PMCA4a antibody (1:100 polyclonal; generated by Brandenburger et al. 2011), LDH antibody (1:1000 polyclonal; BIOTREND Chemikalien, Technologiezentrum, Cologne, Germany), or MIF antibody (1:1000 polyclonal; ABCAM, Cambridge, UK) in blocking buffer at 4 °C overnight. After being washed, membranes were incubated with enhanced ECL-anti-mouse IgG-POD antibody (1:3000; Amersham Biosciences, Freiburg, Germany) or ECL-anti-rabbit IgG-POD antibody (1:3000; Amersham Biosciences) in blocking buffer for 1 h at room temperature. The peroxidase reaction was visualized by enhanced chemiluminescence detection (Amersham Biosciences) according to the manufacturer’s instructions.
Immunocytochemistry
For immunocytochemistry, smears of sperm isolated from various portions of the epididymis were prepared and air-dried. Sperm were permeabilized with 1% Triton X-100 in PBS for 10 min at room temperature. Thereafter, the detergent was removed by repeated washes in PBS. Non-specific binding sites were blocked by a 30-min incubation in 10% normal goat serum, 0.1% Triton X-100 in PBS. Primary antibodies were added in blocking medium overnight at 4 °C (anti-PMCA4, 1:50). After the incubation with secondary antibody (goat anti-mouse IgG Cy3, 1:250; 1 h at room temperature in the dark) and subsequent incubation with 4,6-diamidino-2-phenylindole (DAPI; 0.1 μg/ml PBS; 15 min at room temperature in the dark), slides were mounted with IMMU-MOUNT (Thermo Electron, Pittsburgh, USA). Images were analyzed by fluorescence microscopy. To visualize the transfer of biotinylated epididymosomal proteins to sperm, Streptavidin Alexa 488 (Dianova, Hamburg, Germany) was used in 10% normal goat serum and 0.1% Triton X-100 in PBS instead of primary antibodies.
Extraction and separation of lipids
The lipids were extracted in low-binding polymere tubes (BioSciences, Franklin Lakes, N.J., USA). For extraction of lipids, 100 μl methanol and 200 μl chloroform was added to epididymosomes (150 μg protein) or sperm (107). Thereafter, the mixture was treated with ultrasound for 10 min. Following the addition of 1 ml KCl and centrifugation at 3000g for 1 min, the mixture formed a nonpolar lower phase (LP) and a polar upper phase (UP). The lipids were enriched in the nonpolar lower phase. A synthetic phase was prepared for washing the LP and UP. In the synthetic phase, the sample was replaced by water. The remaining lipids in the UP were extracted by washing the UP with the synthetic lower phase (sLP) and subsequent centrifugation at 3000g for 1 min. These steps were repeated and the LP were pooled with sLP. The pooled LP/sLP were washed with the synthetic upper phase and were immediately centrifuged at 3000g for 1 min. The new lower phase was dried in a Speed-Vac (Bachofer) and lipids were dissolved for further analyses in 20 μl chloroform/methanol mix (2:1, v/v). Thin layer chromatography (TLC) was used for the separation of phospholipids. Silica gel plates (Sigma Aldrich) were used as stationary phase. Extracted lipids (2 μl) and various concentrations (1 μg, 0.5 μg, 0.1 μg) of pure standards (phosphatidylserine [PS], phosphatidylinositol [PI], phosphatidylcholine [PC], phosphatidylethanolamine [PE], sphingomyeline [SM]; Sigma Aldrich) were spotted onto the plates and were analyzed by using three different running solvents with decreasing polarity in series as mobile phases: solvent 1, methyl acetate:propan-2-ol:chloroform:methanol:0.25% (w/v) aqueous KCl (25:25:25:10:9 [v/v]); solvent 2, n-hexane:diethyl ether:acetic acid (75:23:2 [v/v]); solvent 3, n-hexane. Plates were dried between each solvent step. Before sample application, TLC plates were conditioned in solvent 1 and were subsequently activated by being heated in an oven for 35–40 min at 110 °C. After separation, phospholipid spots were visualized by using a staining solution containing 10% (w/v) CuSO4 and 8.5% (v/v) H3PO4 and subsequent heating at 170 °C for 10 min. Densitometrical analyses of the spots were performed by using the image and analyzing program SCION IMAGE.
Phospholipid analysis
The total concentration of phospholipids in epididymosomes and sperm was determined by using the method of Fiske and Subbarow (1925). The assay was performed in phosphorus-free tubes. An aliquot of 0.7 ml 70% perchloric acid was added to the dried lipid extracts and to 50 μl of standard reagent. Dissolved lipids were incubated at 180 °C for 3 h. The samples were then chilled for 15 min at room temperature and incubated for 5 min on ice. Subsequently, 2 ml 0.5% ammonium molybdate solution and 100 μl Fiske-Subbarow reagent were added to the samples, which were boiled for 10 min in a water bath and transferred to ice. The samples were measured at 830 nm. The phenylphosphate-di-sodium salt was used as a standard reagent at a concentration of 32 nM phosphoric acid in 50 μl H2O to calculate the total amount of phospholipids in nanomoles per milligram (nM/mg) sample protein.
Cholesterol analysis
The concentration of cholesterol in epididymosomes and sperm was determined by the colorimetric method of Liebermann-Burchardt (Burke et al. 1974). Samples were measured against a blank value (50 μl water, 2 ml cholesterol reagent solution) at λ=620 nm. The total amount of cholesterol (in nM/mg sample protein) was calculated by using a standard cholesterol curve.
Ca2+-ATPase assay
Mg2+-dependent Ca2+-ATPase activity was measured in epididymosomes and sperm according to the method previously described by Sikdar et al. (1991). To assay the activity of Mg2+-dependent Ca2+-ATPase, 2 μg epididymosomal proteins or 106 sperm and 3 mM ATP were added to reaction buffer containing 54.5 mM histidine hydrochloride, 2 mM EGTA, 0.2 mM dithioerythritol, 50 mM sucrose, 1 mM MgCl2 and 4 mM CaCl2 in a total volume of 250 μl. The reaction was stopped by adding 70 μl ice-cold trichloroacetic acid after 30 min. Thereafter, the samples were diluted with 550 μl distilled water. An aliquot of 100 μl 1.75% ammonium molybdate and of 100 μl 2% ascorbic acid was added to the samples to determine the amount of released inorganic phosphate (Pi). After a 10-min incubation, the complexed Pi was measured colorimetrically at 820 nm. Enzyme activity was calculated in micromoles Pi per milligram protein per hour (μM Pi/mg protein per h). Ca2+-ATPase activity was defined as the difference of activity in the presence or absence of Ca2+.
Electron microscopy
For transmission electron microscopy, epididymosomes were fixed in Ito-Karnovsky-mixture (2.5% glutaraldehyde, 2.5% paraformaldehyde, 0.05% picric acid) and washed in 100 mM cacodylate buffer (pH 7.3). The isolated vesicles were processed for routine electron microscopical examination as previously described (Triphan et al. 2007).
Statistical analysis
Statistical analyses were performed by using the one-way analysis of variance (ANOVA). In all cases, a P-value <0.05 was considered to have statistical significance.
Results
Ultrastructure of bovine epididymosomes
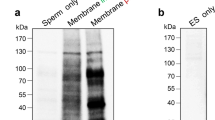
The ultrastructure of epididymosomes isolated from diverse parts of the bovine epididymis was analyzed by transmission electron microscopy. Several differences were obvious between the two populations, indicating a high heterogeneity of epididymosomes along the epididymal tract. Caput epididymosomes (90–450 nm) were smaller than epididymosomes from the cauda epididymidis (150–750 nm). In addition, the caput epididymosomes displayed a higher electron density than the cauda epididymosomes. Both vesicle populations were free of organelles (Fig. 1). To verify that the apocrine-secreted epididymosomes were vesicles and not merely cell debris, Western blotting experiments were performed by using antibodies against MIF and LDH. MIF, described to be secreted via the apocrine pathway by principal cells of the epididymis (Eickhoff et al. 2001), was detected in the caput and cauda epididymosomes (Fig. 2a). In addition, the classical residential cytoplasmic protein LDH, which has been shown not to be transferred into aposomes during apocrine secretion (Wilhelm et al. 1998), was absent in the isolated bovine epididymosomes (Fig. 2b).
Transmission electron micrographs of vesicles (epididymosomes) isolated from bovine caput epididymidis (a) and cauda epididymidis (b). Epididymosomes are represented by a heterogeneous population of vesicles. The size of the epididymal vesicles from the caput segment ranged from 90 to 450 nm and from the cauda segment from 150 to 750 nm. Bars 0.75 μm
Western blot analysis of epididymosomes (epid.) purified from the caput and cauda epididymidis; 15 μg protein per lane was loaded in a and 30 μg protein per lane was loaded in b. a Macrophage migration inhibitory factor (MIF) was detected by using a polyclonal antibody against MIF. An immunoreactive double-band was obvious in caput and cauda vesicles (12 kDa monomer, 24 kDa dimer). b Lactate dehydrogenase (LDH) was detected by using a polyclonal antibody against LDH. LDH was only detected in the epididymal tissue homogenate and in the cytoplasmic fraction of epididymal cells and not in epididymosomes. Positive controls were recombinant MIF protein (2 μg protein per lane) in a and recombinant LDH protein (0.5 μg protein per lane) in b. Representative immunoblots from at least three independent experiments with similar results are shown
In vitro fusion of epididymosomes and sperm
In this study, the fusion of sperm and epididymosomes was analyzed by evaluating the relief of fluorescence self-quenching of R18. As was evident, the in vitro fusion process was dependent on the proton concentration. A high extent of fusion was measured at pH 5 in all sperm/vesicle combinations. In contrast, less fusion ability was shown at pH 7 and no fusion was detectable at pH 8 (Fig. 3a, b). At pH 5, a fast initial fusion increase occurred within the first 180 s. The fusion rate slowed down gradually over a period of 10 min (Fig. 3a). The extent of fusion was different in caput sperm compared with cauda sperm. Cauda sperm showed a lower potential for fusion with the epididymosomes (33%) than did caput sperm in the same time span (caput sperm with caput vesicle: 52%; caput sperm with cauda vesicle: 54%; Fig. 3a). In addition, the results were verified by microscopic fluorescence analyses at pH 5.0 and 7.0 and were extended by analyses at the epididymal physiological pH of 6.5 (Fig. 4). After in vitro fusion, increments of fluorescence signals were predominantly visible in the neck and mid-piece of the sperm tails and in the sperm head (Fig. 4).
In vitro fusion of sperm (s) with octadecyl rhodamine-B (R18)-loaded epididymosomes (e). a Extent of fusion versus time at pH 5. b Effects of various pH values (pH 5, pH 7, pH 8) on the extent of in vitro fusion of sperm with epididymosomes. The fusion experiments were performed for 10 min. The protein ratio between epididymosomes and sperm was 1:2. The fusion was calculated as a percentage of fluorescence, taking 100% as the complete intermixing of the lipid phases after addition of 0.1% Triton X-100. The calculations were carried out by using the formula of Corazzi et al. (1991). Data presented are the average of four experiments and vertical bars represent SE
Transfer of membrane constituents from R18-loaded epididymosomes to bovine spermatozoa by in vitro fusion. Fusion experiments were carried out for 10 min at various pH values: pH 5 (a–c), pH 6.5 (d–f), pH 7 (g–i). The protein ratio between epididymosomes and sperm was 1:2. Transfer of membrane constituents was identified in the head, neck and mid-piece of sperm tail at pH 5 and 6.5. No transfer of membrane constituents was detected at pH 7. Negative controls (j, k) consisted in non-fused caput sperm and cauda sperm. The corresponding pH was achieved by using the following buffers: 0.32 mol/l sucrose in 20 mmol/l MES at pH 5 or pH 6.5 or in 2 mmol/l HEPES at pH 7. Emission at λ=580 nm (n=3). Bars 6 μm
Lipid composition of epididymosomes and epididymal spermatozoa before and after in vitro fusion
Epididymosomes
The cholesterol (Chol) and the total phospholipid (Pl) concentrations were 1.3-fold and 1.5-fold significantly higher in the caput epididymosomes than in the cauda epididymosomes. In contrast, the Chol/Pl ratio increased slightly by a factor of 1.1 during epididymal transit. The major phospholipids in the epididymosomes were PE, PC and SM, whereas PS and PI occurred in lower quantities. Nevertheless, all phospholipid classes were found in similar concentrations in both epididymosome populations (caput, cauda; Table 1).
Epididymal spermatozoa
The quantity of cholesterol was significantly decreased by a factor of 1.3 from caput to cauda spermatozoa, whereas the concentration of total phospholipids was unchanged along the epididymal tract. In consequence, the Chol/Pl ratio decreased from 1.1 to 0.8 from caput to cauda epididymal spermatozoa (Table 2). In bovine epididymal spermatozoa, PC, PE and SM were the predominant phospholipids. PE (3.3-fold), PC (1.8-fold) and SM (1.7-fold) were decreased significantly in cauda sperm compared with caput sperm (Table 2).
Epididymal spermatozoa after in vitro fusion
After in vitro fusion, caput spermatozoa possessed a decreased Chol/Pl ratio compared with non-fused caput spermatozoa, because the concentration of total phospholipids was increased significantly by 1.3-fold (fusion of caput spermatozoa with caput epididymosomes) and 1.2-fold (fusion of caput spermatozoa with cauda epididymosomes). The quantity of cholesterol remained unchanged. Cauda spermatozoa displayed no alteration in the Chol/Pl ratio after in vitro fusion with cauda epididymosomes, although an increased cholesterol concentration of 1.2-fold was measured. No significant changes were observed for total phospholipids after the fusion of cauda spermatozoa with cauda epididymosomes. However, no significant variances were detected in the composition of single phospholipid classes after in vitro fusion of spermatozoa with epididymosomes, independent of the spermatozoa vesicle combination (Table 2).
In summary, the Chol/Pl ratio in spermatozoa decreased along the epididymal tract and after in vitro fusion of caput sperm with caput or cauda epididymosomes.
Detection of PMCA4 in bovine epididymosomes and localization of PMCA4 in caput and cauda sperm before and after in vitro fusion with epididymosomes
Epididymosomes
Western blotting analyses were performed with a specific antibody against PMCA isoform 4 (recognizing the splice variants PMCA4a and PMCA4b) and a splice-variant-specific antibody against PMCA4a. In the caput epididymosomes, a strong immunoreactive band, corresponding to PMCA4b (∼133 kDa) and a faint band corresponding to PMCA4a (128 kDa) were detected by using the antibody against PMCA4 isoenzymes (Fig. 5a). In contrast, in cauda epididymosomes, a dominant immunoreactive band corresponding to PMCA4a (∼128 kDa) was visible by using the same antibody (Fig. 5b). In addition, with the splice-variant-specific antibody, PMCA4a was detected in both epididymosome populations, with a stronger signal in the cauda epididymosomes than in the caput epididymosomes. Taken together, these results suggest that, along the epididymal tract, a splice variant shift occurs from PMCA4b to PMCA4a from caput to cauda epididymosomes.
Detection of PMCA4 in epididymosomes by Western Blot analyzes. a PMCA4 splice variants (4a and 4b) were detected by using the specific monoclonal pan-PMCA4 antibody JA9. A strong immunoreactive band corresponding to PMCA4b (∼133 kDa) and a weak immunoreactive band corresponding to PMCA4a were seen in caput epididymosomes. An immunoreactive band corresponding to PMCA4a (∼128 kDa) was visible in cauda epididymosomes. b The presence of PMCA4a was demonstrated by using the new polyclonal PMCA4a antibody. PMCA4a was detected in caput and cauda epididymosomes, where it increased strongly from caput to cauda epididymosomes; 15 μg protein per lane was loaded. Representative results from three independent experiments with similar results are shown
Epididymal spermatozoa
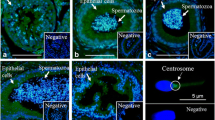
By performing immunofluorescence studies on sperm smears, PMCA4 was predominately localized in the neck and mid-piece of cauda spermatozoa. In caput spermatozoa, the fluorescence signal was faint (Fig. 6).
Immunohistological analyses of biotinylated proteins (a–e) and PMCA4 (f–o) transferred from epididymosomes to bovine spermatozoa by in vitro fusion. Transferred proteins from biotin-labeled epididymosomes (a–e) were identified in the postacrosomal region, neck, mid-piece and principal piece. Biotinylated proteins were revealed with streptavidin Alexa 488 reagent (green transferred proteins). Spermatozoa not incubated with epididymosomes were used as negative controls. Representative immunofluorescence results from two independent experiments with similar results are shown. Bars 6 μm. PMCA4 (f–j) was predominately identified with clearly visible fluorescence signals by using the monoclonal pan-PMCA4 antibody (JA9) in the neck and mid-piece of non-fused cauda spermatozoa and after in vitro fusion of caput or cauda spermatozoa with cauda epididymosomes. In caput spermatozoa and in caput spermatozoa fused with caput epididymosomes, the fluorescence signals were slightly visible. Nuclei were fluorescently labeled by using DAPI (k–o). Representative immunofluorescence results from three independent experiments with similar results are shown. Exposure time: 1.44 s. Bars 10 μm
Epididymal sperm after in vitro fusion
In vitro fusion of spermatozoa with epididymosomes did not change the PMCA4 localization in spermatozoa in any of the sperm/vesicle combinations. However, caput sperm that had been incubated with caput epididymosomes displayed a low-fluorescence signal. Interestingly, caput sperm incubated with cauda epididymosomes showed a clear fluorescence signal (Fig. 6). In cauda sperm, the fluorescence signal was approximately the same before and after in vitro fusion with cauda epididymosomes. These results allow us to suggest that PMCA4 is transferred from epididymosomes to caput sperm by in vitro fusion.
Transfer of protein from epididymosomes to epididymal sperm
To localize transferred proteins after in vitro fusion, caput and cauda spermatozoa were incubated with biotin-labeled epididymosomes. Subsequently, the extensively washed spermatozoa were smeared onto slides and the transferred proteins were revealed with streptavidin Alexa 488 (Dianova; Fig. 6). Protein transfer occurred at the mid-piece, the postacrosomal region and the principal piece (Fig. 6) in all vesicle/spermatozoa combinations. In addition, protein transfer was detected at the neck region after in vitro fusion of the caput spermatozoa with epididymosomes (Fig. 6). Spermatozoa not incubated with biotin-labeled epididymosomes remained unlabeled (Fig. 6).
Determination of Mg2+-dependent Ca2+-ATPase activity in bovine epididymosomes and in spermatozoa before and after in vitro fusion with epididymosomes
Epididymosomes and epididymal sperm
Ca2+-ATPase activity increased from caput to cauda epididymosomes (2.5-fold) and in sperm (1.5-fold; Fig. 7).
Determination of Mg2+-dependent Ca2+-ATPase activity in epididymosomes (a) and bovine sperm (106; b) from caput epididymidis and cauda epididymidis. a Cauda vesicles displayed a higher Ca2+-ATPase activity than caput vesicles. Data are expressed as means ± SD from five independent experiments. b Ca2+-ATPase activity in sperm was measured before and after in vitro fusion. Untreated cauda sperm displayed a higher activity than untreated caput sperm. After in vitro fusion, the caput sperm showed a higher Ca2+-ATPase activity than untreated caput sperm. Data are expressed as means ± SD from seven independent experiments. Vertical bars indicate SD. *Statistically significant differences in sperm before and after in vitro fusion (P<0.05); ♦statistically significant differences between caput and cauda vesicle or sperm (P<0.05)
Epididymal spermatozoa after in vitro fusion
We observed an increment of the Ca2+-ATPase activity in caput sperm after in vitro fusion with epididymosomes (Fig. 7b). The Ca2+-ATPase activity in bovine epididymal caput sperm was two-fold higher after in vitro fusion with epididymosomes than before fusion. In contrast, in-vitro-fused cauda sperm with cauda epididymosomes showed no changes. These results suggest that the Mg2+-dependent Ca2+-ATPase activity was increased by the in vitro fusion of caput sperm with epididymosomes.
Discussion
During their passage through the epididymis, biochemical modifications of spermatozoa are induced by epididymal factors, including changes in the lipid and in the plasma membrane protein composition (for reviews, see Caballero et al. 2011; Cooper 1998, 1999). Apocrine secreted vesicles of the epididymis, called epididymosomes, have been hypothesized to play an important role in sperm maturation (Hermo and Jacks 2002). We have therefore studied the effects of epididymosomes on sperm plasma membrane protein and lipid composition under various experimental conditions in vitro.
Electron microscopy of epididymosomes isolated from caput and cauda epididymal secretion has shown heterogeneity in size (caput vesicles: 90 nm–450 nm; cauda vesicles: 150 nm–750 nm) and electron density. The difference in the appearance of caput and cauda vesicles substantiates the presumption that vesicles secreted in the proximal epididymal segment do not cross into the cauda epididymidis, which is in agreement with observations made by Sullivan and co-workers (Frenette et al. 2006; Girouard et al. 2011). The group of Sullivan (Frenette et al. 2010) has described their bovine cauda epididymosomes as being in the range of 50–250 nm, which is, on average, smaller than our isolated vesicles. Isolated epididymosomes originating from other species differ in size and range: ram from 25 to 75 nm (Gatti et al. 2005), hamster from 50 to 200 nm (Legare et al. 1999), mouse from 50 to 800 nm (Rejraji et al. 2006) and rat from 0.2 to 3.5 μm (Fornes et al. 1995). The last mentioned size of rat epididymosomes seems large and resembles the sizes of vesicles measured on ultrathin sections of mouse (1–5 μm) or bovine (2–5 μm) epididymal tissues (Eickhoff et al. 2006; Rejraji et al. 2006) and not of isolated vesicles. We have checked the presence of the apocrine secreted protein MIF (Eickhoff et al. 2006, 2001) and the absence of the cytoplasmic resident protein LDH (Wilhelm et al. 1998) in isolated vesicles, indicating their nature as true apocrine secreted epididymosomes.
Various groups have reported that epididymosomes interact with spermatozoa and that they transfer proteins to them (Caballero et al. 2011; Frenette et al. 2002; Frenette and Sullivan 2001; Yanagimachi et al. 1985). Nevertheless, the fusion process between epididymosomes and spermatozoa has not been convincingly shown to date. The fusion process of prostasomes (vesicles secreted by the human prostate) with human spermatozoa has been successfully studied by Arienti and co-workers (Arienti et al. 1997; Carlini et al. 1997) by using the dye R18, which dissolves into the lipid core of the membrane (Hoekstra et al. 1984). Adopting this method for the in vitro fusion of epididymal spermatozoa with epididymosomes, we have clearly demonstrated that caput spermatozoa show a higher potential for fusion with epididymosomes than cauda spermatozoa. This seems physiologically consistent, as cauda spermatozoa have already been saturated during epididymal transit. The highest fusion rates have been observed at slightly acidic pH values (pH 5–6.5). No in vitro fusion has been detected at neutral or alkaline pH values (pH 7.0, pH 8.0). The acidic pH range is equivalent to the pH value of the epididymal fluid measured in rat in which the pH increases from 6.48 in the distal caput to 6.85 in the distal cauda epididymidis (Levine and Marsh 1971). Fluorescent microscopy analyses have demonstrated an increased fluorescent signal in the sperm head, mid-piece and neck region after the in vitro fusion of bovine spermatozoa with R18-loaded epididymosomes.
The lipid composition of epididymosomes and of epididymal spermatozoa has been studied with regard to changes occurring in spermatozoa after in vitro fusion with epididymosomes. In epididymosomes, the cholesterol, total phospholipid content and Chol/Pl ratio differs in vesicles isolated from the caput epididymidis from vesicles isolated from the cauda epididymidis. The Chol/Pl ratio of bovine epididymosomes from the caput and cauda epididymosomes is, at 0.77 and 0.87, higher than the Chol/Pl ratio in mouse vesicles (0.26 and 0.48; Rejraji et al. 2006). The cholesterol and total phospholipid concentrations are higher in bovine caput epididymosomes than in bovine cauda epididymosomes. Interestingly, in mouse epididymosomes, the total phospholipid amount is consistent but the cholesterol concentration is reversed (Rejraji et al. 2006). The main phospholipids in bovine caput and cauda epididymosomes are SM, PC and PE. In mouse epididymosomes, the concentration of the main phospholipids SM and PC is distinctive between the caput and cauda epididymidis (Rejraji et al. 2006). Recently, the group of Sullivan (Frenette et al. 2010) has separated two populations of bovine cauda epididymosomes showing different amounts of cholesterol and single phospholipids.
The Chol/Pl ratio in bovine epididymal spermatozoa decreases from caput to cauda spermatozoa, resulting in a reduction in the amount of cholesterol. The concentration of total phospholipids is similar in caput and cauda spermatozoa. Although the absolute values of the Chol/Pl ratio are higher for bovine spermatozoa than in all other mentioned species, a decreasing Chol/Pl ratio from caput to cauda spermatozoa has also been shown in the rat (Hall et al. 1991) and has been demonstrated as a trend, although not significantly, in the boar (Nikolopoulou et al. 1985). In contrast, an increased Chol/Pl ratio from caput to cauda sperm has been detected in mouse, ram and goat (Parks and Hammerstedt 1985; Rana et al. 1991; Rejraji et al. 2006). The decreased cholesterol concentration from bovine caput to cauda spermatozoa measured in our study is in agreement with observations in other species such as rat, boar and mouse (Hall et al. 1991; Nikolopoulou et al. 1985; Rejraji et al. 2006). With regard to the total phospholipids, an unchanged amount of total phospholipids has only been shown for boar spermatozoa (Nikolopoulou et al. 1985). In contrast to our observations, in the other species, the concentration of total phospholipids decreases from caput to cauda spermatozoa of the mouse (Rejraji et al. 2006) and rat (Hall et al. 1991). The main phospholipids in bovine epididymal sperm are PC, PE and SM, which decrease significantly from caput to cauda and which are also the main phospholipids in the other species.
In order to determine whether the changes in lipids occur as a result of the exchange of lipids between epididymosomes and spermatozoa, we have analyzed the lipid content of spermatozoa after the in vitro fusion of caput spermatozoa with caput or cauda vesicles. We have found that only the Chol/Pl ratio changes equivalently to the Chol/Pl ratio from unfused cauda sperm. This change is attributable to an increase in total phospholipids, unlike in vivo. The individual phospholipids do not change significantly after in vitro fusion.
Previously, we have shown that cauda spermatozoa contain a much higher level of PMCA4a than caput spermatozoa. Because PMCA4 knockout mice are infertile (Okunade et al. 2004; Schuh et al. 2004), PMCA4 appears to be essential for male fertility. As bovine epididymal spermatozoa express RNA for neither PMCA4 nor PMCA4a, our findings allow us to hypothesize that PMCA4a is transferred via epididymosomes onto bovine spermatozoa membranes in the cauda epididymidis (Brandenburger et al. 2011). The transfer of proteins from epididymosomes to sperm has been shown in several studies. For example, ADAM7 and P25b have been shown to be transferred from epididymosomes to sperm (Frenette and Sullivan 2001; Oh et al. 2009). Furthermore, MIF, GPX5, SPAM1 and CD52/HE5 are associated with epididymosomes, and the respective authors assume that these proteins are transferred to spermatozoa during epididymal transit (Deng et al. 2000; Eickhoff et al. 2001; Kirchhoff and Hale 1996; Rejraji et al. 2002). Protein transfer has been studied by in vitro fusion experiments with biotin-labeled epididymosomes and has been visualized by immunohistochemistry. In contrast to Frenette et al. (2002), who have found that the transferred proteins are preferentially localized in the acrosomal cap and only occasionally in the mid piece, the proteins in our study have been predominately detected in the postacrosomal region and in the neck and mid-piece. Interestingly, these are the same sperm regions as shown previously in the R18 experiments. In our current and previous study (Post et al. 2010), we have also located PMCA4 in the same sperm regions. Interestingly, in our current study, we have been able to demonstrate that, in bovine sperm, an obvious increase occurs in the PMCA4 fluorescence signal in the mid-piece, progressing from caput to cauda. Notably, after in vitro fusion of bovine caput sperm with cauda epididymosomes, an increase of the fluorescence signal apparently reaching the fluorescence intensity of epididymal cauda sperm has been observed by using the antibody against PMCA4. These findings substantiate the postulated transfer of PMCA4 from cauda epididymosomes to caput sperm. Unfortunately, as the polyclonal antibody against PMCA4a does not work on sperm smears, experiments on PMCA4a in such preparations have had to be disregarded (data not shown). Nevertheless, Western blot analysis of epididymosomes of caput and cauda origin has showed a splice variant shift from PMCA4b in caput epididymosomes to PMCA4a in cauda epididymosomes co-indicated with an increase of PMCA4a from caput to cauda vesicles. This is consistent with our previous results showing a switch from PMCA4b in caput epididymidis to PMCA4a in cauda epididymidis by quantitative polymerase chain reaction. Furthermore, PMCA4a is exclusively visible in cauda epididymal epithelium and is absent in caput and corpus epididymal epithelium (Brandenburger et al. 2011). In comparison with other species, e.g., the rat, a divergent distribution of PMCA4 is obvious in the different parts of the epididymis (Wilhelm et al. 2008). In epididymal sperm of mice (cauda) and rat (caput, corpus, cauda), unlike in the bull, PMCA4 protein is predominately located in the principal piece and not in the mid-piece (Okunade et al. 2004; Wilhelm et al. 2008), indicating clear-cut species-specific differences.
We have previously demonstrated that the Mg2+-dependent Ca2+-ATPase activity increases significantly from caput to cauda sperm (Sanchez-Luengo et al. 2004). Currently performed assays have emphasized the alteration of Mg2+-dependent Ca2+-ATPase activity after in vitro fusion. Along the epididymal tract and after in vitro fusion of caput sperm with epididymosomes (caput or cauda), the Ca2+-ATPase activity increases significantly and is even higher than in isolated epididymal cauda sperm. Cauda sperm fused in vitro with cauda epididymosomes show no changes in activity. Similarly, the epididymosomes isolated from the cauda epididymidis exhibit a significantly higher activity than the caput vesicles. The Ca2+-ATPase activity experiments are in agreement with PMCA4 detection.
Taken together, our results provide strong evidence that bovine epididymosomes participate in the changes of the lipid and protein composition of epididymal sperm and particularly in the transfer of PMCA4 onto sperm. Recent studies indicate that the Ca2+-ATPase activity is influenced by the environmental lipid spectrum (Zhang et al. 2009). Future studies need to clarify whether a functional link exists between lipid and PMCA4/Ca2+-ATPase activity in bovine epididymosomes and sperm.
References
Arienti G, Carlini E, Palmerini CA (1997) Fusion of human sperm to prostasomes at acidic pH. J Membr Biol 155:89–94
Aumüller G, Wilhelm B, Seitz J (1999) Apocrine secretion—fact or artifact? Anat Anz 181:437–446
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brandenburger T, Strehler EE, Filoteo AG, Caride AJ, Aumuller G, Post H, Schwarz A, Wilhelm B (2011) Switch of PMCA4 splice variants in bovine epididymis results in altered isoform expression during functional sperm maturation. J Biol Chem 286:7938–7946
Burke RW, Diamondstone BI, Velapoldi RA, Menis O (1974) Mechanisms of the Liebermann-Burchard and Zak color reactions for cholesterol. Clin Chem 20:794–801
Caballero J, Frenette G, Sullivan R (2011) Post testicular sperm maturational changes in the bull: important role of the epididymosomes and prostasomes. Vet Med Int 2011:757194
Carlini E, Palmerini CA, Cosmi EV, Arienti G (1997) Fusion of sperm with prostasomes: effects on membrane fluidity. Arch Biochem Biophys 343:6–12
Cooper TG (1998) Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Suppl 53:119–136
Cooper TG (1999) Epididymis. Encycl Reprod 2:17
Corazzi L, Pistolesi R, Arienti G (1991) The fusion of liposomes to rat brain microsomal membranes regulates phosphatidylserine synthesis. J Neurochem 56:207–212
Deng X, He Y, Martin-Deleon PA (2000) Mouse Spam1 (PH-20): evidence for its expression in the epididymis and for a new category of spermatogenic-expressed genes. J Androl 21:822–832
Eickhoff R, Wilhelm B, Renneberg H, Wennemuth G, Bacher M, Linder D, Bucala R, Seitz J, Meinhardt A (2001) Purification and characterization of macrophage migration inhibitory factor as a secretory protein from rat epididymis: evidences for alternative release and transfer to spermatozoa. Mol Med 7:27–35
Eickhoff R, Jennemann G, Hoffbauer G, Schuring MP, Kaltner H, Sinowatz F, Gabius HJ, Seitz J (2006) Immunohistochemical detection of macrophage migration inhibitory factor in fetal and adult bovine epididymis: release by the apocrine secretion mode? Cells Tissues Organs 182:22–31
Fiske CH, Subbarow Y (1925) The colormetric determination of phosphorus. J Biol Chem 66:26
Fornes MW, Barbieri A, Cavicchia JC (1995) Morphological and enzymatic study of membrane-bound vesicles from the lumen of the rat epididymis. Andrologia 27:1–5
Frenette G, Sullivan R (2001) Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol Reprod Dev 59:115–121
Frenette G, Lessard C, Sullivan R (2002) Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol Reprod 67:308–313
Frenette G, Girouard J, Sullivan R (2006) Comparison between epididymosomes collected in the intraluminal compartment of the bovine caput and cauda epididymidis. Biol Reprod 75:885–890
Frenette G, Girouard J, D’Amours O, Allard N, Tessier L, Sullivan R (2010) Characterization of two distinct populations of epididymosomes collected in the intraluminal compartment of the bovine cauda epididymis. Biol Reprod 83:473–480
Gatti JL, Metayer S, Belghazi M, Dacheux F, Dacheux JL (2005) Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod 72:1452–1465
Girouard J, Frenette G, Sullivan R (2011) Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl 34:e475–e486
Groos S, Wilhelm B, Renneberg H, Riva A, Reichelt R, Seitz J, Aumuller G (1999) Simultaneous apocrine and merocrine secretion in the rat coagulating gland. Cell Tissue Res 295:495–504
Hall JC, Hadley J, Doman T (1991) Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl 12:76–87
Hermo L, Jacks D (2002) Nature’s ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev 63:394–410
Hoekstra D, Boer T de, Klappe K, Wilschut J (1984) Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 23:5675–5681
Kirchhoff C, Hale G (1996) Cell-to-cell transfer of glycosylphosphatidylinositol-anchored membrane proteins during sperm maturation. Mol Hum Reprod 2:177–184
Legare C, Berube B, Boue F, Lefievre L, Morales CR, El-Alfy M, Sullivan R (1999) Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol Reprod Dev 52:225–233
Levine N, Marsh DJ (1971) Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol (Lond) 213:557–570
Nikolopoulou M, Soucek DA, Vary JC (1985) Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta 815:486–498
Oh JS, Han C, Cho C (2009) ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol Cells 28:441–446
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE (2004) Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279:33742–33750
Palmerini CA, Carlini E, Nicolucci A, Arienti G (1999) Increase of human spermatozoa intracellular Ca2+ concentration after fusion with prostasomes. Cell Calcium 25:291–296
Parks JE, Hammerstedt RH (1985) Development changes occurring in the lipids of ram epididymal spermatozoa plasma membrane. Biol Reprod 32:653–668
Post H, Schwarz A, Brandenburger T, Aumuller G, Wilhelm B (2010) Arrangement of PMCA4 in bovine sperm membrane fractions. Int J Androl 33:775–783
Rana AP, Majumder GC, Misra S, Ghosh A (1991) Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim Biophys Acta 1061:185–196
Rejraji H, Vernet P, Drevet JR (2002) GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol Reprod Dev 63:96–103
Rejraji H, Sion B, Prensier G, Carreras M, Motta C, Frenoux JM, Vericel E, Grizard G, Vernet P, Drevet JR (2006) Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol Reprod 74:1104–1113
Sanchez-Luengo S, Aumuller G, Albrecht M, Sen PC, Rohm K, Wilhelm B (2004) Interaction of PDC-109, the major secretory protein from bull seminal vesicles, with bovine sperm membrane Ca2+-ATPase. J Androl 25:234–244
Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, Neyses L (2004) Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J Biol Chem 279:28220–28226
Sikdar R, Ganguly U, Pal P, Mazumder B, Sen PC (1991) Biochemical characterization of a calcium ion stimulated-ATPase from goat spermatozoa. Mol Cell Biochem 103:121–130
Sullivan R, Saez F, Girouard J, Frenette G (2005) Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis 35:1–10
Triphan J, Aumuller G, Brandenburger T, Wilhelm B (2007) Localization and regulation of plasma membrane Ca(2+)-ATPase in bovine spermatozoa. Eur J Cell Biol 86:265–273
Wandernoth PM, Raubuch M, Mannowetz N, Becker HM, Deitmer JW, Sly WS, Wennemuth G (2010) Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS One 5:e15061
Wennemuth G, Babcock DF, Hille B (2003) Calcium clearance mechanisms of mouse sperm. J Gen Physiol 122:115–128
Wilhelm B, Keppler C, Hoffbauer G, Lottspeich F, Linder D, Meinhardt A, Aumuller G, Seitz J (1998) Cytoplasmic carbonic anhydrase II of rat coagulating gland is secreted via the apocrine export mode. J Histochem Cytochem 46:505–511
Wilhelm B, Meinhardt A, Renneberg H, Linder D, Gabius HJ, Aumuller G, Seitz J (1999) Serum albumin as a potential carrier for the apocrine secretion of proteins in the rat coagulating gland. Eur J Cell Biol 78:256–264
Wilhelm B, Brandenburger T, Post H, Aumuller G (2008) Expression and localization of PMCA4 in rat testis and epididymis. Histochem Cell Biol 129:331–343
Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H (1985) Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat 172:317–330
Zhang J, Xiao P, Zhang X (2009) Phosphatidylserine externalization in caveolae inhibits Ca2+ efflux through plasma membrane Ca2+-ATPase in ECV304. Cell Calcium 45:177–184
Acknowledgments
The authors are greatly indebted to Anne Henkeler, Gudrun Hoffbauer and Claudia Keppler for their expert technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwarz, A., Wennemuth, G., Post, H. et al. Vesicular transfer of membrane components to bovine epididymal spermatozoa. Cell Tissue Res 353, 549–561 (2013). https://doi.org/10.1007/s00441-013-1633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1633-7