Abstract
To investigate extrinsic origins of calcitonin gene-related peptide immunoreactive (CGRP-IR) nerve fibres in the sheep ileum, the retrograde fluorescent tracer Fast Blue (FB) was injected into the ileum wall. Sections of thoraco-lumbar dorsal root ganglia (DRG) and distal (nodose) vagal ganglia showing FB-labelled neurons were processed for CGRP immunohistochemistry. The distribution of CGRP-IR in fibres and nerve cell bodies in the ileum was also studied. CGRP-IR enteric neurons were morphometrically analysed in myenteric (MP) and submucosal plexuses (SMP) of lambs (2–4 months). Sensory neurons retrogradely labelled with FB were scattered in T5-L4 DRG but most were located at the upper lumbar levels (L1-L3); only a minor component of the extrinsic afferent innervation of the ileum was derived from nodose ganglia. In the DRG, 57% of retrogradely labelled neurons were also CGRP-IR. In cryostat sections, a dense network of CGRP-IR fibres was observed in the lamina propria beneath the epithelium, around the lacteals and lymphatic follicles (Peyer's platches), and along and around enteric blood vessels. Rare CGRP-IR fibres were also present in both muscle layers. Dense pericellular baskets of CGRP-IR fibres were observed around CGRP-negative somata. The only CGRP-IR nerve cells were well-defined Dogiel type II neurons localised in the MP and in the external and internal components of the SMP. CGRP-IR neurons in the myenteric ganglia were significantly larger than those in the submucosal ganglia (mean profile areas: about 1,400 μm2 for myenteric neurons, 750 μm2 for submucosal neurons). About 6% of myenteric neurons and 25% of submucosal neurons were CGRP-IR Dogiel type II neurons. The percentages of CGRP-IR neurons that were also tachykinin-IR were about 9% (MP) and 42% (SMP), whereas no CGRP-IR neurons exhibited immunoreactivity for vasoactive intestinal peptide, nitric oxide synthase or tyrosine hydroxylase in either plexus. Thus, CGRP immunoreactivity occurs in the enteric nervous system of the sheep ileum (as in human small intestine and MP of pig ileum) in only one morphologically defined type of neuron, Dogiel type II cells. These are probably intrinsic primary afferent neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substantial neuroanatomical and pharmacological evidence has been obtained showing that the 37-amino-acid neuropeptide, calcitonin gene-related peptide (CGRP), produced by alternative calcitonin gene expression (Amara et al. 1982; Rosenfeld et al. 1983), plays a crucial role in many physiological and pathological regulatory functions of the enteric nervous system (ENS) of vertebrate species, including the regulation of gastrointestinal smooth muscles and motility (Palmer et al. 1986; Bartho et al. 1987; Rasmussen et al. 1992; Grider 1994), sensory functions (Sternini 1991; Rasmussen et al. 2001), intestinal microcirculation (Vanner 1994; Kawasaki 2002), secretion (Taché 1992), amino acid absorption (Barada et al. 2000), lymphatic microcirculation and lymphocyte function (Ichikawa et al. 1991, 1994).
The distribution of CGRP immunoreactivity has been previously investigated in the pig (Scheuermann et al. 1987, 1991) and man (Timmermans et al. 1992). Work on human small intestine (Timmermans et al. 1992) has indicated that CGRP immunoreactivity is mainly found in a neuronal population morphologically similar to the CGRP-immunoreactive (IR) somata observed in the porcine small intestine (Scheuermann et al. 1987, 1991) and mouse colon (Furness et al. 2004b), i.e. in Dogiel type II neurons. Brehmer et al. (2002) have shown that CGRP immunoreactivity also occurs in another neuronal population in the porcine myenteric plexus (MP), viz. type V neurons of the ileum. We have recently analysed the distribution of calbindin (CALB)-IR neurons in both plexuses of unweaned and mature sheep (Chiocchetti et al. 2004) and found the co-localisation of immunoreactivity for CALB and CGRP mainly in Dogiel type II neurons. Immunochemical and radioimmunoassay studies have demonstrated that CGRP immunoreactivity in the gastrointestinal tract arises from a set of neurons in the MP and submucosal plexus (SMP; Clague et al. 1985; Feher et al. 1986; Sternini 1992) and from primary afferent neurons whose cell bodies are located in the nodose and dorsal root ganglia (DRG; Gibbins et al. 1985; Sternini et al. 1987; Franco-Cereceda et al. 1987; Green and Dockray 1987; Su et al. 1987).
Although CGRP immunoreactivity has been investigated in the ENS of several mammalian species, where it occurs in Dogiel type II neurons and extrinsic primary afferent neurons (see above), the sources of CGRP innervation have not been examined in ruminants. Ruminants are the natural hosts of prion diseases, which include scrapie in sheep. We have recently shown that prions infect the ovine ENS and that the ENS or nerves innervating the intestine may be a route of prion infection (Chiocchetti et al. 2005). To analyse further the nature of prion infection of the ENS, the neuron types in the sheep intestine, especially the afferent neurons, have to be identified. Thus, we have investigated the location of CGRP immunoreactivity in the extrinsic and intrinsic innervation of the sheep ileum to determine whether it is as good a marker of afferent neurons as it is in some monogastric species.
Materials and methods
Cryostat sections and whole-mounts were obtained from the ileum of lambs in the age range 3 days to 4 months, of mixed breeds and of both sexes, slaughtered at a public slaughterhouse or killed under ethical approval of the Committee of Animal Experimentation of Bologna University. In order to block axonal transport and obtain the maximum enhancement of immunoreactivity of the nerve cells, two lambs, under deep anaesthesia and weighing 20 kg, were injected intraperitoneally with an inhibitor of microtubular transport (5 mg/kg colchicine; Sigma-Aldrich Chemie, Steinheim, Germany) dissolved in saline, 2 h before death. Sheep in which the ileum had been infiltrated with the retrograde fluorescent tracer Fast Blue (FB) were deeply anaesthetised as described in detail below and killed by means of intravenous administration of embutramide, mebenzonium iodide and tetracaine hydrochloride (Tanax, Intervet, Munich, Germany).
Injection of the FB fluorescent tracer
Surgical procedures
Three lambs were maintained on a diet of hay and water ad libitum for 1 week before the experiment. One day before surgery, the animals were kept without food. Anaesthesia was induced by intravenous administration of sodium thiopental (10 mg/kg i.v.) and was maintained with isofluorane, nitrous oxide and oxygen via a cuffed endotracheal tube of 8 mm internal diameter. A gastroesophageal tube was subsequently introduced to allow the emission of gastric gas. Following a midline laparotomy, the ileum was exposed and intramurally slowly infiltrated with FB in aqueous solution (2%), injected at multiple sites by means of a microsyringe. The fluorescent tracer FB produces blue fluorescent labelling of cytoplasm at an excitation wavelength of 360 nm. A total of 170 μl FB was injected into the mesenteric and anti-mesenteric borders of the ileum. At least 17 injections of FB were made in a segment of ileum of about 15 cm in length, beginning at the ileo-caecal junction. The syringe was gently withdrawn and any tracer leakage was removed from the surface of the ileum. The animals were allowed to recover from surgery and began to eat hay on the first post-operative day.
Post-injection survival time
The optimal timing for maximal cell labelling was established on the basis of our own experience with the retrograde transport of fluorescent tracers in the visceral neural pathways of sheep (Chiocchetti et al. 2003). For this study, we chose a survival period of 3 weeks.
Tissue preparation
At the end of the chosen survival time, the animals were deeply anaesthetised and killed by means of embutramide, mebenzonium iodide and tetracaine hydrochloride (Tanax) administration. The T5-S2 spinal cord surrounded by the dural sack was immediately exposed over its full length through a dorsal laminectomy. In doing so, we took care not to cut the spinal roots to ensure the later precise identification of the various segments of the cord and the collection of the DRGs. After freeing the cord of the spinal dura, the spinal cord was divided into segments. Segmental boundaries were localised by means of the spinal roots and by counting them from the last thoracic spinal nerve located just caudal to the 13th rib. The right and left T5-S2 DRGs and the distal vagal (nodose) ganglia (DVG) were quickly removed, fixed for 48h in 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2) at 4°C, rinsed overnight in phosphate-buffered saline (PBS: 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2) and stored at 4°C in PBS containing 30% sucrose and sodium azide (0.1%). The tissues were frozen in isopentane cooled in liquid nitrogen and then mounted in Tissue Tek (Sakura Finetek Europe) mounting medium. Serial longitudinal DRG sections (12 μm thickness starting from the dorsal surface of the ganglion) were cut on a cryostat and mounted on gelatin-coated slides. The sections were not coverslipped and were examined within 2 h of cutting. The slides selected for immunohistochemistry were processed for immunostaining or stored at −20°C. Immunohistochemical studies were performed only on the frozen sections in which FB-stained neurons were previously observed.
Light microscopy
Whole-mount processing
The technique followed in this investigation was as described in detail previously (Chiocchetti et al. 2004). Briefly, we collected segments of ileum (15–20 cm in length), beginning about 2 cm oral to the ileocecal junction; these were immediately immersed in ice-cold oxygenated Krebs solution containing the L-type calcium channel blocker, nicardipine, as a muscle relaxant (10−6 M; Sigma-Aldrich Chemie). Segments of ileum were cut open along the mesenteric border and the tissues were vigorously flushed out with fresh oxygenated Krebs solution at room temperature. In order to inhibit axonal transport from the cell bodies and thus to optimise the immunoreactivity of the nerve cells for neuropeptides, the ileum specimens (but not those from lambs treated with colchicine before killing) were incubated overnight in tissue culture medium (Dulbecco's modified Eagle's medium with glutamine; Sigma-Aldrich Chemie) to which penicillin (100 U/ml), streptomycin (100 μg/ml) and colchicine (2.5×10−4 M; Sigma) were added (Messenger and Furness 1990). Tissue for whole-mount preparation was pinned on a balsa board, mucosal surface facing down, and fixed in 2% paraformaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer (pH 7.0) at 4°C overnight and subsequently washed in dimethylsulphoxide (DMSO; 3×10 min) followed by PBS (3×10 min). All tissue was stored at 4°C in PBS containing azide (0.1%).
Ileum cryosections
Because the mucosa of whole-mounts was removed to reduce preparation thickness, cryosections were prepared to study the vertical projections of the CGRP-IR fibres and the presence of these in the two muscle layers and the mucosa. For tissue to be examined in section, segments of ileum were removed, pinned to balsa wood without stretching, and fixed overnight at 4°C. Tissue was cleared in DMSO as described above, placed in PBS-sucrose-azide (PBS containing 0.1% sodium azide and 30% sucrose as a cryoprotectant) and stored at 4°C overnight. The following day, small segments of tissue were transferred to a mixture of PBS-sucrose-azide and OCT compound (Tissue Tek) at a ratio of 1:1 for a further 24 h before being embedded in 100% OCT. To prepare frozen sections, sheep intestine was frozen in isopentane and liquid nitrogen, following which sections of 12 μm thickness were cut, collected onto gelatin-coated slides and left to dry for 1 h at room temperature.
Immunohistochemistry
Cryostat sections of ileum, DRG and DVG and whole-mount preparations were processed for single- and double-labelling. For single-labelling, we utilised rabbit anti-CGRP (Peninsula; polyclonal), whereas for double-labelling, we co-localised rabbit anti-CGRP antiserum with anti-neuron-specific enolase (NSE), human neuronal protein (Hu), tachykinin (TK), vasoactive intestinal peptide (VIP), nitric oxide synthase (NOS) and tyrosine hydroxylase (TH) antibodies. Tissues were incubated in 10% normal goat serum in PBS containing 1% Triton X-100 for 30 min at room temperature to reduce non-specific binding of the secondary antibody and to permeabilise the tissue to the antisera. The rabbit anti-CGRP antiserum was used at a concentration of 1:200. The other antibodies that were tested in double-labelling studies are listed in Table 1. All primary antibodies were diluted in antibody diluent (1.8% NaCl in 0.01 M sodium phosphate buffer containing 0.1% sodium azide). Following incubation in a single antibody or in combined primary antibodies for 1 night (cryostat sections) or 2–3 nights (whole-mounts) at 4°C in a humid chamber, preparations were washed (3×10 min) in PBS and then incubated for 1 h (cryostat sections) or 3 h (whole-mounts) at room temperature with the appropriate secondary antibodies (Table 2). The cryostat sections and whole-mount preparations were cover-slipped with buffered glycerol (pH 8.6).
Specificity of antibodies
Absorption tests have been previously carried out on sheep ENS for the guinea-pig anti-VIP and rat anti-substance P (SP) antibodies (Pfannkuche et al. 2002) and the specificity of the mouse anti-NOS antibody was tested by the same authors. In this investigation, we carried out adsorption studies for the rabbit anti-CGRP serum. Adsorption of the diluted rabbit anti-CGRP antibody with 10−5 M rat CGRP peptide (Sigma) for 24 h at 4°C prior to application to the tissue strongly reduced CGRP immunostaining in SMP neurons or completely prevented it in MP neurons and in perivascular CGRP-IR fibres. The specificity of the secondary antibodies was tested by applying these antisera without use of the primary antibodies. No stained neurons and fibres were seen after omitting the primary antisera.
Diaminobenzidine immunohistochemistry
The diaminobenzidine reaction was applied to define more accurately the CGRP staining and the shapes of cells and fibres. After incubation in the primary rabbit anti-CGRP antibody, whole-mount specimens were incubated overnight with biotinylated goat anti-rabbit IgG (10 μg/ml; Vector Labs). After being washed (3×10 min) in PBS, the specimens were treated overnight with the avidin-biotin-peroxidase-complex (ABC kit; Vector). After three washes (10 min each) in PBS, immunoreactive sites were visualised by treating tissues with 3-3′–diaminobenzidine (DAB kit; Vector), with nickel ammonium sulphate enhancement. Immunostained specimens were mounted on chrome-alum-coated slides, covered with a double tissue paper layer and maintained flat with the apposition of another slide (sandwich technique), until ethanol dehydratation. The specimens were finally cleared with xylene and cover-slipped with Entellan (Merk, Darmstadt, Germany).
Fluorescence microscopy
Preparations were examined on a Zeiss Axioplan microscope equipped with a filter system providing excitation light of 360 nm wavelength to reveal blue FB fluorescent labelling of neuronal cytoplasm; the microscope was also equipped with the appropriate filter set for discriminating between fluorescein isothiocyanate (FITC) and Alexa 594 fluorescence (or tetrametyl rhodamine isothiocyanate, TRITC). We used filter set 10 for FITC (450–nm to 490-nm excitation filter and 515–nm to 565-nm emission filter) and filter set 00 for Alexa 594 and TRITC (530–nm to 585-nm excitation filter and 615-nm emission filter). Images were recorded by using a Polaroid DMC digital photocamera (Polaroid, Cambridge, Mass., USA) and DMC 2 software. Images were further processed by using Corel Photo Paint and Corel Draw software programs. KS300 Zeiss software (Kontron Elektronik, Germany) was utilised for the morphometric analysis of CGRP-labelled nerve cells. The somatic cross-sectional area of CGRP-IR somata was measured after manual tracing of the cell outline; 500 CGRP-stained neurons in the MP and 400 in the SMP were measured in two lambs (2 and 4 months old).
Quantitative analysis
Whole-mounts
The proportions of neurons that were immunoreactive for a particular chemical marker and that were also positive for other neurochemicals were determined by examining fluorescently labelled, double-stained whole-mount preparations. Neurons were first located by the presence of a fluorophore that labelled one antigen and then the filter was switched to determine whether the neuron was labelled for a second antigen located with a fluorophore of a different colour. In this way, proportions of neurons labelled for pairs of antigens were determined. For each staining combination, two whole-mount preparations of the myenteric and submucosal plexus, from three to five different animals, were used. The cohort size was 50 neurons and at least 200 cells were counted from each animal. The percentages of neurons that were immunoreactive for a particular marker and that were also immunoreactive for a second neurochemical were calculated and expressed as mean±standard deviation (SD). The proportions of neurochemically identified populations were then expressed relative to the number of NSE- or Hu-IR neurons, which was considered to represent 100% of the enteric neurons.
We consider that the ENS cells stained with the general markers (NSE and Hu) probably represent a large proportion of the neuronal population on the basis of the specificity of these markers for neuron-specific protein in other species. In addition, the anti-NSE antibody is generally accepted to stain the entire neuronal population of the sheep ENS (Pfannkuche et al. 2002). Our personal observations of the validity of NSE and Hu staining have shown that the anti-NSE antibody stains about 98% of the Hu-IR neurons and that 100% of the Hu-stained cells are NSE-IR, suggesting that both are good pan-neuronal markers of sheep enteric neurons. We have also tested the possibility that NSE is a marker of ENS glial cells by comparing immunoreactivity for NSE with that for glial fibrillary acid protein (GFAP). We did not observe any co-localisation between NSE-IR and GFAP-IR cells.
Student's t test was used to evaluate differences between soma sizes of the CGRP-IR neuronal population of MP and SMP in growing lambs (2–4 months old). Differences were considered statistically significant at values of P<0.001.
DRG and DVG cryostat sections
The proportion of neurons positive for the retrograde tracer FB and immunoreactive for CGRP was determined by examining fluorescently labelled, single-immunostained preparations. Neurons were first located by the presence of FB staining and then the filter was switched to determine whether the neuron was labelled for CGRP, which was located with a fluorophore of a different colour. The neurons with FB labelling were counted and their CGRP-IR was determined. We counted, for each ganglionic level, the total number of FB-labelled sensory neurons.
Results
Extrinsic source of CGRP-IR primary afferent fibres
The retrogradely labelled neurons visualised in the DRG, following injection of FB into the ileum wall, were scattered at T5-L4 levels but the vast majority were located at the upper lumbar levels (L1-L3). The largest number of FB-labelled neurons counted in one lamb was 577, with the left ganglia harbouring the majority of FB-labelled cells (Fig. 1). The proportion of FB-labelled DRG neurons that were immunolabelled for CGRP was 57% (Fig. 2a, a′). CGRP-containing afferent neurons did not exhibit viscerotopic segregation within spinal ganglia and were distributed throughout each individual ganglion.
CGRP immunoreactivity in dorsal root ganglion (DRG) neurons labelled after injection of FB (2%) into the ileum wall. a, a′ The medium-sized FB-labelled neuron (arrow) in the L2 DRG (a) exhibits strong cytoplasmic CGRP staining (a′). b, b′ The FB-labelled neuron (arrow) in the T13 DRG (b) is also CGRP-IR (b′). Bar 50 μm
By contrast, and in agreement with other studies carried out on the small intestine of other species (Green and Dockray 1987; Su et al. 1987), only a minor component of the afferent innervation of the ileum was derived from neurons located in the nodose ganglia. We counted only a few FB-stained neurons (14 FB-labelled cells in one lamb) in serial sections through both nodose ganglia; we carried out no immunohistochemical examination of this tissue.
CGRP-IR neurons and fibres in cryostat sections
A few CGRP-IR neurons were seen in both enteric plexuses but they were more numerous in the SMP. Their shapes were revealed better in whole-mounts and are described below. The CGRP innervation of the mucosa was intense, since the CGRP-IR processes formed dense networks extending from the base to the tip of the villi, around the lacteal and beneath the epithelium (Fig. 3a,b). No CGRP immunoreactivity was exhibited by entero-endocrine cells. A dense network of CGRP-IR fibres occurred in submucosal and myenteric ganglia. No CGRP-IR fibres were seen inside the lymphatic follicles, whereas reactive fibres were abundant around all follicles (Fig. 3c). The circular and longitudinal muscle layers harboured few and scarce-to-moderate numbers of CGRP-IR fibres, respectively (Fig. 3c,d).
General appearance of the mucosal, myenteric and submucosal plexuses innervation in cryostat section. Longitudinal (a) and transverse (b) sections of the villi showing CGRP-IR nerve fibres along the major axis of the villi, extending from the base to the tip, around the lacteal and beneath the epithelium. c CGRP-IR fibres were not seen inside the lymphatic nodules (LN), whereas abundant fibres lay around the follicles. Few fibres were present in the circular (CML) and longitudinal (LML) muscle layers. d Note the interconnecting CGRP-IR strand of fibres between ganglia of the SMP and MP. Bar 100 μm
CGRP-IR neurons and fibres in whole-mount preparations
The MP neurons exhibited satisfactory CGRP staining only after colchicine treatment, whereas the SMP cells were strongly CGRP-positive without colchicine treatment.
CGRP-IR neurons and fibres in MP
The percentage of neurons expressing CGRP immunoreactivity in the MP was 6±2% (n=2870 cells from four animals double-stained for Hu or NSE). All the neurons exhibiting CGRP immunoreactivity had Dogiel type II morphology (Fig. 4a). The CGRP-IR somata of the MP were generally ovoid, presenting multipolar, bipolar and pseudounipolar aspects. Numerous CGRP-IR cells possessed four to five visible processes, sometimes having a broad calibre where they arose from the soma (Fig. 4b). Often, they did not seem to give rise to daughter branches or varicosities in the neighbourhood of the soma. We also commonly observed neurons whose processes were not stained for CGRP immunoreactivity. The cells exhibited mainly a smooth outline (sometimes undulated) and an eccentrically placed round or oval CGRP-negative nucleus, encircled by a dense ring of cytoplasmic CGRP-IR condensation. The same percentage of CGRP-IR neurons was seen in non-colchicine-treated tissues but these weakly positive neurons did not exhibit strong perinuclear staining, which was probably Golgi-associated. The CGRP cytoplasmic staining was distributed in a typical granular pattern mainly in the putative Golgi area within the cell bodies and granular staining never extended into proximal processes. CGRP-IR neurons were either single cells (located along the interconnecting strands or outside the ganglia) or were grouped within aggregates of two to twelve cells, clustering or forming a line of neurons with their major axis disposed along the circumference of the gut. We frequently detected no CGRP-IR cell bodies, in specimens up to 1 cm2, in which many CGRP-IR fibres were instead present. The CGRP-IR neurons tended to be at the lateral surfaces of the ganglia and were often observed in close apposition to CGRP-negative cells, which were encircled by dense bright large varicose CGRP-IR processes. We had previously established that some of the CGRP-negative neurons encircled by these CGRP-IR fibres were radially multidendritic CALB-IR neurons (Chiocchetti et al. 2004). The CGRP-IR fibres formed dense networks, wrapping around and often apposing ganglion cells; this pattern suggested that CGRP could be a transmitter at intrinsic synapses. The CGRP-IR fibres were not homogenously distributed in the MP, being dramatically represented only in few areas of the MP. These fibres, presenting numerous varicosities, were observed around CGRP-IR and CGRP-negative neurons, in the primary, secondary and tertiary interconnecting strands and along arterioles at the level of the MP; thick bundles of CGRP-IR fibres (without varicosities) were often seen running in the longitudinal direction covering the whole length of the specimen (maximum: 2 cm; Fig. 4c).
CGRP immunoreactivity in MP whole-mount preparations. a CGRP-IR neurons exhibiting a smooth outline and an eccentrically placed CGRP- negative nucleus (Dogiel type II) grouped in a small cluster. Bar 100 μm. b CGRP-IR MP neurons showing broad-calibre processes arising from the soma. Bar 50 μm. c CGRP-IR MP fibres running along the major axis of the ileum as revealed by diaminobenzidine staining with nickel-ammonium-sulphate enhancement. Bar 100 μm
CGRP-IR neurons and fibres in SMP
In the SMP, the CGRP-IR neurons represented a large percentage of the total neuronal population (25±8%; n=1280 cells from three subjects) and were present in all the main SMP ganglia. All the CGRP-reactive neurons were Dogiel type II in morphology but were smaller than those in the MP. The neurons were mainly pseudo-unipolar (pear-shaped or ovoid) or bipolar; it was not possible to see, in either kind of preparation (fluorescent or peroxidase staining), neurons exhibiting more than two processes (Fig. 5a). In diaminobenzidine preparations, profiles of many CGRP-IR neurons were irregular, being indented by the enteroglia nuclei (Fig. 5a,b). The CGRP-IR SMP cells presented, like the MP neurons, round or oval nuclei that were peripherally placed. As in the MP, CGRP-IR fibres and varicosities (forming complete baskets around cells) outlined CGRP-positive and CGRP-negative neurons. Unlike the MP, the CGRP-IR fibres were homogeneously distributed within the ganglia, forming a regular network of contacts. Reactive fibres were mostly associated with small arteries and arterioles, as previously shown in other species such as rat, guinea-pig and rabbit (Gibbins et al. 1985; Goehler et al. 1988; Eysselein et al. 1991), and were abundant (Fig. 6a,b).
CGRP immunoreactivity located with diaminobenzidine enhanced with nickel-ammonium-sulphate in an SMP whole-mount preparation. CGRP-IR neurons show Dogiel type II morphology and are mainly pseudounipolar or bipolar. The irregular profiles of many CGRP-IR neurons (a, b) are indented by enteroglial nuclei (arrows). Bar 50 μm
Soma sizes of CGRP-IR neurons
CGRP-IR neurons of the MP were significantly larger than the SMP neurons (P<0.001).
Soma sizes of CGRP-IR neurons in MP
The profiles of the CGRP-IR neurons showed a wide range of dimensions, between 412 μm2 and 2979 μm2. The mean value of the profile area of the CGRP-IR somata was 1412±515 μm2 (mean±standard deviation, n=500 neurons). The maximum diameters of cell bodies ranged from 32 μm to 112 μm. Some irregular oval CGRP-IR somata had an elongated narrow profile, with a major axis of 112 μm and a minor axis of 29 μm. These elongated cell bodies formed the upper part of the range of maximum diameters, whereas the oval-shaped bodies formed the lower part of the range, indicating that the range of cell volumes is not as extensive as the range of maximum diameters suggests. A histogram of cell cross-sectional areas of 500 CGRP-IR MP neurons from three lambs is presented in Fig. 7a.
Soma sizes of CGRP-IR neurons in SMP
The average size of the submucosal ganglion neurons in the sheep ileum is much smaller than that in the corresponding MP. The CGRP-IR SMP neurons presented a range between 90 μm2 and 2431 μm2, the average neuron size being 747±477 μm2 (mean±standard deviation, n=400 neurons). The major and the minor neuronal axis observed for the CGRP-IR SMP neuronal population was 78 μm and 8 μm, respectively. Cells with cross-sectional areas of less than 500 μm2 were more common than larger cells. A histogram of cell cross-sectional areas of 400 CGRP-IR SMP neurons from two lambs is presented in Fig. 7b.
Neurochemical coding of CGRP-IR neurons
Immunoreactivity for CGRP and tachykinin
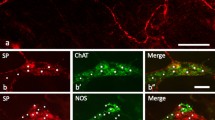
The percentage of neurons expressing TK-IR in the MP was 13±3% (n=992 cells from four animals). We observed the co-localisation of immunoreactivity for CGRP and TK in 9±13% (range: 0% to 27%) of CGRP-IR MP neurons (Fig. 8a, a′). Only 4±5% (range: 0% to 12%) of TK-IR cells showed CGRP immunoreactivity. Co-localisation was noted only in small neurons and was never observed in the largest Dogiel type II somata. The TK staining was faint, like the CGRP immunoreactivity. The CGRP-IR fibres seemed to innervate different target neurons than the SP-IR fibres. Some small TK-IR cells were seen to be encircled, as observed for the radially multidendritic CALB-IR cells, by a dense ring of CGRP-IR varicosities. Strongly stained TK-IR fibres also formed typical baskets around CGRP-negative cells.
Myenteric neurons after simultaneous localisation of CGRP immunoreactivity with SP (a, a′), VIP (b, b′), NOS (c, c′) and TH (d, d′) immunoreactivity. a, a′ Four Dogiel type II-like neurons (asterisks) are immunoreactive for CGRP (a) but not SP-IR (a′). Note the dense varicose basket formed by CGRP-IR fibres around CGRP-negative neurons. Bar 50 μm. b, b′ CGRP immunoreactivity (b) is not co-localised with VIP immunoreactivity (b′). Note a VIP-IR cell (asterisks) that is CGRP-negative. Bar 50 μm. c, c′ CGRP immunoreactivity (c) is not co-localised with NOS immunoreactivity (c′). Note that the CGRP-IR fibres encircle two large NOS-IR neurons. Bar 50 μm. d, d′ Three neurons (asterisks) immunoreactive for CGRP (d) are not TH-IR (d′). Bar 50 μm
The percentage of SMP neurons expressing TK-IR was 38±11% (n=230 cells from one animal). In the SMP, 42±29% of CGRP-IR somata were also TK-IR.
Immunoreactivity for CGRP and VIP
No co-localisation of immunoreactivity for CGRP and VIP was observed in neurons of either plexus (Fig. 8b, b′). The percentage of neurons expressing VIP immunoreactivity in the MP was 6±2% (n=1136 cells from four animals). The percentage of VIP-IR cells in the SMP was 9±7% (n=431 cells from four animals). The pattern of weak VIP staining was cytoplasmic and was similar to that of CGRP (granular pattern). The VIP immunoreactivity was observed mainly in medium-sized neurons. VIP-IR fibres were abundant in the circular muscle layer and also in the thinnest interconnecting strands of fibres (tertiary plexus); like CGRP-IR and TK-IR fibres, VIP-IR fibres formed typical dense varicose baskets around CGRP-negative cells. We noted a rare perivascular VIP-IR innervation. We observed CGRP-IR and VIP-IR fibres around VIP-IR and CGRP-negative somata and vice versa.
Immunoreactivity for CGRP and NOS
The percentage of neurons expressing NOS immunoreactivity in the MP and SMP of this subset of lambs was 32±5% and 21±20%, respectively (n=199 cells in the MP; n=192 cells in the SMP, from two lambs). No co-localisation of immunoreactivity for CGRP and NOS was observed in the neurons of either plexus (Fig. 8c, c′). The NOS-IR neurons were strongly stained and were numerous; they exhibited a broad spectrum of sizes, some NOS-IR cells being larger than the largest CGRP-IR somata. The majority of the longest processes of NOS-IR cells were caudally directed. The circular muscle layer strips that remained attached to whole-mount MP preparations were rich in NOS-IR fibres. NOS-IR fibres were seen encircling CGRP-IR somata and vice versa. Similar to the VIP-IR fibres, fibres immunoreactive for both neurochemicals were seen encircling the two kinds of neurons. A large percentage (97±4%; n=150 cells from three lambs) of neurons expressing VIP-IR were also NOS-IR.
Immunoreactivity for CGRP and TH
The proportions of TH-IR cells in the MP and SMP neuronal populations were, in two lambs (20-day-old subjects), 3.5±3.5% (n=847 cells) and 1±1% (n=519 cells), respectively. No co-localisation of immunoreactivity for CGRP and TH was seen, in either plexus (Fig. 8d, d′). The TH-IR cells were faintly stained, were mainly small and had a Dogiel type I-like shape (these data were supported by diaminobenzidine/horseradish peroxidase-stained specimens that were examined). We did not observe any relationship between TH-IR fibres and CGRP-IR neurons. A large number of small Hu- or NSE-negative but TH-IR cells were seen in both plexuses, primarily in the SMP, where they preferentially aligned along submucosal blood vessels in close apposition to the perivascular TH-IR fibres. They probably represent, as demonstrated in mice, mast-cells; indeed, the mast cells may store high levels of dopamine and occasionally produce noradrenalin and adrenalin (Freeman et al. 2001).
Table 3 reports the analysis of the chemical code of the ENS neurons.
Discussion
CGRP-IR fibres of extrinsic origin
This paper describes, for the first time, the distribution of vagal and thoraco-lumbar spinal afferent neurons that innervate the ileum of sheep. Spinal primary afferent neurons were distributed between the thoracic and lumbar dorsal root ganglia, T5-L4. Few labelled cells were also found in the left and right DVG. Our results are in agreement with the data obtained by Cottrell and Greenhorn (1987) who investigated the sensory innervation of the gastro-duodenal junction of sheep. They found labelled neurons in the vagal ganglia and in the DRGs at levels T6-L3. The present investigation clearly demonstrates that the vagal contribution to the ileum afferent innervation is modest (about 3% of innervating neurons) in comparison with the abundance of DRG components (about 97%) and that a significant proportion of DRG neurons are CGRP-IR (57%). The small number of FB-labelled neurons in the T5-L4 DRG (577 cells in one lamb) and DVG (14 cells in one lamb) might be attributable to the relatively small length of the ileum (15 cm) that was injected. The proportion of DRG neurons projecting to the gut that are CGRP-IR has also been determined in the rat, mouse and guinea-pig, where the neurons are 50%–90% CGRP-IR, depending on species and region (Green and Dockray 1988; Sternini and Anderson 1992).
Intrinsic CGRP-IR neurons
The only intrinsic nerve cells that contained CGRP-IR were neurons with Dogiel type II morphology, most of which were large neurons. This resembles the morphology of the intrinsic primary afferent (intrinsic sensory) neurons (IPANs) in the guinea-pig small intestine (Furness et al. 2004a). Pharmacological studies have demonstrated that IPANs utilise CGRP as a transmitter in the rat colon and human jejunum (Grider 1994; Grider et al. 1996). Reflexes that are initiated by stimulation of the mucosal endings of IPANs are blocked by CGRP receptor antagonists. Moreover, slow synaptic potentials elicited in second order neurons when submucosal IPANs in the guinea-pig small intestine are stimulated are blocked by a CGRP receptor antagonist (Pan and Gershon 2000). IPANs, identified by their morphology and projections in the mouse colon, are also CGRP-IR (Furness et al. 2004b). Furthermore, Dogiel type II neurons, presumed to be IPANs, are CGRP-IR in the pig small intestine (Scheuermann et al. 1991) and a proportion are CGRP-IR in human (Timmermans et al. 1992; Brehmer et al. 2004). Thus, by analogy with other species, we deduce that the CGRP-IR Dogiel type II neurons in sheep are IPANs. IPANs provide a dense innervation of the musosa and of enteric ganglia but provide few fibres in the circular muscle (Bornstein et al. 1991; Kirchgessner et al. 1992; Furness et al. 2004a,b). This is consistent with the distribution that we have found here for CGRP-IR fibres in the ovine ileum, supporting the conclusion that the IPANs in the sheep small intestine, like IPANs in rat, mouse, guinea-pig, and pig, contain CGRP. In human, although a few IPANs contain CGRP, better markers are calretinin, SP and somatostatin (Brehmer et al. 2004).
The present work confirms the greater average sizes of Dogiel type II neurons compared with other neuron types. Gabella and Trigg (1984) analysed neuronal cell profiles in all neurons displaying a nucleus, in semi-thin tangential sections in the MP of the sheep ileum. They observed a wide range of neuronal sizes, with profiles from less than 100 μm2 up to 1500 μm2 in area (average neuronal cell profile area =441 μm2). We have recorded a range of the CGRP-IR Dogiel type II MP neurons from 412 μm2 to 2979 μm2 (mean=1412, n=500 cells). A similar difference in soma size between the whole population and CGRP-expressing neurons was observed in the SMP, where the data from semi-thin sections showed an average neuronal cell profile area of 237 μm2, whereas CGRP neurons had profile areas of 747±477 μm2 (n=400 cells).
CGRP-IR fibres
The present work confirms other investigations showing that the CGRP innervation of the alimentary tract has a dual origin, viz. from extrinsic and intrinsic neurons. We have found that the CGRP-IR processes innervate all layers of the sheep ileum, although few such fibres are present in the muscle. The large supply of CGRP to arteries and arterioles is consistent with the innervation of the vessels by extrinsic afferent nerve fibres. The DRG neurons send their peripheral processes to gastrointestinal microvessels via the perivascular plexus (Vanner and Surprenant 1996). Moreover, extrinsic CGRP sensory nerves mediate neurogenic vasodilation of ENS arterioles (Vanner 1994). Gates et al. (1989) have shown, in the canine gastrointestinal tract, that only small arteries, and not veins or venules, express CGRP-binding sites. This observation correlates well with previous studies (Brain and Williams 1985) demonstrating that CGRP is a potent vasodilator affecting only resistance vessels (arteries and arterioles) and not capacitance vessels (veins and venules). Although we cannot rule out CGRP occurring in intrinsic vasodilator neurons in the SMP of guinea-pig small intestine, intrinsic vasodilation is mediated via VIP-positive neurons (Neild et al. 1990). We have not observed any co-localisation of immunoreactivity for CGRP and VIP in the SMP of the sheep ileum and thus we speculate that the CGRP-IR neurons are not involved in intrinsic blood-flow regulation. The CGRP-IR fibres form dense networks, wrapping around and often apposing ganglion cells; this pattern is similar to the distribution of the terminals of IPANs (Bornstein et al. 1991), whereas the terminals of extrinsic afferent neurons are sparse in enteric ganglia (Gibbins et al. 1985). Functional studies have shown that CGRP causes excitation of myenteric neurons (Palmer et al. 1986) and that it is a transmitter of IPANs (see above). Thus, CGRP might exert some of its effects within the digestive tract via connections of IPANs with interneurons or motor neurons. We have observed that CGRP-IR fibres make contacts with TK-IR, VIP-IR or NOS-IR neurons. TK-IR neurons seem to play a motor functional role in the sheep ENS, whereas VIP/NOS neurons seem to play an inhibitory role (Pfannkuche et al. 2002) and so we can speculate that CGRP-IR Dogiel type II neurons play a physiological sensory role and act on both excitatory and inhibitory motor neurons.
Chemical coding
We identified distinct classes of neurons expressing different chemical codes: CGRP-IR, VIP-IR, NOS-IR and TH-IR neurons. Immunoreactivity for CGRP and TK is poorly co-localised in the somata of the MP (about 9% of CGRP-IR neurons were TK-IR in the MP, 42% in the SMP, see Table 3). The co-localisation of immunoreactivity for CGRP‐IR and TK in enteric neurons is unusual and does not appear to occur in other species. Immunoreactivity for TH and VIP is not co-localised in sheep ileum neurons and almost all (97±4%) of the VIP-IR cells are also NOS-IR but only a half (58±27%) of NOS-IR neurons co-express VIP immunoreactivity (Chiocchetti et al. 2004). Although, in the guinea-pig, pig and human intestine, small neurons expressing immunoreactivity for TK and VIP are two mutually exclusive groups of neurons in the submucosal ganglia and are probably involved in controlling the secretion of the mucosa, we have observed that about 30% of SP-IR cells are also VIP-IR and that about 60% of VIP-IR somata are also SP-IR. VIP is a non-cholinergic transmitter of secretomotor neurons (Furness et al. 2003). We have shown that CGRP-IR, like TK-IR and VIP-IR, fibres (Keast et al. 1985) are the most prevalent of peptidergic nerves in the gut mucosa. As demonstrated by Anlauf et al. (2003) in the human and by Li et al. (2004) in murine small intestine (and observed in the sheep ileum by Chiocchetti et al. 2003), a population of intrinsic catecholamine-containing neurons occurs in the ileum of adults. Catecholamines seem to play an antisecretory activity, and TH-IR fibres also occur in the mucosa, but their presence is sparse in comparison with that of CGRP-IR, TK-IR and VIP-IR fibres. Previously published results indicate that three distinct classes of neurons are present in the MP of the sheep rumen (Pfannkuche et al. 2002), i.e. cells reactive for choline acetyltransferase (ChAT) and SP (68%), NOS and VIP (26%) and ChAT (5%). We have shown that the CGRP-IR cells are not NOS-IR, VIP-IR or TH-IR and a few CGRP-IR cells are also TK-IR (about 9%); thus, we speculate that another class of neurons exists in the sheep ileum.
Concluding remarks
In conclusion, the major sources of CGRP fibres innervating the ileum of the sheep are primary afferent neurons of the DRG and intrinsic primary afferent neurons. Thus, if either the extrinsic or intrinsic primary afferent neurons are a route of infection by the prion proteins of scrapie or by other infective agents, this might be conveniently investigated by an examination of CGRP-IR neurons.
Reference
Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240–244
Anlauf M, Schafer MK, Eiden L, Weihe E (2003) Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol 459:90–111
Barada KA, Saade NE, Atweh SF, Khoury CI, Nassar CF (2000) Calcitonin gene-related peptide regulates amino acid absorption across rat jejunum. Regul Pept 90:39–45
Bartho L, Lembeck F, Holzer P (1987) Calcitonin gene-related peptide is a potent relaxant of intestinal muscle. Eur J Pharmacol 135:449–451
Bornstein JC, Hendriks R, Furness JB, Trussell DC (1991) Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J Comp Neurol 314:437–451
Brain SD, Williams TJ (1985) Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol 86:855–860
Brehmer A, Schrödl F, Neuhuber W (2002) Correlated morphological and chemical phenotyping in myenteric type V neurons of porcine ileum. J Comp Neurol 453:1–9
Brehmer A, Croner R, Dimmler A, Papadopoulos T, Schrödl F, Neuhuber W (2004) Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Autonomic Neurosci 112:49–59
Chiocchetti R, Clavenzani P, Barazzoni AM, Grandis A, Bombardi C, Lalatta Costerbosa G, Petrosino G, Bortolami R (2003) Viscerotopic representation of the subdiaphragmatic tracts of the digestive apparatus within the vagus complex in the sheep. Brain Res 961:32–44
Chiocchetti R, Grandis A, Bombardi C, Clavenzani P, Lalatta Costerbosa G, Lucchi ML, Furness JB (2004) Characterisation of neurons expressing calbindin immunoreactivity in the ileum of the unweaned and mature sheep. Cell Tissue Res 318:289–303
Chiocchetti R, Clavenzani P, Mazzoni M, Albanese V, Di Guardo G, De Grossa L, Bortolami R, Lalatta Costerbosa G (2005) The sheep enteric nervous system after scrapie (PrPsc) infection. Autonomic Neurosci 119:144–145
Clague JR, Sternini C, Brecha NC (1985) Localization of calcitonin gene-related peptide-like immunoreactivity in neurons of the rat gastrointestinal tract. Neurosci Lett 56:63–68
Cottrell DF, Greenhorn JG (1987) The vagal and spinal innervation of the gastro-duodenal junction of sheep. Q J Exp Physiol 72:513–524
Eysselein VE, Reinshagen M, Cominelli F, Sternini C, Davis W, Patel A, Nast CC, Bernstein D, Anderson K, Khan H, Snape WJ (1991) Calcitonin gene-related peptide and substance P decrease in the rabbit colon during colitis. A time study. Gastroenterology 101:1211–1219
Feher E, Burnstock G, Varndell IM, Polak JM (1986) Calcitonin gene-related peptide-immunoreactive nerve fibres in the small intestine of the guinea-pig: electron-microscopic immunocytochemistry. Cell Tissue Res 245:353–358
Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hokfelt T, Fischer JA (1987) Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides 8:399–410
Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton AL, Narasimhachari N, Domen J, Siméon N, Couderc F, Stewart JK (2001) Catecholamines in murine bone marrow derived mast cells. J Neuroimmunol 119:231–238
Furness JB, Clerc N, Vogalis F, Stebbing MJ (2003) The enteric nervous system and its extrinsic connections. In: Yamada T (ed) Textbook of gastroenterology. Lippincott, Williams & Wilkins, Philadelphia, pp 12–34
Furness JB, Jones C, Nurgali K, Clerc N (2004a) Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72:143–164
Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K (2004b) Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317:1–12
Gabella G, Trigg P (1984) Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. J Neurocytol 13:49–71
Gates TS, Zimmerman RP, Mantyh CR, Vigna SR, Mantyh PW (1989) Calcitonin gene-related peptide-alpha receptor binding sites in the gastrointestinal tract. Neuroscience 31:757–770
Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S (1985) Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57:125–130
Goehler LE, Sternini C, Brecha NC (1988) Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea-pig: distribution and colocalization with substance P. Cell Tissue Res 253:145–150
Green T, Dockray GJ (1987) Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett 76:151–156
Green T, Dockray GJ (1988) Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience 25:181–193
Grider JR (1994) CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol 266:G1139–G1145
Grider JR, Kuemmerle JF, Jin JG (1996) 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol 270:G778–G782
Ichikawa S, Shiozawa M, Iwanaga T, Uchino S (1991) Immunohistochemical demonstration of peptidergic nerve fibers associated with the central lacteal lymphatics in the duodenal villi of dogs. Arch Histol Cytol 54:241–248
Ichikawa S, Sreedharan SP, Goetzl EJ, Owen RL (1994) Immunohistochemical localization of peptidergic nerve fibers and neuropeptide receptors in Peyer's patches of the cat ileum. Regul Pept 54:385–395
Kawasaki H (2002) Regulation of vascular function by perivascular calcitonin gene-related peptide-containing nerves. Jpn J Pharmacol 88:39–43
Keast JR, Furness JB, Costa M (1985) Distribution of certain peptide-containing nerve fibres and endocrine cells in the gastrointestinal mucosa in five mammalian species. J Comp Neurol 236:403–422
Kirchgessner AL, Tamir H, Gershon MD (1992) Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12:235–248
Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD (2004) Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci 24:1330–1339
Messenger JP, Furness JB (1990) Projections of chemically-specified neurons in the guinea-pig colon. Arch Histol Cytol 53:467–495
Neild TO, Shen KZ, Surprenant A (1990) Vasodilation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol (Lond) 420:247–265
Palmer JM, Schemann M, Tamura K, Wood JD (1986) Calcitonin gene-related peptide excites myenteric neurons. Eur J Pharmacol 132:163–170
Pan H, Gershon MD (2000) Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci 20:3295–3309
Pfannkuche H, Schemann M, Gäbel G (2002) Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res 309:347–354
Rasmussen TN, Gregersen H, Harling H, Holst JJ (1992) Calcitonin gene-related peptide: effect on contractile activity and luminal cross-sectional area in the isolated, perfused porcine ileum. Scand J Gastroenterol 27:787–792
Rasmussen TN, Schmidt P, Poulsen SS, Holst JJ (2001) Localisation and neural control of the release of calcitonin gene-related peptide (CGRP) from the isolated perfused porcine ileum. Regul Pept 98:137–143
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135
Scheuermann DW, Stach W, De Groodt-Lasseel MHA, Timmermans J-P (1987) Calcitonin gene-related peptide in morphologically well-defined type II neurons of the enteric nervous system in the porcine small intestine. Acta Anat 129:325–328
Scheuermann DW, Krammer H-J, Timmermans J-P, Stach W, Adriaensen D (1991) Fine structure of morphologically well-defined type II neurons in the enteric nervous system of the porcine small intestine revealed by immunoreactivity for calcitonin gene-related peptide. Acta Anat 142:236–241
Sternini C (1991) Tachykinin and calcitonin gene-related peptide immunoreactivities and mRNAs in the mammalian enteric nervous system and sensory ganglia. Adv Exp Med Biol 298:39–51
Sternini C (1992) Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann NY Acad Sci 657:170–186
Sternini C, Anderson K (1992) Calcitonin gene-related peptide-containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Motor Res 9:45–59
Sternini C, Reeve JR, Brecha NC (1987) Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology 93:852–862
Su HC, Bishop AE, Power RF, Hamada Y, Polak JM (1987) Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neuroscience 7:2674–2687
Taché Y (1992) Inhibition of gastric acid secretion and ulcers by calcitonin gene-related peptide. Ann NY Acad Sci 657:240–247
Timmermans J-P, Scheuermann DW, Barbiers M, Adriaensen D, Stach W, Van Hee R, De Groodt-Lasseel MHA (1992) Calcitonin gene-related peptide-like immunoreactivity in the human small intestine. Acta Anat 143:48–53
Vanner S (1994) Co-release of neuropeptides from capsaicin-sensitive afferents dilates submucosal arterioles in the guinea-pig ileum. Am J Physiol 267:G650–G655
Vanner S, Surprenant A (1996) Neuronal reflexes controlling intestinal microcirculation. Am J Physiol 271:G223–G230
Acknowledgements
We thank Valeria Albanese and Beatrice Travostini for their invaluable assistance and advice in the preparation of tissue for immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Ricerca Fondamentale Orientata (RFO) and Fondazione Del Monte di Bo e Ra.
Rights and permissions
About this article
Cite this article
Chiocchetti, R., Grandis, A., Bombardi, C. et al. Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum: a morphometric and neurochemical investigation. Cell Tissue Res 323, 183–196 (2006). https://doi.org/10.1007/s00441-005-0075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0075-2