Abstract
We have examined the innervation of the gut-associated lymphoid system of the sheep ileum, with a view to identifying potential sites for neuroinvasion by pathogens, such as prions (PrPSc). Special attention has been paid to the follicles of Peyer’s patches (PPs), which are major sites of PrPSc accumulation during infection. Evidence exists that the enteric nervous system, together with the parasympathetic and sympathetic pathways projecting to the intestine, are important for PrPSc entry into the central nervous system. Thus, PrPSc might move from PPs to the neurons and nerve fibres that innervate them. We investigated, by immunohistochemistry and retrograde tracing (DiI) from the follicles, the distribution and phenotype of enteric neurons innervating the follicles. Antibodies against protein gene product 9.5, tyrosine hydroxylase, dopamine β hydroxylase, choline acetyltransferase, calbindin (CALB), calcitonin gene-related peptide (CGRP), and nitric oxide synthase were used to characterise the neurons. Immunoreactivity for each of these was observed in fibres around and inside PP follicles. CGRP-immunoreactive fibres were mainly seen at the follicular dome. Retrograde tracing revealed submucosal neurons that contributed to the innervation of PPs, including Dogiel type II neurons and neurons immunoreactive for CALB and CGRP. The major source of the adrenergic fibres are the sympathetic ganglia. Our results thus suggest that enteric and sympathetic neurons are involved during the first stage of neuroinvasion, with neurons connecting to them acting as potential carriers of PrPSc to the central nervous system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small intestine of young ruminants contains two types of organized intestinal lymphoid aggregates, ileal and jejunal Peyer’s patches (PPs). The ileal PP is a primary lymphoid tissue, being responsible for the majority of B cells that are generated within the ileum and for the diversification of the pre-immune antibody repertoire (Gerber et al. 1986; Reynaud et al. 1991). The ileal PP undergoes involution at around sexual maturity, whereas jejunal PP persist in adult animals (Landsverk et al. 1991).
PPs are relatively sparsely innervated, although they express neuropeptide receptors under a range of inflammatory conditions (Stead 1992). This suggests dynamic interplay between the immune and nervous systems during gut inflammation. Studies of PP innervation in cattle (Balemba et al. 1999) and pigs (Krammer and Kühnel 1993) have failed to reveal any nerve fibres within lymphoid follicles, probably because fibres reaching PPs are extremely thin and/or show low levels of immunoreactivity (IR). By contrast, other investigations in cattle (Defaweux et al. 2007), pigs (Kulkarni-Narla et al. 1999), cats (Feher et al. 1992), hamster (Pfoch and Unsicker 1972), mouse (Ottaway et al. 1987; Defaweux et al. 2005; Ma et al. 2007), rat (Shtylik et al. 1998), rabbit (Driessen et al. 1992) and sheep (Heggebø et al. 2003; Press et al. 2004; Lalatta-Costerbosa et al. 2007) have revealed innervation inside PP follicles. Recent investigations have demonstrated the presence of a dense neuronal network at the level of suprafollicular dome region, but not within the germinal centers, in porcine jejunal (Green et al. 2003; Vulchanova et al. 2007) and human ileal (Vulchanova et al. 2007) PPs.
The aim of the present study, which has been carried out on Sarda breed lambs, has been to determine whether part of the innervation of PP is from enteric neurons and to identify these enteric neurons in order to be able to evaluate them as possible intermediates in the transfer of a number of neurotropic agents including prions (PrPSc; Prusiner 1998; Mabbott and MacPherson 2006) into the enteric nervous system (ENS) and to the central nervous system (CNS). Indeed, the ENS, most likely plays, together with PPs, a key role in the early pathogenesis of several animal and human transmissible spongiform encephalopathies (Mabbott and MacPherson 2006; Beekes and McBride 2007). Prion uptake from the gut lumen is believed to occur via the epithelial microfold (M) cells overlying PPs. These cells are specialized for the transepithelial transport of macromolecules and their transfer to follicular dendritic cells (FDCs) and probably represent a crucial prion replication site (Heppner et al. 2001; Beekes and McBride 2007; Defaweux et al. 2007).
Materials and methods
Tissue preparation
Six Sarda breed lambs, aged 2–5 months, were killed with ethical approval of the Committee of Animal Experimentation of Bologna University. They were deeply anaesthetised and killed by means of Tanax (0.5 ml/kg; Intervet Italia) administration. Segments of ileum (about 20 cm in length), beginning about 2 cm oral to the ileocecal junction, were collected and immersed in phosphate-buffered saline (PBS) containing nicardipine (10−6 M; Sigma-Aldrich Chemie).
Tissues were pinned to balsa wood without stretching and fixed overnight at 4°C in 4% paraformaldehyde in PBS, washed in PBS (3×10 min), cut into pieces of about 1×1 cm, placed in PBS containing sodium azide (0.1%) and sucrose (30%) and stored at 4°C overnight. The following day, tissues were transferred to a mixture of PBS-sucrose-azide and OCT compound (Tissue Tek, Sakura Finetek Europe, The Netherlands) at a ratio of 1:1 for a further 24 h before being embedded in 100% OCT. To prepare frozen sections, tissues were frozen in isopentane and liquid nitrogen, following which serial sections were cut at a thickness of 15 μm, collected onto gelatin-coated slides and left to dry for 1 h at room temperature (RT). Specimens of ileum were also cut on a freezing microtome into sections (50 μm) tangential to the longitudinal muscle layer and the sections were serially mounted on gelatine-coated slides and left to dry for 1 h at RT.
Immunohistochemistry
Cryosections were processed for single- and double-labelling. Tissues were incubated in 10% normal goat serum in PBS containing 1% Triton X-100 for 30 min at RT. The antibodies that were used are listed in Table 1. Following incubation in single or combined primary antibodies overnight at 4 °C in a humid chamber, preparations were washed in PBS (3×10 min) and then incubated for 1 h at RT with appropriate secondary antibodies (Table 2). The sections were cover-slipped with buffered glycerol, pH 8.6.
Tissue preparation for DiI tracing
Small crystals of DiI (Molecular Probes, Eugene, Ore., USA) were diluted at 3% in 100% ethanol and evaporated onto small glass beads (about 200 μm; Sigma) that were placed in the middle of PP follicles, after gently removing the overlying mucosa with a blade. In so doing, the follicles (pear-shaped and often breaching the muscularis mucosae) were “decapitated”; this allowed us to place beads inside follicles by the use of entomological forceps.
DiI tracing in organotypic culture (supravital tracing)
Lambs were killed as specified above. Segments of ileum were collected, immediately immersed in ice-cold oxygenated sterile Krebs solution containing nicardipine, cut open along the mesenteric border and flushed out with fresh ice-cold oxygenated Krebs solution. The segments (2×3 cm) were pinned out with the mucosa facing upwards in a Sylgard-lined Petri dish filled with oxygenated Krebs solution (4°C), which was replaced every 10 min. In the middle of the specimens, the mucosa was removed for viewing the “decapitated” top of the follicles and DiI (evaporated on glass beads) was placed inside one or more follicles. Subsequently, tissues were placed in Dulbecco’s modified Eagle’s medium (Sigma), supplemented with an antibiotic-antimycotic mixture (Sigma; 100 mg/ml penicillin, 100 μg/ml streptomycin, 20 μg/ml gentamycin, 2.5 μg/ml amphotericin B), including 10% fetal bovine serum (Sigma) and 25 mM NaHCO3. After 24 h of organotypic tissue culture, the preparations were fixed overnight in modified Zamboni’s fixative containing 2% paraformaldehyde and 0.2% picric acid in 0.1 M sodium phosphate buffer and trimmed into small pieces (1×1 cm). We carried out immunohistochemical investigations by using both anti-calbindin (CALB) and anti-calcitonin gene-related peptide (CGRP) antibodies on tangential cryosections in which DiI-labelled cells were observed.
DiI tracing in fixed tissue
Ileal segments were distended, mucosa-face up, on balsa wood and fixed in 4% paraformaldehyde in PBS overnight at 4°C. After fixation, tissues were washed in PBS, cut into pieces of 2×3 cm and stretched in Sylgard-lined Petri dishes or on balsa wood. After the application of DiI crystals into PPs, tissues were immersed in PBS azide (1%) and stored in an oven at 37°C. Tissues were observed in tangential cryosections after preparing small pieces of tissues (1×1 cm) and in whole-mount preparations (myenteric plexus: MP; submucosal plexus: SMP) made as described in Chiocchetti et al. (2004). Briefly, to obtain SMP whole-mount preparations, the remaining mucosa and the submucosal lymphatic components had to be scraped away with a scalpel blade. Further microdissections were required to separate the SMP from the circular muscle layer. The component of the SMP remaining firmly attached to the connective tissue encircling the follicles was delicately removed with forceps and added to SMP specimens to be observed. MP whole-mount preparations were obtained by separating the remaining two muscular layers, thereby producing the longitudinal muscle layer with the attached MP.
Specificity of antibodies
The specificity of the mouse anti-nitric oxide synthase (NOS) and rabbit anti-CGRP antibodies had been previously tested (Pfannkuche et al. 2002; Chiocchetti et al. 2006). In this investigation, we carried out absorption studies for the rabbit anti-CALB serum. Absorption of the diluted rabbit anti-CALB antibody with 10−5 M of the rat CALB peptide (Swant) for 24 h at 4°C prior to application to the tissue prevented CALB immunostaining. Antibodies to peripheral choline acetyltransferase (pChAT), tyrosine hydroxylase (TH) and dopamine β hydroxylase (DBH) have been well characterised in other species (Tooyama and Kimura 2000; Chiocchetti et al. 2004) and, as they have been raised against highly conserved peptides and proteins, they probably recognise the appropriate antigens in sheep. However, this was not directly tested. The specificity of the secondary antibodies was tested by applying these antisera without use of the primary antibodies. No stained neurons or fibres were seen after omitting the primary antisera.
Fluorescence microscopy
Preparations were examined on a Zeiss Axioplan microscope equipped with the appropriate filter cubes for discriminating between fluorescein isothiocyanate (FITC) and Alexa 594 fluorescence. Images, recorded by using a Polaroid DMC digital camera (Polaroid, Cambridge, Mass., USA) and DMC 2 software, were further processed by Corel Photo Paint and Corel Draw software programs (Corel, Dublin, Ireland).
Results
Immunoreactivity in the ileal wall
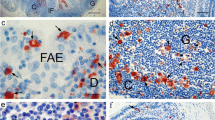
Protein gene product 9.5 (PGP 9.5)-IR was detected in perifollicular ganglia of the SMP and in nerve fibres (Fig. 1a). Thin PGP 9.5-immunoreactive fibre bundles were observed inside the capsule, in the external follicular layers (Fig. 1b), with PGP 9.5-immunoreactive fibres being additionally detected around crypts (Fig. 1c), around lacteals and close to the basal membranes of enterocytes (Fig. 1d).
Cryosections showing PGP 9.5 immunoreactivity (PGP 9.5-IR; a–d), tyrosine hydroxylase (TH-IR; e–i), and dopamine β hydrossxylase (DBH-IR; i’) in the lamb ileum. a PGP 9.5-immunoreactive neurons in a submucosal ganglion (g) and fibres of SMP located between two follicles (Foll) within an ileal PP. b A small PGP 9.5-immunoreactive fibre bundle (arrows) inside a follicle (Foll). c PGP 9.5-immunoreactive fibres (arrows) in mucosa crypts, around the glandular epithelium. d A PGP 9.5-immunoreactive fibre (arrows) in contact with the epithelial basal membrane of a villus. e Several TH-immunoreactive fibres (arrow) reach and apparently penetrate a submucosal follicle (Foll). f A long TH-immunoreactive fibre running in the centre of the follicle (arrows). g TH-immunoreactive fibres running along a blood vessel (arrows) and reaching a lymphatic follicle (Foll) in the SMP; note that the follicle expresses non-specific red fluorescence, probably attributable to the numerous macrophages present inside PP. h TH-immunoreactive fibre (arrows) along the major axis of a villus in the mucosa; note the numerous TH-immunoreactive non-neuronal cells (probably mast cells). i, i’ Co-localization of TH-IR (i) and DBH-IR (i’) in nerve fibres reaching and, apparently, penetrating the follicle (arrows). Bars 50 μm (a–c, i, i’), 100 μm (d–h)
TH-IR was observed in nerve fibres, along the blood vessels approaching the follicles, around and inside PP follicles, mainly confined to the subcapsular space (Fig. 1e–g) and in the suprafollicular dome area. TH-immunoreactive fibres with varicosities were found close to the mucosal glands and along the lacteals, where they reached numerous large elongated TH-immunoreactive mast cells (Fig. 1h), which in sheep, cattle and some other species contain dopamine (Falck et al. 1964; Huntley et al. 1992; Freeman et al. 2001).
DBH-IR was co-localized in nerve fibres reaching the follicles and in the suprafollicular dome area. Not all TH-immunoreactive fibres expressed DBH-IR, which was fainter in comparison with TH-IR (Fig. 1i–i’).
pChAT-IR was expressed by neurons of the SMP and by nerve fibres around (Fig. 2a,b) and inside the subcapsular area of PP follicles (Fig. 2c), around crypts (Fig. 2d), and in some enterocytes (probably enterochromaffin cells; Fig. 2e). However, nerve fibres within paravascular nerve bundles did not exhibit pChAT-IR.
Cryosections showing peripheral choline acetyltransferase (p-ChAT-IR; a–e), calbindin (CALB-IR; f), calcitonin gene-related peptide (CGRP-IR; g) and nitric oxide synthase (NOS-IR; h) immunoreactivity in the lamb ileum. a Two large pChAT-immunoreactive neuronal aggregates (arrows) in the SMP around a large follicle (Foll). b Strongly labelled pChAT-immunoreactive neurons and fibres in the SMP. c Strongly labelled pChAT-immunoreactive fibres (arrows) approaching the follicle (Foll); note that the immunoreactivity of fibres seems to stop at the periphery of the follicle. d Strong pChAT-IR neuronal fibre (arrow) around a crypt in the mucosa. e Note the pChAT-immunoreactive epithelial cell (probably an enterochromaffine cell, arrow). f CALB-IR in a freezing microtome tangential section (50 μm thickness). SMP CALB-immunoreactive fibres (arrows) encircle and penetrate the follicle (Foll). g CGRP-immunoreactive fibres (arrows) in the dome area of a follicle (Foll) within a PP. h A large bundle of neuronal NOS-immunoreactive fibres in apparent contact with a follicle (Foll). Bars 50 μm (b–e, g), 100 μm (a, h), 200 μm (f)
CALB-IR was noted in bundles of fibres inside PP follicles (Fig. 2f). CALB-IR was not seen in paravascular nerve fibres, whereas it was strong in perifollicular fibres and nearby neurons. CGRP-immunoreactive varicose fibres encircled follicles and penetrated their dome area (Fig. 2g). This preponderance of fibres in the dome was not observed with other neuronal markers.
NOS-immunoreactive fibres were seen around PP follicles and also inside them (Fig. 2h). However, NOS-IR was not seen in paravascular fibres.
Some background fluorescent spots were observed within PP follicles; these had the appearance of macrophages, which are known to exhibit natural fluorescence (Defaweux et al. 2005, 2007).
DiI tracing: organotypic cultures
We analysed retrogradely labelled neurons only after 24 h of incubation, since we observed that, after longer incubation times, the dye migrated into the lymphatic vessels and perifollicular spaces. Indeed, PP vessels, lined by a high endothelium, are permeated by large numbers of migrating lymphocytes (Yamaguchi and Schoefl 1983). After 3–4 days of incubation, we observed numerous DiI-labelled immune cells (and some DiI crystals) dispersed along the perifollicular spaces; this increased the background so much that it obscured the retrograde labelling of neurons.
After 24 h of DiI transport from the follicles in living tissue, a few DiI-labelled nerve cell bodies were observed in the external SMP. DiI-labelled neurons occurred close to the follicles in which DiI crystals had been inserted. DiI-labelled fibres were also observed between DiI-labelled follicles and nerve cells. Immunohistochemistry showed that some DiI-labelled neurons were CALB-IR (Fig. 3a–b’) and some neurons were CGRP-IR (Fig. 3c–c’). Some of the neurons with CALB-IR or CGRP-IR were Dogiel type-II-shaped. Other DiI-labelled cells were neither CALB-IR nor CGRP-IR.
Cryosections showing DiI-filled neurons in submucosal ganglia labelled after application of the tracer in lymphatic follicles in organotypic cultures from lamb ileum. a, a’ Two DiI-labelled neurons that were not CALB-immunoreactive (stars). Note that the strongly fluorescent CALB-IR neurons (a’) showed an apparent Dogiel type I morphology. DiI was not present at the nucleus level. b, b’ A DiI-filled Dogiel type II neuron that was also CALB-immunoreactive (star). c, c’ Three faint DiI-labelled neurons that were CGRP-immunoreactive (stars). Bars 50 μm
DiI tracing: postmortem tissue
Small DiI-coated beads adhered to the follicles following their placement by using microforceps (Fig. 4a) and remained in place for the length of incubation (up to 8 weeks). During the incubation, slight diffusion of DiI occurred around the bead but this halo of diffusion remained inside the follicle (Fig. 4b). In frozen sections, the DiI tracer was observed throughout the entirety of follicles to which DiI was applied (Fig. 4c,d). Sometimes, it was more concentrated at the level of the connective tissue capsule (Fig. 4e–h). No spread of dye between neighbouring follicles was observed (Fig. 4c–e). No DiI-labelled immune cells could be detected in the perifollicular spaces.
Beads coated with the DiI tracer (diameter=~200 μm) were applied to follicles (Foll) of lamb PPs in fixed ileum (postmortem tracing). a, b Stereomicroscopy. c–h Tangential cryosections of submucosa. i–k Whole-mount preparations of SMP. a A glass bead with DiI (large arrow) just applied to a follicle (small arrows follicles that become smaller, underdeveloped and not homogeneously packed in older lambs of 4–5 months). b A follicle in which one glass bead with DiI was applied (large arrow); note that the tracer, after a 2-week incubation, was confined inside the Foll capsule (dotted line). The follicles, belonging to a 1-month-old lamb, are large, packed and homogeneously developed. c Three glass beads with DiI were applied to three follicles (Foll); note that the tracer was homogeneously distributed inside the follicles (star position of glass bead application). d Two close follicles (Foll); the DiI glass bead was applied only to one follicle (bright orange fluorescence). Two large bundles of neuronal DiI-labelled fibres were seen in contact with the DiI-labelled follicle. No spread of dye between neighbouring follicles was observed. e A DiI-labelled smalll SMP neuron (arrow) located between three follicles. The glass bead with tracer was applied only to the follicle right in which it was more concentrated at the level of the connective tissue capsule. f A small SMP DiI-labelled neuron (arrow) with an apparent Dogiel type II shape. g Four DiI-labelled SMP neurons (arrows) located close to a DiI-labelled follicle (Foll). h A small DiI-labelled SMP neuron (arrow) close to a DiI-labelled follicle (Foll). i, j, k Whole-mount preparations showing DiI-labelled neurons with an apparent multipolar (i, j) and elongated (k) morphology. Bars 800 μm (a, b), 400 μm (c), 200 μm (d), 100 μm (e, h), 50 μm (f, g, i, j, k)
DiI-labelled nerve cells and fibres were observed in the submucosa after 11 days of incubation, the earliest time examined. DiI-labelled nerve fibres, sometimes forming large bundles, encircled and closely approached the follicle capsules (Fig. 4d). Some DiI-labelled fibres were seen within the paravascular nerve bundles that followed SMP blood vessels and some fibres were in the SMP itself. Small and medium-sized DiI-labelled neurons were located in the external SMP, between follicles (Fig. 4e–k). Some neurons were clearly recognizable as having Dogiel type II morphology, whereas others had polygonal or elongated shapes and could not be readily allocated to established shape classifications. In whole-mounts, DiI-labelling was of Dogiel type II, multipolar or elongated neurons (Fig. 4i–k).
We attempted to identify labelled neurons immunohistochemically after tracing in fixed tissue, in which tissue had been maintained in PBS azide at 37°C during dye transport, but we were unable to obtain adequate staining.
Discussion
Sources of innervation of PPs
Nerve fibres with TH-IR, DBH-IR, pChAT-IR, CALB-IR, NOS-IR and CGRP-IR were found in the sub-capsular region of the follicles and adrenergic and above all CGRP-immunoreactive fibres were also located in the dome of the follicles. The possible origins of each of these fibre types are discussed below.
The presence of labelled nerve cells in submucosal ganglia after application of DiI to PP follicles indicates that there is an intrinsic innervation of the follicles. This is the first evidence, in sheep, for an intrinsic innervation of PPs; however, Shtylik et al. (1998) have observed, by the use of the retrograde tracer primulin, an intrinsic (SMP) and extramural innervation of PPs in the rat. Immunohistochemical investigations have shown that CALB- and CGRP-immunoreactive neurons contribute to the intrinsic innervation and that some of these immunoreactive neurons have Dogiel type II morphology. DiI tracing in fixed tissue has confirmed that submucosal Dogiel type II neurons innervate the follicles. Analysis of the responses of Dogiel type II neurons to physiological stimuli indicates that they are intrinsic sensory neurons of the gut wall (Furness et al. 2004; Mao et al. 2006) and determination of their patterns of projection in various mammalian species supports this functional identification (Hens et al. 2000, 2001; Brehmer et al. 2006). All the processes of Dogiel type II neurons are axon-like, so whether the processes that innervate the follicles subserve a sensory or a motor function remains uncertain. However, the output processes of Dogiel type II neurons appear to be in enteric ganglia, where they innervate interneurons and motor neurons of enteric reflex pathways, suggesting that the intra-follicular nerve endings are sensory. On the other hand, axon collaterals of Dogiel type II neurons that innervate the mucosa have motor functions (Furness 2006) and so an efferent function of the PP innervation cannot be discounted. In submucosal ganglia of the sheep, all CALB-immunoreactive Dogiel type II neurons are also immunoreactive for CGRP and most are immunoreactive for ChAT (Chiocchetti et al. 2004). Thus, at least part of the ChAT-IR innervation of the follicles is accounted for by the intrinsic sensory neurons. As the product of ChAT, acetylcholine (ACh), is a vasodilator, release from these nerve endings could increase follicular blood flow. Furthermore, ACh receptors have been demonstrated on some immune cells populations (Ma et al. 2007) and neuropeptide receptors, such as substance P (SP) and vasoactive intestinal peptide (VIP) receptors (Bienenstock et al. 1989; Pascual et al. 1994). SP has been shown to enhance the production of lymphocytes and IgA, whereas VIP and somatostatin decrease mucosa antibody production (Payan et al. 1987; Bienenstock et al. 1989).
Neither CALB-immunoreactive nor CGRP-immunoreactive neurons in the sheep ENS express TH-IR (Chiocchetti et al. 2004, 2006); furthermore, no sheep ENS neurons express DBH-IR, indicating that the TH axons could come from a different source, although not all the TH-immunoreactive fibres also express DBH-IR. This last piece of evidence might be related to a low level of expression of the DBH enzyme. The most likely source is from noradrenergic sympathetic neurons innervating the ileum, which all express TH- and DBH-IR (unpublished), although a small proportion of nerve cell bodies in the submucosal ganglia (1%) exhibits TH-IR (Chiocchetti et al. 2006); an extrinsic denervation of a small ileal segment would definitely confirm this hypothesis. A sympathetic origin would be consistent with the evidence that sympathetic neurons control the lymphatic and blood flow of gut-associated lymphoid tissue (Yamaguchi and Schoefl 1983). PPs do not have a separate blood supply and Yamaguchi and Schoefl (1983) have demonstrated, in mice, that major arterial trunks divide near PPs into two or three smaller vessels that enter the superficial region of PPs. These authors have observed constrictions at points where arterioles branched off to enter the follicles. The arterioles and venules are restricted to the superficial zone of the nodule, whereas the deeper lymphoid parenchyma is permeated only by a mesh of capillaries. Consequently, the subcapsular sympathetic innervation of follicular blood vessels could be related to the control of the blood supply to the follicles.
Furthermore, a growing body of evidence suggests that sympathetic innervation of primary and secondary lymphoid organs provides an important functional link between the nervous and immune systems and may be involved in regulating immune development (Felten et al. 1985, 1987, 1988; Sharkey 1992). Gonzales-Ariki and Husband (2000) have observed that sympathetic innervation is an influential factor in the ontogeny of IgA plasma cells in the lamina propria of rat small intestine; these cells originate in the PPs and their migration to the lamina propria seems to be antigen-independent; the authors suggest that sympathetic neurons might regulate the migratory patterns of lymphocytes. The proximity of both PP lymphocytes and lamina propria mast cells with TH-immunoreactive nerve fibres confirms that both immune and neural factors are involved in immunity and inflammation.
We have not yet identified the origins of the NOS-immunoreactive fibres that innervate the PP. NOS-immunoreactive neurons are common in both the submucosal and myenteric ganglia, where they compose about 20% and 30% of neurons, respectively (Chiocchetti et al. 2006; Lalatta-Costerbosa et al. 2007). NOS-immunoreactive neurons are mostly motor and interneurons in the MP and most probably motoneurons in the external SMP (Mazzuoli et al. 2007). CGRP and NOS are not co-localised, although a small proportion of NOS neurons is CALB-IR (Chiocchetti et al. 2004, 2006). NOS is found in few sympathetic post-ganglionic neurons (Young et al. 2000) but its very presence means that a sympathetic origin of the subcapsular fibres cannot be eliminated. Moreover, a small number of NOS axons occur in the vagus (Young et al. 2000) and NOS may also be released from the endings of spinal afferent neurons in the gut wall, where is causes vasodilation (Holzer et al. 1995). Moreover, we have identified several NOS-IR dorsal root ganglion (DRG) neurons innervating the ovine ileum (unpublished).
In addition to the intrinsic source of CGRP that has been demonstrated, some of these fibres in the follicles may be of extrinsic origin. The only fibre type with a pattern of innervation distinct from the other fibre types is the CGRP-IR class of fibres that selectively innervates the domes of follicles. CGRP-immunoreactive fibres have also been found along blood vessels and CGRP-IR is well established to occur in DRG neurons supplying an afferent gut innervation (Green and Dockray 1987; Chiocchetti et al. 2006). Therefore, the CGRP-immunoreactive innervation could also represent a spinal afferent innervation. In unpublished studies, we have found a few CGRP-immunoreactive neurons in the distal vagal (nodose) ganglion, indicating that CGRP-immunoreactive fibres could contribute to the vagal afferent of the intestine. Interestingly, in vitro studies have shown that CGRP enhances the proliferation of T lymphocytes (Payan et al. 1987).
Few other studies have investigated the chemical phenotypes of neurons that innervate PP and, indeed, none as comprehensively as in the present work. In the cat, Feher et al. (1992) have observed, at the ultrastrucutural level, sparse somatostatin-immunoreactive fibres. In the pig, Kulkarni-Narla et al. (1999) have reported cholinergic fibres inside follicles and TH-immunoreactive fibres in the dome area. In human, Vulchanova et al. (2007) have detected peptidergic (SP-, VIP-, and CGRP-immunoreactive) innervation only in the dome area. In cattle, Defaweux et al. (2007) have seen TH-immunoreactive fibres inside follicles.
Our research group (Lalatta-Costerbosa et al. 2007) has recently observed nitrergic intrafollicular fibres in sheep. In the present work, we have confirmed this observation and added new elements for understanding the neuroanatomical connections of PP; the presence of cholinergic (pChAT-immunoreactive), peptidergic (CGRP-immunoreactive) and adrenergic (TH- and DBH-immunoreactive) fibres inside PPs are consistent with the presence of cholinergic and peptidergic receptors on immune cells (lymphocytes, dendritic cells, macrophages) observed in other species (Ichikawa et al. 1994; Carucci et al. 2000; Goode et al. 2000) and with the modulation of lymphocyte production and migration by sympathetic neurons (Gonzales-Ariki and Husband 2000).
Considerations regarding prion disease pathogenesis
This study provides a plausible anatomic basis through which infectious prions may gain access to the ENS and CNS. Following oral ingestion or experimental introduction of prions protein into the intestinal lumen of sheep and other animals, PrP Sc is soon observed (within hours or a few days) in epithelial M cells overlying PPs and in the PP themselves (Aguzzi and Polymenidou 2004; Aguzzi and Heikenwalder 2006; Mabbott and MacPherson 2006). The present study indicates that PrPSc could be transferred both to enteric neurons (intrinsic to the gut wall) and to extrinsic neurons, notably sympathetic post-ganglionic neurons, as the first step in neuroinvasion. Consistent with this, in naturally and experimentally infected animals, PrPSc has been detected in both ENS neurons (Heggebø et al. 2003; Chiocchetti et al. 2005; Marruchella et al. 2007) and in sympathetic neurons (Glatzel et al. 2001; Haik et al. 2003). In the next stage of transfer to the CNS, PrPSc may be transferred to other neurons with which enteric and sympathetic neurons connect. The Dogiel type II neurons are well placed to effect this transfer, as they are multi-axonal neurons that make synaptic connections with a high proportion of enteric neurons, including interneurons and motor neurons (Furness 2006). Thus, they may transfer PrPSc “synaptically”, resulting in a rapid dissemination of infective prion protein throughout the ENS. Dogiel type II neurons also innervate intestinofugal neurons that send axons outside the gastrointestinal tract and make synaptic connections in sympathetic ganglia (Sharkey et al. 1998). Therefore, PrPSc might reach sympathetic ganglia in two ways: by orthograde transport via the axons of intestinofugal neurons and by retrograde transport within the sympathetic post-ganglionic neurons. Furthermore, enteric neurons of the small intestine receive synaptic connections from both vagal efferents (Powley 2000) and extrinsic sensory axons (Furness 2006). Thus, enteric neurons could be intermediates in the neuroinvasion of the brain-stem and spinal cord.
Prions might also reach nerve endings after crossing a leaky mucosa, without first entering PP follicles, as nerve endings from enteric neurons, sympathetic neurons and extrinsic primary afferent neurons lie close to the enterocytes of the small intestine. Of note, several nerve fibres are also closely surrounded by immunocytes in the mucosa of mouse (Ma et al. 2007), pig and human (Vulchanova et al. 2007) small intestine. This leads us to postulate that this continuity of lymphoid cells and nerve fibres outside PPs could represent another potential site of entry for pathogens into the nervous system.
In conclusion, the sources of PP innervation that we have characterized suggest that foreign agents entering the gut by this route may colonize both intrinsic and extrinsic neurons directly, being subsequently carried to the CNS through connections with these neurons. The present work thus provides a plausible anatomic basis for “prion neuroinvasion” following the transfer of luminal PrPSc to PPs as part of the normal gut defence system.
References
Aguzzi A, Heikenwalder M (2006) Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol 4:765–775
Aguzzi A, Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116:313–327
Balemba OB, Mbassa GK, Semuguruka WD, Assey RJ, Kahwa CK, Hay-Schmidt A, Dantzer V (1999) The topography, architecture and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat 195:1–9
Beekes M, McBride PA (2007) The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J 274:588–605
Bienenstock J, Croitoru K, Ernst PB, Stead RH, Stanisz A (1989) Neuroendocrine regulation of mucosal immunity. Immunol Invest 18:69–76
Brehmer A, Schrödl F, Neuhuber W, Tooyama I, Kimura H (2006) Co-expression pattern of neuronal nitric oxide synthase and two variants of choline acetyltransferase in myenteric neurons of porcine ileum. J Chem Neuroanat 27:33–41
Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M, Steinman RM, Mojsov S (2000) Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J Immunol 164:3494–3499
Chiocchetti R, Grandis A, Bombardi C, Clavenzani P, Lalatta-Costerbosa G, Lucchi ML, Furness JB (2004) Characterisation of neurons expressing calbindin immunoreactivity in the ileum of the unweaned and mature sheep. Cell Tissue Res 318:289–303
Chiocchetti R, Clavenzani P, Mazzoni M, Albanese V, Di Guardo G, De Grossi L, Bortolami R, Lalatta-Costerbosa G (2005) The sheep enteric nervous system after scrapie (PrPSc) infection. Autonomic Neurosci 119:144–145
Chiocchetti R, Grandis A, Bombardi C, Lucchi ML, Tadini Dal Lago D, Bortolami R, Furness JB (2006) Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum. A morphometric and neurochemical investigation. Cell Tissue Res 323:183–196
Defaweux V, Dorban G, Demonceau C, Piret J, Jolois O, Thellin O, Thielen C, Heinen E, Antoine N (2005) Interfaces between dendritic cells, other immune cells, and nerve fibres in mouse Peyer’s patches: potential sites for neuroinvasion in prion diseases. Microsc Res Tech 6:1–9
Defaweux V, Dorban G, Antoine N, Piret J, Gabriel A, Jacqmot O, Falisse-Poirier N, Flandroy S, Zorzi D, Heinen E (2007) Neuroimmune connections in jejunal and ileal Peyer’s patches at various bovine ages: potential sites for prion neuroinvasion. Cell Tissue Res 329:35–44
Driessen A, Creemers J, Gebos K (1992) The innervation of the lymphoid tissue at the ileocolonic transition: an enzyme and immunohistochemical study. Acta Anat (Basel) 144:304–310
Falck B, Nystedt T, Rosengren E, Stenflo J (1964) Dopamine and mast cells in ruminants. Acta Pharmacol Toxicol 21:51–58
Feher E, Fodor M, Burnstock G (1992) Distribution of somatostatin-immunoreactive nerve fibres in Peyer’s patches. Gut 33:1195–1198
Felten DL, Felten SY, Carlson SL, Olschowaka JA, Livnat S (1985) Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol 135:755S–765S
Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S (1987) Noradrenergic sympathetic neural interactions with the immune system. Structure and function. Immunol Rev 100:225–260
Felten SY, Felten DL, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowka JA, Livnat S (1988) Noradrenergic sympathetic innervation of lymphoid organs. Prog Allergy 43:14–36
Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton AL, Narasimhachari N, Domen J, Siméon N, Couderc F, Stewart JK (2001) Catecholamines in murine bone marrow derived mast cells. J Neuroimmunol 119:231–238
Furness JB (2006) The enteric nervous system. Blackwell, Oxford, pp 64–66
Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K (2004)Projections and chemistry of Dogiel type II neurons in the mouse colon.Cell Tissue Res 317:1–12
Gerber HA, Morris B, Trevella W (1986) The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci 64:201–203
Glatzel M, Heppner FL, Albers KM, Aguzzi A (2001) Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31:25–34
Gonzales-Ariki S, Husband AJ (2000) Ontogeny of IgA+ cells in lamina propria: effects of sympathectomy. Dev Comp Immunol 24:61–69
Goode T, O’Connell J, Ho WZ, O’Sullivan GC, Collins JK, Douglas SD, Shanahan F (2000) Differential expression of neurokinin-1 receptor by human mucosal and peripheral lymphoid cells. Clin Diagn Lab Immunol 7:371–376
Green T, Dockray GJ (1987) Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett 76:151–156
Green BT, Lyte M, Kulkarni-Narla A, Brown DR (2003) Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J Neuroimmunol 141:74–78
Haïk S, Faucheux BA, Sazdovitch V, Privat N, Kemeny JL, Perret-Liaudet A, Hauw JJ (2003) The sympathetic nervous system is involved in variant Creutzfeldt-Jakob disease. Nat Med 9:1121–1129
Heggebø R, Gonzales L, Press CM, Gunnes G, Espenes A, Jeffrey M (2003) Disease associated PrP in the enteric nervous system of scrapie-affected sheep. J Gen Virol 84:1327–1338
Hens J, Schrödl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW, Schemann M, Timmermans JP (2000) Mucosal projections of enteric neurons in the porcine small intestine. J Comp Neurol 421:429–436
Hens J, Vanderwinden JM, De Laet MH, Scheuermann DW, Timmermans JP (2001)Morphological and neurochemical identification of enteric neurones with mucosal projections in the human small intestine. J Neurochem 76:464-471
Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A (2001) Transepithelial prion transport by M cells. Nat Med 7:976–977
Holzer P, Wachter C, Heinemann A, Jocic M, Lippe IT, Herbert MK (1995) Diverse interactions of calcitonin gene related peptide and nitric oxide in the gastric and cutaneous microcirculation. Can J Physiol Pharmacol 73:991–994
Huntley JF, Haig DM, Irvine J, Inglis L, MacDonald A, Rance A, Moqbel R (1992) Characterisation of ovine mast cells derived from in vitro culture of haemopoietic tissue. Vet Immunol Immunopathol 32:47–64
Ichikawa S, Sreedharan SP, Goetzl EJ, Owen RL (1994) Immunohistochemical localization of peptidergic nerve fibres and neuropeptide receptors in Peyer’s patches of the cat ileum. Regul Pept 54:385-395
Krammer HJ, Kühnel W (1993) Topography of the enteric nervous system in Peyer’s patches of the porcine small intestine. Cell Tissue Res 272:267–272
Kulkarni-Narla A, Beitz AJ, Brown DR (1999) Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res 298:275–286
Lalatta-Costerbosa G, Mazzoni M, Clavenzani P, Di Guardo G, Mazzuoli G, Marruchella G, De Grossi L, Agrimi U, Chiocchetti R (2007) NOS-immunoreactivity and NADPH-d histochemistry in the enteric nervous system of Sarda breed sheep with different PrP genotypes in wholemount and cryostat preparations. J Histochem Cytochem 55:387–401
Landsverk T, Halleraker M, Aleksandersen M, McClure S, Hein W, Nicander L (1991) The intestinal habitat for organized lymphoid tissues in ruminants; comparative aspects of structure, function and development. Vet Immunol Immunopathol 28:1–16
Ma B, Wasielewski R von, Lindenmaier W, Dittmar KEJ (2007) Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol 36:62–74
Mabbott NA, MacPherson GG (2006) Prions and their lethal journey to the brain. Nat Rev Microbiol 4:201–211
Mao Y, Wang B, Kunze W (2006) Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol 96:998–1010
Marruchella G, Ligios C, Albanese V, Cancedda MG, Madau L, Lalatta-Costerbosa G, Mazzoni M, Clavenzani P, Chiocchetti R, Sarli G, De Grossi L, Agrimi U, Aguzzi A, Di Guardo G (2007) Enteroglia and neuron involvement without apparent neuronal loss in ileal enteric nervous system plexi from scrapie-affected sheep. J Gen Virol 88:2899–2904
Mazzuoli G, Mazzoni M, Albanese V, Clavenzani P, Lalatta-Costerbosa G, Lucchi ML, Furness JB, Chiocchetti R (2007) Morphology and neurochemistry of descending and ascending myenteric plexus neurons of sheep ileum. Anat Rec 290:1480–1491
Ottaway CA, Lewis DL, Asa SL (1987) Vasoactive intestinal peptide-containing nerves in Peyer’s patches. Brain Behav Immun 1:148–158
Pascual DW, Kiyono H, McGhee JR (1994) The enteric nervous system: interactions for mucosal immunity and inflammation. Immunomethods 5:56–72
Payan DG, McGillis JP, Renold FK, Mitsuhashi M, Goetzl EJ (1987) Neuropeptide modulation of leukocyte function. Ann N Y Acad Sci 496:182–191
Pfannkuche H, Schemann M, Gäbel G (2002) Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res 309:347–354
Pfoch M, Unsicker K (1972) Electron microscopic study on the innervation of Peyer’s patches of the Syrian hamster. Z Zellforsch Mikrosk Anat 123:425–429
Powley TL (2000) Vagal input to the enteric nervous system. Gut 47:iv30–iv32
Press CM, Heggebø R, Espenes A (2004) Involvement of gut-associated lymphoid tissue of ruminants in the spread of transmissible spongiform encephalopathies. Adv Drug Deliv Rev 56:885–899
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Reynaud CA, Mackay CR, Muller RG, Weill JC (1991) Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer’s patches. Cell 64:995–1005
Sharkey KA (1992) Substance P and calcitonin gene-related peptide (CGRP) in gastrointestinal inflammation. Ann N Y Acad Sci 664:425–442
Sharkey KA, Lomax AEG, Bertrand PP, Furness JB (1998) Electrophysiology, shape and chemistry of intestinofugal neurons projecting from guinea pig distal colon to inferior mesenteric ganglia. Gastroenterology 115:909–918
Shtylik AV, Otellin VA, Nozdrachev AD (1998) Innervation of grouped lymphoid nodules (Peyer’s patches) by the enteric nervous system and the topography of their interior neural elements in the rat. Morfologiia 114:34–39
Stead RH (1992) Innervation of mucosal immune cells in the gastrointestinal tract. Reg Immunol 4:91–99
Tooyama I, Kimura H (2000) A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers. J Chem Neuroanat 17:217–226
Vulchanova L, Casey MA, Crabb GW, Kennedy WR, Brown DR (2007) Anatomical evidence for enteric neuroimmune interactions in Peyer’s patches. J Neuroimmunol 185:64–74
Yamaguchi K, Schoefl GI (1983) Blood vessels of the Peyer’s patches in the mouse. I. Topographical studies. Anat Rec 206:391–401
Young HM, Anderson CR, Furness JB (2000) Nitric oxide in the peripheral autonomic nervous system. In: Steinbusch HWM, De Vente J, Vincent SR, Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 17. Elsevier Science, Amsterdam, pp 215–226
Acknowledgements
The careful technical assistance of Caterina Mongardi-Fantaguzzi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR, PRIN 2006), from the Fondazione del Monte di Bologna e Ravenna and from the National Health and Medical Research Council of Australia (grant no. 400020).
Rights and permissions
About this article
Cite this article
Chiocchetti, R., Mazzuoli, G., Albanese, V. et al. Anatomical evidence for ileal Peyer’s patches innervation by enteric nervous system: a potential route for prion neuroinvasion?. Cell Tissue Res 332, 185–194 (2008). https://doi.org/10.1007/s00441-008-0583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0583-y