Abstract
Although aging is a conserved phenomenon across evolutionary distant species, aspects of the aging process have been found to differ between males and females of the same species. Indeed, observations across mammalian studies have revealed the existence of longevity and health disparities between sexes, including in humans (i.e. with a female or male advantage). However, the underlying mechanisms for these sex differences in health and lifespan remain poorly understood, and it is unclear which aspects of this dimorphism stem from hormonal differences (i.e. predominance of estrogens vs. androgens) or from karyotypic differences (i.e. XX vs. XY sex chromosome complement). In this review, we discuss the state of the knowledge in terms of sex dimorphism in various aspects of aging and in human age-related diseases. Where the interplay between sex differences and age-related differences has not been explored fully, we present the state of the field to highlight important future research directions. We also discuss various dietary, drug or genetic interventions that were shown to improve longevity in a sex-dimorphic fashion. Finally, emerging tools and models that can be leveraged to decipher the mechanisms underlying sex differences in aging are also briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
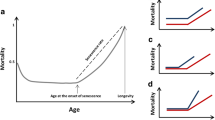
A large number of metazoans species have evolved with sexual reproduction. In most cases, these species have two sexes, which often differ in many biological aspects. At the most fundamental biological level, both genetic and hormonal mechanisms can underlie phenotypic sex differences (Regitz-Zagrosek et al. 2015). To note, the term “gender” is primarily used to refer to the social aspects of male to females differences, whereas the term “sex” refers to the genetic/biological determination level (Haig 2004). Thus, biological sex, which we will focus on in this review, is primarily determined by sex chromosome karyotype (i.e. XY vs. XX in mammals), and secondarily by gonadal identity (i.e. testes vs. ovaries, leading to a predominance of estrogens or androgens) (Schurz et al. 2019). Although aging is thought to be very stereotypical, accumulating evidence has revealed strong sex dimorphism in aging and longevity phenotypes. For instance, female life expectancy always exceeds that of males in an analysis of 54 countries (Rochelle et al. 2015). However, remarkably little is known about the biological pathways which underlie these robust differences.
Phenotypic sex differences can result from the fundamental differences in sex chromosome complement between females and males (Schurz et al. 2019). The mammalian X and Y chromosomes started their evolution from ordinary autosomes ~ 180 million years ago (Schurz et al. 2019). Because mammalian females usually carry two X chromosomes, dosage compensation mechanisms evolved, leading to one of Xs to become repressed early in development through a mechanism called random X-chromosome inactivation (XCI) (Brown et al. 1992; Cordaux and Batzer 2009). Though rare, sex chromosome aneuploidies exist and can lead to various human diseases, including Turner syndrome (i.e. X monosomy) or Klinefelter syndrome (i.e. XXY) (Skuse et al. 2018). The mechanisms by which these aneuploidies lead to diseases are thought to stem both from dosage imbalance arising from the small number of genes that can escape XCI, and from the downstream endocrine impact of sex chromosomes (Skuse et al. 2018). Conversely, the fact that X aneuploidies lead to disease supports the notion that both X chromosomes are required for mammalian female health and may contribute to sex-dimorphic phenotypes.
The other driver of phenotypic sex differences can be sex-steroid signaling (Schurz et al. 2019). The most common estrogen species is 17-β-estradiol (commonly referred to as E2), and it can bind to classical estrogen receptors (ERs) ERα and ERβ. E2-bound ERs translocate to the nucleus, where these receptors are recruited to estrogen response elements (EREs), or indirectly bind to DNA via interaction with other transcription factors to regulate gene transcription (Menazza and Murphy 2016). Alternatively, E2 can bind to the membrane-bound forms of ERα and ERβ, or to the more recently discovered G protein-coupled estrogen receptor 1 (GPER1), activating intracellular signaling cascades leading to altered gene expression (Medzikovic et al. 2019; Menazza and Murphy 2016). Accumulating evidence supports the complexity of E2/ER signaling, for instance because ERα and ERβ control gene expression in different ways (Menazza and Murphy 2016). Importantly, relative expression levels for ERα and ERβ differ among cell types, sex, and disease status (Medzikovic et al. 2019). In males, testosterone is synthesized at high levels by Leydig cells in the testes (Hammes and Levin 2019) and performs most of its cellular effects through binding to the androgen receptor (AR). Similar to estrogen, androgens can signal through both nuclear and extranuclear compartments of different cell types and tissues (Hammes and Levin 2019). Estrogens are thought to be protective against a wide variety of diseases, whereas testosterone seems to enhance the risk of disease progression (Clocchiatti et al. 2016; Ostan et al. 2016). Consistently, the risks of hypertension and developing Alzheimer’s disease (AD), two major causes of death in females, are remarkably inversely correlated with estrogen production (Ostan et al. 2016; Pike 2017). Despite the overwhelming evidence of sex differences in health and aging, underlying mechanisms for this phenomenon remain a major knowledge gaps of modern aging research (Austad and Fischer 2016; Clocchiatti et al. 2016; Ostan et al. 2016).
Here, we discuss the current knowledge of sex disparities in health, longevity, and longevity interventions, which will provide an important basis to frame new studies. Where the interplay between sex- and age-related differences has not yet been explored, we also present the current knowledge of each effect separately to delineate important gaps in knowledge in the field. Finally, we also discuss emerging tools which are available to effectively address these questions experimentally in the laboratory.
Sex-dimorphic outcomes in aging and longevity
Although numerous longevity and health-promoting interventions (e.g., genetic, dietary, drug) have been identified, much of the preclinical research supporting these interventions have not systematically and thoroughly explored sex-dimorphic effects (Miller et al. 2017). Indeed, most existing studies in the field have overwhelmingly favored the use of male samples. To address this fundamental gap, NIH guidelines now mandate the inclusion of sex as a biological variable in experimental design. However, except in rare cases, sex is still often treated by the field as a confounding factor rather a variable of interest in its own rights.
Sex dimorphism in mammalian health and longevity
The cohort of human supercentenarians (i.e. individuals aged over 110) reveals a surprising predictor for achieving such exceptional longevity: being female. Indeed, out of recorded 34 currently living supercentenarians, 33 are women (Adams 2019). Moreover, despite overall life expectancy increases for both women and men over the last few decades, human longevity has remained highly sex dimorphic, with the life expectancy of women systematically and robustly exceeding that of men (Austad and Fischer 2016). The increased life expectancy of women is likely to stem from the fact that they are less likely to succumb to most of the significant age-related causes of death: women die at a lower age-adjusted rate for 13 out of the 15 leading causes of death in the USA (Xu et al. 2016). In addition to humans, sex differences in lifespan have been observed across animal taxa, with mammalian females being generally longer lived than males (Austad and Fischer 2016; Bronikowski et al. 2011; Clutton-Brock and Isvaran 2007; Finch 1990).
In most reported cases, female laboratory rats live longer than their male counterparts (Berg and Simms 1960; Carlson and Hoelzel 1946; McCay et al. 1935; Nolen 1972). The existence of a similar female advantage for lifespan in laboratory mice is still hotly debated, especially for inbred strains (Austad 2011; Austad and Fischer 2016). However, in standardized husbandry conditions developed for the NIA Interventions Testing Program (ITP), female individuals consistently outlive males at three independent sites (Harrison et al. 2014; Miller et al. 2011; Strong et al. 2008), suggesting that the human female advantage may be recapitulated in laboratory mice, at least in controlled conditions. Intriguingly, a recent report using an elegant genetic model showed that both the presence of two chromosome X and, to a lesser extent, the presence of ovaries led to increased survival in mice (see below) (Davis et al. 2018).
Hormonal inputs in mammalian health and longevity
A key difference in male and female milieu is the presence and endocrine fluctuations of sex-steroid hormones (e.g., 17-β-estradiol). Although these factors decline with age in both sexes, the rate of decline differs greatly between the sexes (Gubbels Bupp 2015). In addition, regular physiological fluctuations in sex-steroid hormone levels are seen throughout the menstrual cycle in women of child-bearing age, with increased estrogen production during the follicular phase, and increased progesterone production in the luteal phase. Similar cyclic fluctuations of sex-steroid hormones are observed throughout mammals and are known as the ‘estrus cycle’ in rodents (Hong and Choi 2018). Hormonal differences between sexes and natural fluctuations of hormonal levels are likely to broadly impact gene regulation, even in somatic cells, with both short and long-term effects. Indeed, as previously mentioned, estrogens and androgens can act through both (1) nuclear receptors (i.e. ER-α, ER-β, AR), which function like transcription factors, and (2) membrane-associated receptors (i.e. mER-α, mER-β, GPER) (Buskiewicz et al. 2016). The complex cross-talk between signaling from different receptors to the same hormone is still poorly understood (Buskiewicz et al. 2016) and may underlie the existence of context-dependent beneficial vs. pathogenic effects of the same hormone.
In addition to their role in sex determination and fertility, accumulating evidence suggests that sex steroids differentially contribute to health and lifespan in females vs. males (Austad and Fischer 2016; Dulken and Brunet 2015). Indeed, supporting a key role for sex-steroid hormones in female aging, later age-at-menopause is a strong predictor of increased woman longevity (Hong et al. 2007; Ossewaarde et al. 2005; Shadyab et al. 2017), and post-menopausal women are more at risk for many age-related afflictions (e.g., osteoporosis, immune decline, neurodegeneration) (Gubbels Bupp 2015). Consistently, many health parameters differ between male and female mice with aging (Fischer et al. 2016). In addition, several reports indicate that key adult stem cell populations (i.e. hematopoietic, neural and muscle stem cells) display higher self-renewal, and regenerative capacity in female vs. males (Deasy et al. 2007; Nakada et al. 2014; Pawluski et al. 2009). Moreover, females generally exhibit increased wound healing ability (Deasy et al. 2007; Gilliver et al. 2008; Yao et al. 2016) and liver regeneration (Tsukamoto and Kojo 1990).
Sex dimorphism in longevity phenotypes upon dietary or drug-based interventions
Dietary restriction (DR), the limitation of total caloric intake or specific nutrients (i.e. amino-acids) without malnutrition, has been generally shown to improve health and longevity outcomes across species (Fontana and Partridge 2015). However, when treating sex as a biological variable, clear differences emerge in the efficacy of DR as a health and longevity-extending intervention (Table 1). For instance, Honjoh and colleagues demonstrated sex-dimorphic responses to DR between hermaphrodites and males in the nematode C. elegans, with hermaphrodites displaying greater lifespan extension than males (Honjoh et al. 2017). The mechanism behind this DR response variation was proposed to involve the sex determination pathway and the worm steroid hormone receptor DAF-12 (Honjoh et al. 2017). Similarly, in Drosophila melanogaster, two studies reported a greater extension of lifespan in females vs. males upon DR, which could result from sex differences in insulin/insulin-like pathway signaling, nutrient-sensing pathways, and intestinal stem cell activity (Magwere et al. 2004; Regan et al. 2016). Although sex dimorphism has been observed in the mammalian response to DR, the better-responding sex is not always the same across studies of laboratory mice (selected examples in Table 1). This may be partly due to slight genetic background differences between studies (Liao et al. 2010), which could interact with genetic sex or reflect more complex interactions. Alternatively, these discrepancies in sex-biases responses to DR may result from cryptic differences (e.g., exact nature of the diet, hormonal status of mice, stress levels). Thus, systematic and well-controlled studies will be needed to establish whether the direction of sex-dimorphic effects of DR is a broadly conserved phenomenon.
In addition to DR, drug-based pro-longevity interventions have also been reported to display sex-dimorphic responses (see Table 2). Rapamycin is one of the best documented examples of such a response (Fischer et al. 2015; Miller et al. 2014). Rapamycin works as a DR mimetic by inhibiting mTOR, a kinase that regulates cell growth through cellular nutrient sensing (Wilkinson et al. 2012). Studies of rapamycin supplementation have reported sex-dimorphic differences in both longevity and health parameters (Table 2). To note, these effects have been suggested to partially stem from sex-dependent rapamycin bioavailability (Fischer et al. 2015; Miller et al. 2014). Another DR mimetic, metformin, is a common anti-hyperglycemic drug that primarily functions by uncoupling the electron transport chain, thereby mimicking a low-energy state (El-Mir et al. 2000). Sex-dimorphic differences in the response to neonatal exposure to metformin have been reported, with a greater lifespan extension observed in males compared to females (Anisimov et al. 2015). Acarbose, a glucosidase inhibitor, was also proposed to act as a DR mimetic to promote health and longevity (Harrison et al. 2014, 2019). Studies have shown that with age, postprandial glycemia becomes less tightly regulated (Frantz et al. 2005; Miyamura et al. 2010), and acarbose is thought to prevent this age-related defect by slowing carbohydrate digestion, thereby reducing postprandial glucose spikes (Harrison et al. 2014). As part of the NIA ITP, acarbose has been well studied in male and female genetically heterogeneous UM-HET3 mice (Garratt et al. 2017; Harrison et al. 2014, 2019). In the context of this strain, male mice displayed substantially greater longevity and health compared to females (Garratt et al. 2017; Harrison et al. 2019). These effects included improved metabolism (i.e. glucose tolerance, mTOR signaling) and reduced microglial activation (Garratt et al. 2017; Sadagurski et al. 2017).
Supplementation with 17-α estradiol was another ITP success with sex-dimorphic impact on aging (Garratt et al. 2017; Harrison et al. 2014). This molecule was selected as a non-feminizing form of estrogen, which was thought to potentially engage estrogen-associated health insurance mechanisms without altering innate sexual characteristics (Garratt et al. 2017; Harrison et al. 2014). Intriguingly, despite not influencing sex characteristics, the longevity- and health-promoting effects of 17-α estradiol are sex dimorphic (Garratt et al. 2017; Harrison et al. 2014). Indeed, 17-α estradiol has been shown to lead to elevated mTORC2 activity in males, but not in females (Garratt et al. 2017). Similar to acarbose, 17-α estradiol also preferentially improved glucose clearance in males compared to females (Garratt et al. 2017).
Genetic longevity models with sex-dimorphic health of longevity phenotypes
More surprisingly than in response to drug supplementation, genetic manipulations have also been shown to exert sex-dimorphic impact on lifespan, leading to either greater lifespan extension in females vs. males (e.g., Igf1r haploinsufficiency), or lifespan extension exclusively in males (e.g., Sirt6 overexpression) (Bokov et al. 2011; Enns et al. 2009; Holzenberger et al. 2003; Kanfi et al. 2012; Selman et al. 2008, 2009; Xu et al. 2014; Yao et al. 2016).
The insulin signaling pathway has been extensively studied in the context of aging and longevity, and mouse knock-out (KO) models have been generated for many genes in the pathway. Interestingly, most of the insulin pathway KO mice display some measure of sex dimorphism. For example, Igf1r haploinsufficiency is thought to work by decreasing the biological activity of insulin growth factor-1 (IGF-1), which promotes anabolism and growth. Intriguingly, the scale of lifespan extension in this model differs significantly between males and females (Holzenberger et al. 2003). Indeed, Igf1r+/− mice from the 129Sv strain were found to display a larger increased in lifespan in females compared to males (Holzenberger et al. 2003). Although the same sex dimorphism in lifespan extension was also observed on the C57BL/6J background (Bokov et al. 2011; Xu et al. 2014), the extent of the sex dimorphism on the 129Sv background is substantially higher on the C57BL/6J background (Xu et al. 2014). This discrepancy was suggested to stem from strain-specific differences in circulating IGF-1 (Xu et al. 2014) and shows that sex-dimorphic phenotypes may be modified by autosomal genetic variation. Conversely, insulin receptor heterozygous knock-out models have been shown to lead to increased lifespan of male mice only (Nelson et al. 2012). Downstream of the receptors, invalidation of the insulin receptor substrate 1 null in mice (Irs1−/−) was found to lead to increased lifespan of female mice only (Selman et al. 2008).
Sirtuins are highly conserved NAD-dependent deacetylases that have been shown to regulate lifespan across taxa. In mammals, overexpression of Sirt6 in mice was found to significantly increase lifespan in males only (Kanfi et al. 2012). Analysis of the Sirt6 overexpression model revealed that the major components of insulin signaling were affected (Kanfi et al. 2012). Other genetic models that may mimic effective pro-longevity drug targets or dietary interventions are KO models of genes encoding protein kinase A RIIβ, and a subunit of the ribosomal S6 kinase (S6K) (Enns et al. 2009; Lamming et al. 2012; Selman et al. 2009). Protein kinase A (PKA) has been shown to be important in yeast longevity (Longo 2003). Intriguingly, mice without the PKA regulatory isoform RIIβ displayed increased lifespan in males but not in females (Enns et al. 2009), and were protected from age-related fatty liver, insulin resistance, and cardiac dysfunction (Enns et al. 2009). Independent studies have shown that null and heterozygous S6K subunit gene KO lead to increased lifespan in female mice but not males (Lamming et al. 2012; Selman et al. 2009). The S6 Kinase is part of the mTOR signaling cascade, and the female advantage on these genetic models is reminiscent of that observed upon rapamycin supplementation (Fischer et al. 2015; Miller et al. 2014).
Although most of the work in Ames dwarf mice has utilized exclusively male mice, a small study revealed that female Ames dwarf mice may live significantly longer than their male counterparts (Brown-Borg et al. 1996). Interestingly, long-lived mice lacking growth hormone (GH) and growth hormone receptor KO (GHRKO) may have a larger effect on females than males (Gesing et al. 2013). Intriguingly, only females GHRKO benefit from DR-induced lifespan extension (Bonkowski et al. 2006), whereas rapamycin treatment of GH mutant mice leads to a larger decrease in lifespan of male vs. females (12.5% vs. 6%) (Fang et al. 2018). Because of their role in GH signaling, a recent study of mice carrying both the Ames dwarf and GHRKO alleles suggested that females may also display a longevity advantage compared to males in this context (Gesing et al. 2017).
Thus, although differences in the bioavailability or metabolism of longevity-promoting compounds may be responsible for sex-dimorphic longevity effects of drug treatments, the existence of sex-dimorphic longevity effects upon genetic manipulation suggests the existence of complex mechanisms regulating sex-dimorphic phenotypes throughout life.
Widespread sex differences are observed in age-related chronic diseases
Sex-steroid hormones, both androgens, and estrogens have been shown to exert a significant impact on energy metabolism, body composition, vascular function, inflammatory responses, and neurogenesis (Gambineri and Pelusi 2019). While aging is associated with progressive metabolic decline and increased prevalence of chronic diseases in both sexes, the presentation and prevalence of these diseases are highly influenced by sex. Indeed, sex difference/dimorphism is increasingly evident in age-related metabolic disease [obesity, type 2 diabetes mellitus (T2DM), and cardiovascular diseases (CVD)]. Understanding how sex differences in age-related diseases are established will be crucial to leverage these differences for the improvement of health in both sexes.
Sex dimorphism in age-related metabolic dysfunction
Obesity and adiposity
Obesity is a growing public health concern and a key risk factor for many age-related diseases, such as T2DM and CVD. In recent years, the prevalence of elderly obesity has steadily increased (Jura and Kozak 2016). In addition to lifestyle changes with retirement, a shift in sex hormone levels in the elderly may also lead to a chronic positive energy balance state (Masternak et al. 2012), leading to excess fat tissue accumulation. In turn, this increase in adiposity then potentiates the development of age-related diseases (Tchkonia and Morbeck 2010). In addition to increased adiposity, aging is also associated with fat redistribution from subcutaneous to abdominal areas (Kok et al. 2009). While aging is associated with increased adiposity across sexes, men and women exhibit different patterns of adipose storage throughout life. Women typically present with ~ 10% higher total body fat than men throughout life and are generally more prone to obesity. Women tend to store more adipose tissue in the hips and thighs, while men have a more central fat distribution pattern. The pear-shaped female fat distribution may confer protection against metabolic diseases, such as T2DM and atherosclerosis (Manolopoulos et al. 2010) (see below). In women, visceral adiposity rises during the peri-menopausal transition, and adipose tissue redistributes toward abdominal area after menopause (Toth et al. 2000; Kozakowski et al. 2017). Similarly, visceral adiposity increases in men with age as testosterone levels decline (Allan et al. 2008).
Type 2 diabetes mellitus (T2DM)
In contrast to type 1 diabetes, an early onset insulin-dependent autoimmune disorder, T2DM is non-insulin-dependent and becomes more common with increasing age (Arum et al. 2014). Independent of sex differences in obesity and adiposity, the risks of T2DM in men and women are directly affected by sex hormones. Indeed, testosterone can influence T2DM pathogenesis. Based on cross-sectional studies, lower levels of testosterone in men and higher levels of testosterone in women are associated with increased T2DM risks (Gambineri and Pelusi 2019). Polycystic ovary syndrome (PCOS) is the most common hyperandrogenic disorder in women (Conway et al. 2014). Intriguingly, the odds of T2DM are four times as high for women with PCOS compared to healthy women, even when matched for body mass index (BMI) (Gambineri and Pelusi 2019; Moran et al. 2010, Morgan et al. 2012). Thus, imbalance in sex hormones (e.g., PCOS) can directly impact the risk of developing T2DM. Conversely, low baseline testosterone levels in men are associated with a dramatically higher risk of developing T2DM in men (Dhindsa et al. 2010). Thus, in addition to sex differences in body composition and fat deposition, sex hormones directly contribute to sex-dimorphic risk for diabetes.
Metabolic syndrome
Although metabolic syndrome affects men more often than women overall, women only seem to be protected before menopause (Regitz-Zagrosek et al. 2007). Indeed, the female risk of metabolic syndrome becomes roughly equivalent to that of male counterparts after the onset of menopause. Consistent with human data, female mice showed fewer markers for Western diet-induced obesity despite having the same amount of energy intake than matched males (Kaliannan et al. 2018). Males appeared more obese than their female counterparts and had higher fat distribution, more evidence of glucose intolerance, non-alcoholic fatty liver disease, and dyslipidemia (Kaliannan et al. 2018). Thus, sex may also act as a modifier for the impact of metabolic imbalance.
Sex dimorphism in cardiovascular disease
Traditionally viewed as a man’s disease, cardiovascular disease (CVD) is substantially more common in men than women, even with age-standardized statistics. The major areas of age-related CVD include ischemic heart disease, heart failure, and hypertension.
Ischemic heart disease, also known as coronary artery disease, occurs when plaque builds up inside blood vessels, leading to an inadequate blood supply to the heart (Regitz-Zagrosek and Kararigas 2017). Men and women are prone to develop different types: occlusive coronary artery disease is more frequent in male patients, while non-obstructive coronary artery disease or microvascular dysfunction is more common in women (Regitz-Zagrosek et al. 2015). In addition, men develop coronary artery disease earlier and usually present with more severe atherosclerosis in their coronary arteries than women. Indeed, myocardial infarction, which is a manifestation of coronary artery disease, occurs ~ 10 years earlier in men than women (Regitz-Zagrosek and Kararigas 2017). The reason for the relative protection of women may be due to the beneficial lipid profile and the role of estrogen signaling (Jiang and Tian 2017; Sudhir et al. 1997). Women with PCOS and men with a disruptive estrogen receptor mutation have been shown to develop early coronary artery disease (Legro 2003; Sudhir et al. 1997). Heart failure is a typical clinical syndrome arising from different pathophysiological conditions, affecting more than 10% of people aged > 70 years in western societies (Regitz-Zagrosek and Kararigas 2017). Though heart failure affects more women than men in terms of raw case numbers (Ambrosy et al. 2014), women have better odds of survival than men, and heart failure in women usually occurs at a later age (Regitz-Zagrosek and Kararigas 2017). In contrast to ischemic heart disease and heart failure, hypertension tends to affect more women in the elderly population (Vigen et al. 2012). Indeed, the percentage of women with hypertension is about twice that of men, and pre-menopausal women have lower rates of hypertension and lower lipid levels than age-matched men (Regitz-Zagrosek et al. 2015). Possible reasons for the shift in hypertension risk after the onset of menopause may be due to increased production of testosterone by the post-menopausal ovary (Maki and Henderson 2012). Consistently, women with PCOS have higher hypertension risk, indicating that higher testosterone levels are a risk factor for hypertension in women (Dworatzek et al. 2016).
Taken together, CVD shows a clear sex-dimorphic presentation during aging. In addition to the role of hormone-specific mechanism in men and women, sex-dimorphic CVD may also be explained by other mechanisms, such as sex-specific ion handling and rhythmicity in cardiovascular cells—women, in general, have faster resting heart rates and longer rate-corrected Q, T intervals, leading to higher susceptibility to drug-induced QT prolongation (Regitz-Zagrosek et al. 2015). Understanding these sex differences and their underlying mechanisms will be crucial to tailor therapeutic strategies that target sex-specific cardiovascular disease mechanisms.
Sex dimorphism in age-related eye disorders
Glaucoma
Glaucoma is a common age-related eye condition in which the optic nerve is damaged and can potentially lead to irreversible blindness. The maintenance of intraocular pressure is crucial to maintain clear vision (Pattabiraman and Toris 2016). Indeed, glaucoma is the leading cause of blindness in people aged > 60 years old, and is more common in women than men, suggesting sex dimorphism in glaucoma pathogenesis (Quigley and Broman 2006). Although the exact cause of this disparity remains poorly understood, anatomical differences, hormonal differences, lifestyle, and family history are thought to contribute to a significant amount (Brandt et al. 2001; Gordon et al. 2002; Høvding and Aasved 1986; Sommer and Tielsch 1996; Wilson et al. 1987). Several forms of glaucoma exist, the most common subtypes being angle-closure glaucoma (ACG) and open-angle glaucoma (OAG) (Lee et al. 2003; Pattabiraman and Toris 2016; Vajaranant and Pasquale 2012). The higher prevalence of glaucoma in women has been suggested to stem from the use of oral contraceptives (Lee et al. 2003; Pasquale and Kang 2011; Vajaranant and Pasquale 2012). Indeed, the use of oral contraceptives for more than 5 years is associated with a 25% increased risk of OAG (Pasquale and Kang 2011; Wang et al. 2016). Glaucoma incidence is also influenced by menopausal status (Hulsman et al. 2001; Newman-Casey et al. 2014), and post-menopausal hormonal replacement therapy (HRT) in women aged > 50 was associated with reduced prevalence of OAG (Newman-Casey et al. 2014). Conversely, pregnancy decreases intraocular pressure, which may reduce the chances of eventually developing glaucoma (Efe et al. 2012; Phillips and Gore 1985). In the case of OAG, sex is not considered a risk factor per se, although females often have more significant progression (Drange et al. 2001).
Age-related macular degeneration
The human eye has a macula lutea situated near the center of the retina. The macula region can be damaged as we age, which leads to a clinical condition called age-related macular degeneration (AMD) (Ambati et al. 2003; Elbay et al. 2019). AMD pathogenesis is multifactorial, with risk factors including heredity, obesity, hypertension, hypermetropia, ethnicity, and smoking. However, late menopause may be associated with a reduced risk of AMD (Snow et al. 2002). Additionally, increased risk in women is reported (Chakravarthy et al. 2010).
Cataract
Cataract is characterized by clouding or opacification of the lens in the eye, which dull the vision and is often age-related (Kahn et al. 1977). Studies have shown a higher prevalence of cataract in women vs. men of age group 65–75 years (~ 25% vs. ~ 15%) (Klein et al. 1992; Lundström et al. 1999, 2002; Mitchell et al. 1997). However, pre-menopausal women and age-matched men show the same risk of developing cataract (Klein et al. 1992; Mitchell et al. 1997). Higher rates of cataract surgery were reported in women (Lundström et al. 1999, 2002), which may result from declining estrogens after menopause. Interestingly, post-menopausal hormone replacement therapy may reduce the incidence of lens opacification (Freeman et al. 2001; Klein et al. 1992; Younan et al. 2002). The protective effects of estrogens may result from anti-oxidant properties since oxidative stress is a major pathway in the pathophysiology of cataract (Beebe et al. 2010). Moreover, in vitro treatment of lens epithelial cells with E2 was found to be protective against oxidative stress (Celojevic et al. 2011; Wang et al. 2003).
Sex dimorphism in age-related neurodegeneration
Neurodegenerative age-related diseases have a particularly staggering sex-dimorphic prevalence pattern. For instance, Alzheimer’s disease tends to occur more commonly in females, while Parkinson’s disease is biased toward presentation in males. All these major neurodegenerative diseases have associated genetic aspects and are incapable of being cured by treatment—current standard treatment practices can only hope to ameliorate symptoms. Thus, understanding sex differences may provide new research avenues for effective therapeutics.
Alzheimer’s disease
Alzheimer’s disease (AD), the most common form of dementia, is characterized by senile plaque and neurofibrillary tangle buildup in the brain and central and peripheral nervous system that leads to profound degeneration of large portions of the brain. This form of dementia accounts for more than 50% of dementia cases worldwide (Bekris et al. 2010). The degradation associated with AD appears to have a progression through the brain, beginning in medial temporal lobe structures before following temporal projections and causing degeneration in connected regions (e.g., subcortical structures, prefrontal structures, corpus callosum, and expansive cortex regions) (Smith 2002; Teipel et al. 2002). AD symptoms include hallmark progressive memory deficits, as well as language disturbance, visuospatial impairment, and higher executive function impairment (Schachter and Davis 2000). Additionally, many patients experience personality changes, judgment impairments, psychosis, mood disturbances, and sleep disturbances (Schachter and Davis 2000). Due to the extent of degeneration experienced by AD patients in specific regions, most patients develop a similar cluster of symptoms.
There is a distinctive pattern of AD presentation in males and females. AD is a disease that is most commonly present in females, with higher AD occurrence levels in all age groups > 60 except for 65–69. Males face a ~ 6% risk of developing AD in their lifetime after age 65, while women face a ~ 12% chance of developing AD in the same timeframe (Podcasy and Epperson 2016; Viña and Lloret 2010). Clinical presentation of AD also tends to be sex dimorphic, with symptoms and degeneration occurring more rapidly in women than in men (Laws et al. 2016; Lin et al. 2015), and men having a shorter lifespan after diagnosis (Kua et al. 2014). The skewed proportions of female to male AD patients may be linked to sex hormone effects. Indeed, estradiol has been illustrated to have neuroprotective effects (Maki and Henderson 2012), and women who experience menopause later in life tend to have a later age of AD onset (Lin et al. 2015). The significant decline in circulating estradiol after menopause can no longer fully exert neuroprotective effects in the brain, while age-matched males are still capable of aromatizing testosterone into estradiol and, therefore, still experience these neuroprotective effects (Podcasy and Epperson 2016). Along the same lines, although HRT in women has not been reported to have strong protective effects, long-term hormone self-administration is associated with reduced AD risk (Imtiaz et al. 2017).
Interestingly, the genotype of APOE, the most impactful genetic risk factor, is located on chromosome 19 and has been associated with increased risk of late-onset AD, particularly for the e4 genotype (Bekris et al. 2010; Corder et al. 1993). The APOE gene may not directly be involved in the pathology of AD, although > 40% of individuals with AD have the e4 genotype, and ~ 40% of e4 carriers have senile plaques (Farrer et al. 1995; Kok et al. 2009). Interestingly, the APOE e4 allele risk factor appears to interact with sex, with males of APOE e3/e4 and APOE e3/e3 genotypes experiencing AD risk level markedly less than the risk for APOE e4/e4 genotype, while females of APOE e3/e4 genotype are nearly twice as likely to develop AD than those of APOE e3/e3 genotype (Poirier et al. 1993; Tsai et al. 1994).
Parkinson’s disease
Parkinson’s disease (PD) is characterized by a loss of dopamine neurons in the substantia nigra pars compacta, a region of the basal ganglia (Deumens et al. 2002; Kalia and Lang 2015). The basal ganglia are a collection of neural structures responsible for the control of movement (Mink 1996). Two pathways work together to allow movement by enabling activity in a subgroup of neurons while preventing excessive movement (Calabresi et al. 2014). It is the substantia nigra pars compacta exerting a dopaminergic connection upon the striatum that both excite the direct (i.e. movement-initiating) pathway and inhibit the indirect (i.e. movement-inhibiting) pathway (Freeze et al. 2013). PD symptoms include tremor at rest, involuntary movement, and rigidity, which are classified as positive motor symptoms, and poverty of movement (i.e. bradykinesia) and posture disruption, which are classified as negative motor symptoms (Deumens et al. 2002). Cognitive impairments, including pain, fatigue, psychiatric disorders, olfactory dysfunction (Kalia and Lang 2015), and other general cognitive impairments are experienced by up to 50% of PD patients within 3 years of diagnosis (Geurtsen et al. 2014). Dementia is also frequently experienced by PD patients (Kalia and Lang 2015). Commonly, cognitive-motor impairments are seen in addition and include a severely impaired ability to make coarse and fine movement adjustments, as well as the transition between walking surfaces. The pathogenic mechanism for PD remains mostly unknown. While it is clear that PD involves dopamine neuron degeneration, there is no single underlying genetic indicator. Autosomal dominant forms of PD have been discovered [e.g., alpha-synuclein, parkin, leucine-rich repeat kinase 2 (LRRK2)] (Klein and Westenberger 2012; Wood-Kaczmar et al. 2006). Other genetic loci have been less conclusively associated with the disease (Klein and Westenberger 2012).
PD is a disease that is most commonly present in men, with reported proportions of disease occurrence that mostly range at 2:1 (Elbaz et al. 2002; Gillies et al. 2014). In addition, the clinical presentation of symptoms can have highly sex-dimorphic patterns, with females experiencing a later age-of-onset and milder PD phenotype than males (Alves et al. 2009; Haaxma et al. 2007; Miller and Cronin-Golomb 2010; Shulman and Bhat 2006). Women are more likely to experience tremor, dyskinesia, nervousness, and depression in their PD pathology (Haaxma et al. 2007; Martinez-Martin et al. 2012), while men are more likely to experience rigidity, rapid eye movement, and reduction in verbal fluency and facial expression recognition (Martinez-Martin et al. 2012). The sex-dimorphic pattern in both disease frequency and pathology cannot be entirely explained, but pre-existing sex-dimorphic patterns of structure, function, and hormone regulation are almost certainly involved (Gillies et al. 2014).
Sex dimorphism in the microbiome throughout life
Over the past decade, there has been increasing interest in the microbiome and its potential role in modulating overall human health and disease. From the ramifications of vaginal delivery vs. cesarean birth in the colonization of the newborn gastrointestinal tract and its effect on later health (Tanaka and Nakayama 2017), to cardiovascular disease and obesity, the human microbiome has now been shown to have a more significant role in maintaining health and homeostasis than previously imagined. Although many different microenvironments have been gaining interest, we will focus here on the gut microbiome, the influence of host sex on the microbiota, and the impact of the aging microbiome on overall health. To note, very little is known about sex-dimorphic features of the aging microbiome, so we will highlight the current knowledge in the fields of (1) sex differences and hormonal interactions to the microbiome in young animals, and (2) age-related effects on the microbiome. Future work integrating both these aspects will be a crucial step in the field.
The gut microbiome and age-related dysbiosis
The human gut microbiome is estimated to be composed of an estimated 1013 to 1014 micro-organisms (Bianconi et al. 2013; Savage 1977; Sender et al. 2016a, b). The microbial community consists of bacteria, viruses, and fungi. Bacteria represent the largest proportion of the microbial community with an estimated 500–1000 different bacterial species (Sender et al. 2016a). Relevant to aging, Elie Metchnikoff proposed in 1907 that age-related dysfunction was driven by chronic systemic inflammation, which occurred as a result of increased colon permeability (Metchnikoff 1907). The inflammation-driven hypothesis of aging is indeed now a leading hypothesis in the field, known as “inflamm-aging” (Franceschi and Campisi 2014). Although much remains to be studied, pioneering studies have started to map out the effect of aging on the gut microbiome (reviewed in (Nagpal et al. 2018)).
Interestingly, studies in D. melanogaster have revealed that age-related changes in the microbiome lead to increased intestinal permeability (Clark et al. 2015), which in turn promotes increased inflammation and mortality (Rera et al. 2012). The impact of the gut microbiome on systemic inflammation in mammals is supported by the fact that the composition of gut microbiome is correlated with inflammatory circulating cytokines in the human elderly (Claesson et al. 2012) and in aged mice (Conley et al. 2016; Thevaranjan et al. 2017). Interestingly, a recent study showed that intestinal barrier function fails with aging in mice, with increased levels of bacteria products in the blood of aged mice (Thevaranjan et al. 2017). Leveraging comparisons between specific pathogen-free and germ-free mice, the authors further demonstrated that intestinal barrier failure is driven by remodeling of gut microbial communities and alters macrophage function, reducing bacteria killing ability and increasing production of pro-inflammatory cytokines (Thevaranjan et al. 2017). Thus, age-related changes in the composition of the gut microbiome may represent a form of “microbial dysbiosis” (Thevaranjan et al. 2017). Although these studies did not explore how sex may interplay with age-related microbial dysbiosis, gut microbiota composition is tightly associated with diet in the elderly (Claesson et al. 2012), suggesting that the effects of pro-longevity interventions may partially act through microbial community remodeling.
Dietary effects on the gut microbiome
Interestingly, high fat diet (HFD) has been shown to lead to reduced diversity in the gut microbes (Xiao et al. 2017), which itself is a form of “gut dysbiosis” (Turnbaugh et al. 2006). This lack of diversity is thought to leave fewer community members available to process toxic metabolites, which leads to an inflammatory response. This inflammatory response weakens cell–cell junctions and, therefore, increases permeability of the gastrointestinal tract and allows for bacterial translocation (Schwabe and Jobin 2013). Bacterial lipopolysaccharide (LPS), one of the main components of Gram-negative bacterial cell walls (Moreira et al. 2012), has been identified as a triggering inflammatory factor which can cause the onset of insulin insensitivity, obesity and diabetes (Cani et al. 2007; Moreira et al. 2012). Indeed, an HFD increases the proportion of LPS-containing microbiota in the gut (Cani et al. 2007), thus promoting systemic inflammation and metabolic endotoxemia. Interestingly, HFD-fed male mice over a 4-week period experienced similar weight gain and insulin resistance as mice that received a steady subcutaneous infusion of LPS (Cani et al. 2008). Since each microenvironment is affected by the diet and geographical environment the host organism is in (Yatsunenko et al. 2012), it would stand to reason that the sex of the animal could also influence the composition of the microbiome as well.
Sex differences in the microbiota: what do we know?
Although this has not been systematically studied in humans, studies in mouse models show that the composition of gut microbiota is very similar between females and males before puberty (Markle et al. 2013; Steegenga et al. 2014). Indeed, the colonic microbial community of 2-week-old C57BL/6 pups consists predominantly of bacteroidetes and firmicutes, with small amounts of actinobacteria and proteobacteria in both sexes (Steegenga et al. 2014). However, puberty may lead to shifts in the microbiome leading to the establishment of sex-dimorphic microbial communities. Indeed, although there were no differences in the gut microbiota before sexual maturation in the non-obese diabetic (NOD) mouse model for type 1 diabetes (Markle et al. 2013), sequencing of the 16S ribosomal RNA repertoire from the gut microbiota of males and females at weaning (3 weeks), puberty (6 weeks), and adulthood (14 weeks) before diabetes onset revealed that differences in the gut microbiome emerged at puberty (6 weeks) and were strengthened in adults (Markle et al. 2013). Thus, it seems that sex hormones may help shape the composition and function of the gut microbiota, thus potentially influencing disease pathogenesis in a sex-specific fashion.
Bidirectional dialog between gut microbiota and sex hormones
In 1978, Lombardi and colleagues showed that the gut microbiome may impact the concentration of circulating estrogens and androgens (Lombardi et al. 1978). Indeed, the human microbiota can hydrolyze estrogen sulfate and glucuronide conjugates, and rat intestinal flora can hydrolyze androgens and glucuronides (Lombardi et al. 1978). The rate of these hydrolytic reactions was proportional to the concentration of feces in the growth medium (Lombardi et al. 1978). The authors showed that with high fecal load, a reduction of the carbonyl group to a hydroxy group at positions 3 and 17 of androgens, and position 17 of estrogens could take place (Lombardi et al. 1978). These redox reactions, mediated by the intestinal microbiota, were capable of producing a shift in local relative amounts of estrone, estradiol, testosterone, and androstenedione (Lombardi et al. 1978). Importantly, subsequent reabsorption of these steroids into the animal can result in effective changes in the circulating concentration of estrogens and androgens.
In addition to its role on the organism’s own cells, accumulating evidence shows that estrogens can directly impact gut microbiome composition and have a role in maintaining gut homeostasis. The terms “microgenderome” and “estrobolome” have been coined to describe this phenomenon, defined as “the gene repertoire of the microbiota of the gut capable of metabolizing estrogens” (Plottel Claudia and Blaser Martin 2011; Vemuri et al. 2019). In pre-menopausal females, estrogens are predominately secreted into the plasma by the ovaries and are immediately bound to sex hormone-binding globulin (SHBG), which is produced by the liver (Anderson 1974). More than 90% of systemic estrogen is bound to SHBG and thus biologically unavailable (Anderson 1974). Recent work has shown that the amount of biologically available estrogen is partially regulated by the gut microbiome in females (Vemuri et al. 2019). The gut microbiota in pre-menopausal women secretes beta-glucuronidase, which deconjugates SHBG from circulating estrogen, thereby making it biologically available and active (Baker et al. 2017; Kwa et al. 2016; Laurent et al. 2016). This microbially mediated increase in circulating estrogens may confer a protective effect on the host. However, when perturbations of the estrobolome arise, either via pathology (e.g., PCOS, metabolic syndrome) or aging (e.g., menopause), many of these benefits can be lost (Vemuri et al. 2019).
Beyond a mere correlation, hormonal inputs may directly shape the composition of microbial communities. Indeed, oral supplementation of 17-β estradiol suppressed the development of Western diet-induced obesity in both males and in ovariectomized (OVX) females, and that there were no significant differences in metabolic syndrome parameters between intact females and E2 supplemented males (Kaliannan et al. 2018). Importantly, the microbiota of each experimental group clustered as a function of hormonal profiles: the OVX female and untreated male microbiota clustered together apart from that of male E2-treated and intact females (Kaliannan et al. 2018). Once the influence of estrogen on the gut microbiome was lost, the protective benefit was lost. This loss of protection was also demonstrated in intact mice, given antibiotics to deplete the microbiome (Kaliannan et al. 2018). Their serum estrogen levels decreased, and their metabolic syndrome markers resembled those of their male counterparts.
Although clear impact of age and sex have been independently observed in the gut microbiome, the interplay between these inputs is till mostly unexplored and will deserve future study in the field. Thus, the microbiome may be an important component to integrate in studies of sex dimorphism in mammalian age-related phenotypes.
Sex-dimorphic gene regulation in youth and during aging
In multi-cellular organisms, the precise control of gene expression patterns is key not only for development, but also for cell/tissue homeostasis in adults, and deregulation of such patterns is associated with aging (Benayoun et al. 2015; Lai et al. 2019; Stegeman and Weake 2017). A complementary layer of the study of gene regulation mechanisms lies at the level of chromatin. Indeed, cellular chromatin states (i.e. the ‘epigenome’) can regulate transcriptional profiles and are governed in part by post-translational modifications of histones (Dong et al. 2012; Dunham et al. 2012; Hoffman et al. 2013; Jenuwein and Allis 2001; Parker et al. 2013; Whyte et al. 2013). Thus, in line with the prevalence of transcriptional alterations with aging, accumulating observations have noted that the general structure of chromatin and specific patterns of chromatin marks are altered with aging across cell types and species (Benayoun et al. 2015 #410; Ucar and Benayoun 2018 #1605; Pal and Tyler 2016 #813). In addition, perturbations in chromatin-modifying enzymes can impact the lifespan of invertebrates, and even age-related cognitive decline in mice (Benayoun et al. 2015 #410; Ucar and Benayoun 2018 #1605; Pal and Tyler 2016 #813), which supports the status of ‘epigenetic alterations’ as a ‘hallmark of aging’ (López-Otín et al. 2013). Although accumulating studies have started to map genomic remodeling with age, these studies have mainly focused on male individuals, or on pooled male/female samples, thus ignoring potentially sex-dimorphic responses. Indeed, in 70 transcriptomic studies of mouse longevity models (compiled from public repositories), we identified 51 studies including only male samples (~ 73%), 12 including only female samples (~ 17%), and 7 including both sexes (~ 10%). Thus, we will highlight the current knowledge of sex dimorphism in gene expression regulation, and discuss how these differences may drive aspects of sex differences in aging.
Transcriptional signatures of aging across sexes
Thousands of genes can be regulated in a sex-dimorphic manner across a range of youthful, healthy somatic tissues (e.g., brain, liver, heart, muscle) from various mammalian species (Berchtold et al. 2008; Isensee et al. 2008; Naqvi et al. 2019; Qureshi and Mehler 2010; Yang et al. 2006). Genes expressed in a sex-dimorphic manner are located on autosomes as well as on sex chromosomes (Berchtold et al. 2008; Isensee et al. 2008; Qureshi and Mehler 2010; Yang et al. 2006), and many of these genes are not directly targeted by sex hormones (Mayne et al. 2016). Functional enrichment analyses of sex-dimorphic gene regulation have identified differentially enriched pathways between male and female tissue transcriptomes, including immune response, oxidoreductase activity, and lipid metabolism (Yang et al. 2006). The existence of large transcriptional differences between male and female cells holds true in pure cell populations, with evidence that microglia purified from female vs. male brains of young mice show widespread gene expression and functional differences (Guneykaya et al. 2018; Villa et al. 2018). Although these studies only investigated such differences in young adults, since these functional processes are related to “hallmarks of aging” (López-Otín et al. 2013), sex-dimorphic regulation of gene expression could thus have an important impact on the aging process.
Studies in flies have revealed that the sex dimorphism in the expression of the mitochondrial Lon protease mediates sex- and age-specific adaptation to oxidative stress and the sex-dimorphic expression of this protease may be conserved in mice (Pomatto et al. 2017). In landmark studies, high sex dimorphism in the transcriptomic response to dietary restriction (Mitchell et al. 2016) and short-term fasting (Della Torre et al. 2018) were observed in mouse liver. Moreover, DR has been reported to feminize the gene expression profiles of male livers (Estep et al. 2009). Finally, genes expressed in a sex-dimorphic manner in the human brain were proposed to act as mediators of stress susceptibility and depressive symptoms (Labonte et al. 2017), consistent with the idea that sex-dimorphic gene expression can broadly impact human health and physiology. These observations raise the intriguing possibility that sex-dimorphic gene expression may play a key role in aging and response to longevity interventions. Thus, systematically understanding the transcriptional underpinnings of sex differences in aging and longevity would provide crucial molecular handles to develop therapeutic strategies to slow down age-related functional decline and diseases.
Sex dimorphism in aging: a role for chromatin regulation?
Complementary to pure transcriptional regulation, regulation of chromatin states (i.e. the ‘epigenome’), which are governed in part by histone post-translational modifications, can help tune transcriptional programs (Dong et al. 2012; Dunham et al. 2012; Hoffman et al. 2013; Jenuwein and Allis 2001; Parker et al. 2013; Whyte et al. 2013).
The impact of X chromosome inactivation in XX mammalian cells
The most obvious epigenetic impact of sexual differentiation is the inactivation via heterochromatinization of additional X chromosomes into a “Barr body” in cells carrying more than one X chromosome. Although X chromosome inactivation (XCI) has evolved to compensate for a differential dosage of X chromosome genes in XX cells, evidence shows that many X-linked genes can be expressed in a bi-allelic fashion in XX cells: on average ~ 15% of X-linked genes in human and ~ 3% in mice (Berletch et al. 2011). Indeed, a number of genes which are known to “escape” XCI are chromatin remodelers, including histone demethylases UTX, KDM5C, methyl-CpG-binding protein MeCP2, and chromatin remodeler ATRX (Chow and Heard 2009; Qureshi and Mehler 2010). The degree of expression of these escape genes varies by tissue, between 5 and 80% in others relative to the active X chromosome (Chow and Heard 2009). Numerous studies have looked at dysregulation of XCI in diseases, including cancers, Alzheimer’s disease, and physiological aging.
Sex dimorphism in chromatin modifications throughout life
In line with the prevalence of transcriptional alterations with aging, accumulating evidence suggests that the chromatin landscape may be altered with aging across cell types and species. In addition, perturbations in chromatin-modifying enzymes can impact the lifespan of invertebrates, or even age-related cognitive decline in mice (reviewed in details in Benayoun et al. 2015 #410; Ucar and Benayoun 2018 #1605; Pal and Tyler 2016 #813), which supports the status of ‘epigenetic alterations’ as a ‘hallmark of aging’ (López-Otín et al. 2013). Recent studies comparing male and female epigenomic profiles across tissues and cell types have revealed robust sex-dimorphic chromatin features, specifically for chromatin accessibility in human T cells (Qu et al. 2015) and histone modifications across human tissues (Yen and Kellis 2015). Though these differences could result from differential sex chromosome ploidy, a number of the observed differences were identified on autosomes, suggesting that chromatin can indeed be differentially regulated in male vs. female cells (Hadad et al. 2016; Qu et al. 2015; Yen and Kellis 2015). These observations may be explained in part by the presence of genes encoding chromatin regulators on sex chromosomes (e.g., methyl-CpG-binding protein MeCP2, H3K27 demethylases UTX and UTY, H3K4 demethylase KDM5C, etc.) (Qureshi and Mehler 2010).
Sex-specific differences in DNA methylation profiles throughout life
In one of the few studies to examine aging as well as sex differences in epigenetic regulation, Agba et al. (2017) revealed a complex, tissue and sex-specific changes with age at 2 loci, the Nr3c1 promoter and the Igf2/H19 imprinting control region. Interestingly, in several tissues (hippocampus, hypothalamus, skin and liver) the most considerable difference was seen at the intermediate age (9 months) as female rats increased methylation at the Igf2/H19 ICR with the methylation levels becoming most similar at the oldest age (24 months) (Agba et al. 2017). At multiple sites in different tissues, the direction of methylation change with age was opposite between sexes. Few studies have looked at genome-wide, sex-specific DNA methylation divergences with age. In one of these studies, while there no overall change was observed in the level of DNA methylation in male or female mouse hippocampi between 3 and 24 months of age, there was a large amount of change in the methylation of specific CpG and CpH sites (Masser et al. 2017). Importantly, these changes were predominantly sexually divergent (> 90%), with age-related change in methylation going in opposite directions in male and female tissues (Masser et al. 2017). Additionally, many sites were found to maintain sex specificity throughout age (either higher in males than females at both young and old age or vice versa). In contrast, another study of mice hippocampi found more subtle changes in DNA cytosine methylation and hydroxymethylation on autosomal chromosomes and large differences in X-chromosomal methylation and hydroxymethylation (Hadad et al. 2016). Finally, a recent study using the DNA methylation epigenetic clock found an association between age of menopause and “biological” aging, as measured by the DNA methylation profile (Levine et al. 2016).
The extent to which these epigenomic changes are simply mediating hormonal signaling or represent a distinct—if overlapping—mechanism of sex dimorphism remains to be fully elucidated. Further, much of the research done to-date on the epigenetics of sex dimorphism has, with reason, centered on the brain or germ cells, where differences were extremely likely to occur. Whether other tissues demonstrate equivalent divergences with sex and age will be an important future research avenue for the field.
Sex dimorphism in transposable element-driven genomic instability in youth and during aging
Transposable elements (TEs), sometimes called “jumping genes,” are a type of repetitive DNA with the ability to move within host genomes (McClintock 1953). Two main classes have been described: (1) class I transposons, or RNA-mediated transposons, act through a “copy and paste” mechanism, and (2) class II transposons, or DNA-mediated transposons, act through a “cut and paste” mechanism (Chénais et al. 2012). More specifically, RNA-mediated transposons have enzymes to reverse transcribe their transcripts and to integrate the resulting complementary DNA (cDNA) into host genomes. DNA-mediated transposons, similarly, have transposases that recognize, cleave, and re-integrate TE DNA (Chénais et al. 2012). In humans, the two most common TE families—long interspersed nuclear elements (LINEs) and short interspersed nuclear elements (SINEs)—compose about 33% of the genome (Lander et al. 2001). Mechanistically, TEs can disrupt protein-coding sequences or alter gene expression through insertion into regulatory sequences (Kidwell and Lisch 1997). Accumulating evidence supports the notion that TEs are progressively de-repressed with organismal aging across several organisms (Benayoun et al. 2019; De Cecco et al. 2013; Dennis et al. 2012; Li et al. 2013; Wood et al. 2016). The excessive mobilization of TEs is believed to contribute to age-related genomic instability (De Cecco et al. 2013; Li et al. 2013; Maxwell et al. 2011; Wood et al. 2016). Intriguingly, recent evidence suggests that LINE-1 reactivation may contribute to chronic age-related inflammation by promoting inflammatory cytokine secretion (De Cecco et al. 2019; Simon et al. 2019). Although it is now established that TEs may contribute to age-related phenotypes, whether sex may influence their activation and impact on cell health remains largely unknown. Here, we discuss the current knowledge related to the impact of circulating sex hormones and of sex chromosomes on TE activity levels.
A potential role for hormonal regulation in sex-specific TE activation?
As highlighted in previous sections, differences in hormone levels may directly contribute to differences between male and female individuals, partially mediated by sex-steroid signaling (Paterni et al. 2014). Evidence suggests that a tight interaction exists between estrogen receptors and TEs, which may implicate TEs in estrogen-influenced processes, including aging. Specifically, an analysis of chromatin immunoprecipitation (ChIP) data for ER-α in the MCF-7 breast cancer cell line found that a large proportion (~ 20%) of ERα-binding sites overlapped mammalian-wide interspersed repeat (MIR) sequences, a type of SINE element (Bourque et al. 2008). Similarly, Mason et al. (2010) conducted a broad characterization of estrogen response element (ERE) sequences in MCF-7 cells using ChIP-on-chip. They estimated that 19–36% of EREs reside in repetitive (repeat-masked) genomic elements. Though the most common elements belonged to the Alu family, EREs were also detected in non-Alu SINE elements, LINEs, long terminal repeat (LTR) retrotransposons, non-LTR retrotransposons, microsatellites, and DNA transposable elements. Mason et al. (2010) also observed binding of ERα-containing complexes to 2 MIR-b elements in vitro by electrophoretic mobility-shift assay (EMSA) and noted that mutagenesis of either MIR or Alu-EREs reduced readout in their luciferase assays, indicative of their role in modulating transcription of nearby genes. A third study analyzed MCF-7 ChIP-seq datasets corresponding to two modes of estrogen treatment: one where cells were exposed to chronic, low levels of estrogen, and one where cells were treated with an acute higher dose of estrogens (Testori et al. 2012). Consistent with prior studies, they identified an enrichment of ERα at DNA sequences corresponding to a variety of TEs (Testori et al. 2012). Noteworthy, some of these TE-ERα interactions were unique to each dosage scheme, and within TEs that interact with ERα, they identify enrichment of binding sequences corresponding to transcription factors and known cofactors of ERα (Testori et al. 2012). Finally, they note that MIR-like and endogenous retrovirus (ERV)-like elements that interact with ERα are frequently located close to the transcription start site of regulated genes (Testori et al. 2012).
Interactions between TEs and androgen signaling have also been found. A putative androgen response element (ARE) was identified within the promoter of a LINE-1 element family (L1Hs) (Morales et al. 2003). They further demonstrated that this promoter could alter the activity of their LacZ reporter gene in the presence of testosterone in the JEG-3 human choriocarcinoma cancer cell line (Morales et al. 2003), suggesting the potential for androgens to alter the expression of genes downstream of ARE-containing TE regulatory elements. Interestingly, LINE-1 ORF1-p, one of the proteins produced by LINE-1 elements, was found to act as an androgen receptor co-activator with the ability to promote the growth of human prostate carcinoma cells (Lu et al. 2013). Thus, sex-steroid hormones may be directly regulating TE activity in responsive cells.
To add to the complexity, researchers have also sought to characterize TE methylation levels with respect to age, sex, and hormone levels (El-Maarri et al. 2011; Lu et al. 2018). An analysis of peripheral blood mononuclear cells found marginally higher LINE-1 methylation in males compared to females (El-Maarri et al. 2011). However, they did not detect any significant effect on LINE-1 methylation across ages or throughout different stages of the menstrual cycle (El-Maarri et al. 2011). An inverse relationship between methylation levels and age of menopausal onset, however, has been noted (Lu et al. 2018). Thus, the ability of hormones to enhance or initiate gene expression combined with the apparent lack of correlation with TE methylation suggests that their TE-mediated effect may predominantly act at the transcriptional rather than epigenetic level.
Together, these results suggest that the interaction between TEs and sex-steroid hormone signaling can elicit sex-specific regulation of TE levels. On the one hand, TEs may provide the means for enhanced or possibly diminished, expression of neighboring genes, depending on circulating hormone levels and receptor activation. On the other hand, TEs such as LINE-1 and Alu, respectively, contain internal Pol II and Pol III promoters (Elder et al. 1981; Fuhrman et al. 1981; Lavie et al. 2004), and the presence of ERE/ARE motifs within these elements raises the possibility that their transcription may also be influenced by hormonal status. Differences in sex hormone levels are thus likely to drive transcriptional differences through both mechanisms. Moreover, these differences are very likely to be dynamic across time, and especially so within females, as (1) the hormonal cycle is characterized by repeated cyclic fluctuations of circulating estrogens and progesterone (Reed and Carr 2000), and (2) estrogen and testosterone levels tend to decrease throughout life in humans (Ferrini and Barrett-Connor 1998; Harman et al. 2001; Horstman et al. 2012), albeit more dramatically in females. Thus, whether sex-steroid hormone receptors differentially modulate TE transcription/integration or TE-influenced gene expression in males vs. females throughout life deserves further investigation, as it may reveal underlying mechanisms to sex dimorphism in organismal phenotypes.
The contribution sex chromosomes to sex-specific TE regulation
As highlighted in previous sections, sex chromosomes are responsible for determining the gender of an organism, which may impact differences observed between the sexes in organisms with XX/XY sex-determination system (Hodgkin 1992). In the process of understanding the evolution and contents of the Y chromosome in mammals, the field has unraveled many exciting features of this chromosome, including the existence of a large portion of euchromatin in the center, in contrast to its largely heterochromatic ends (Quintana-Murci and Fellous 2001). Indeed, the human Y chromosome, and the Y chromosome in general, is known to be highly repetitive (Hughes and Rozen 2012; Skaletsky et al. 2003; Smith et al. 1987). Research in the field of epigenetics has unraveled the loss of heterochromatin marks with age (Pal and Tyler 2016). Thus, the highly repetitive nature of the Y chromosome, coupled to a prevalent loss of heterochromatin with age, may lead to a sex-specific de-repression of Y-linked TEs (Marais et al. 2018). Indeed, in flies, the Y chromosome was reported to broadly influence the chromatin states of autosomes (Lemos et al. 2010). With age, the Y chromatin becomes de-repressed, and TEs tend to activate, which could further promote age-related phenotypes (Brown and Bachtrog 2017). Intriguingly, studies of longevity in XY, XO, XYY males and XX, XXY females showed that the presence of a Y chromosome accelerates aging in flies (Brown and Bachtrog 2017), which was proposed to be the result of its particularly high TE content (Brown and Bachtrog 2017; Marais et al. 2018).
Thus, although there is still limited information about the link between sex chromosome karyotype, TEs and aging, it is likely that this would be a productive future research avenue.
Current and emerging experimental models for the study of sex-dimorphic mechanisms
The most prevalent genetic model for understanding the relative role of sex chromosome complement (i.e. XX vs. XY) and gonadal sex (i.e. ovary vs. testes) in mammalian phenotypes is the “four core genotype” (FCG) model (Arnold 2009; Arnold and Chen 2009). Thanks to the combination of a null mutation of the Y chromosome testis-determining gene Sry, and the insertion of an Sry transgene in an unmapped autosomal location, sex chromosome complement (XX vs. XY) is effectively decoupled from the animal’s gonadal sex in this model (Arnold 2009; Arnold and Chen 2009). This model has been used to study various aspects of karyotype vs. hormonal effects (Arnold and Chen 2009). Importantly, FCG mice were recently used to demonstrate that the presence of two X chromosomes (and, to a lesser extent, of ovaries) can lead to increased longevity (Davis et al. 2018). Known caveats of the FCG model include potentially incomplete gonadal reprogramming in some genetic backgrounds, and premature gonadal exhaustion has been reported on the C57BL/6 background, the most common background in aging research (Burgoyne and Arnold 2016). To note, FCG leads to gonadal sex reversal during the initial determination of the bipotential gonad, thus decoupling karyotypic and gonadal sex from the beginning of gonadal development (Arnold 2009; Arnold and Chen 2009). This timeline means that the FCG cannot disentangle the organizational effects of gonadal hormones (i.e. impact on the development of distal somatic sites), from their activational effects (i.e. effects on mature tissues after the end of development, and throughout life) (Arnold and Breedlove 1985).
To address the relative importance of endocrine vs. genetic mechanisms in sex-dimorphic phenotypes, it may become useful to leverage a previously described mouse model of adult somatic sex reprogramming: the adult inducible knock-out (KO) of Foxl2 (Uhlenhaut et al. 2009). Remarkably, deletion of Foxl2 in adult mice leads to upregulation of testis-specific genes in the gonad, and within 3 weeks of Foxl2 loss, somatic ovary to testis transdifferentiation occurs (Uhlenhaut et al. 2009). Further, induced Foxl2 knock-out mice acquire circulating testosterone levels comparable to those of ‘normal’ XY male littermates (Uhlenhaut et al. 2009). This genetic model could provide a unique opportunity to study the impact of a female vs. male hormonal milieu in adult genetically female XX individuals, thus allowing to study the non-organizational effect of sex-steroids on aging. This model could thus open a window on the post-developmental effects of gonadal hormones regardless of karyotypic sex. To note, the adult Foxl2 KO model has never been evaluated with aging, and its use could open interesting new research avenues in the field.
Generally, leveraging the power of the FCG and the Foxl2 inducible KO mice should help dissect the adult contribution of gonadal hormones vs. sex chromosomes karyotype in age-related sex-dimorphic phenotypes.
Conclusion and final remarks
In this review, we discussed recent advances in the research of sex differences in aging and longevity. Although differences between women and men aging have been known for decades, the mechanism by which these disparities among the sexes remains poorly understood. Increasing evidence supports the impact of sex hormones on lifespan. However, the molecular dissections of genetic vs. hormonal contributions to these differences are only starting to be touched upon by researchers. To further understand these differences and the molecular pathways that underlie them, it will be crucial to leverage genetic and surgical models that can decouple specific aspects linked to sex differences, including genetic/hormonal sex swaps (e.g., Four Core Genotype, Foxl2 somatic sex reprogramming), hormonal signaling disruptions (e.g., inducible or tissue-specific knock-outs for estrogen receptor or androgen receptor genes), or surgical gonadectomy. Information from these various sources will finally help understand the molecular regulation of sex differences in health and lifespan. Moving forward, leveraging these tools to understand the bases of sex differences in responses to pro-longevity interventions and in the prevalence of age-related diseases will be crucial. In this light, tailoring interventions based on sex will be the first (small) step for aging research toward personalized medicine.
References
Adams JM (2019) GRG world supercentenarian rankings list. http://www.grg.org/SC/WorldSCRankingsList.html. Accessed 10 May 2019
Agba OB, Lausser L, Huse K, Bergmeier C, Jahn N, Groth M, Bens M, Sahm A, Gall M, Witte OW, Kestler HA, Schwab M, Platzer M (2017) Tissue-, sex-, and age-specific DNA methylation of rat glucocorticoid receptor gene promoter and insulin-like growth factor 2 imprinting control region. Physiol Genom 49:690–702. https://doi.org/10.1152/physiolgenomics.00009.2017
Allan CA, Strauss BJG, Burger HG, Forbes EA, McLachlan RI (2008) Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 93:139–146. https://doi.org/10.1210/jc.2007-1291
Alves G, Müller B, Herlofson K, HogenEsch I, Telstad W, Aarsland D, Tysnes OB, Larsen JP (2009) Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 80:851–857. https://doi.org/10.1136/jnnp.2008.168211
Ambati BK, Anand A, Joussen AM, Kuziel WA, Adamis AP, Ambati J (2003) Sustained inhibition of corneal neovascularization by genetic ablation of CCR5. Investig Ophthalmol Vis Sci 44:590–593. https://doi.org/10.1167/iovs.02-0685
Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63:1123–1133. https://doi.org/10.1016/j.jacc.2013.11.053
Anderson DC (1974) Sex-hormone-binding globulin. Clin Endocrinol 3:69–96. https://doi.org/10.1111/j.1365-2265.1974.tb03298.x
Anisimov V, Popovich IG, Zabezhinski MA, Egormin PA, Yurova MN, Semenchenko AV, Tyndyk ML, Panchenko AV, Trashkov AP, Vasiliev AG, Khaitsev NV (2015) Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle 14:46–55. https://doi.org/10.4161/15384101.2014.973308
Arnold AP (2009) Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol 21:377–386. https://doi.org/10.1111/j.1365-2826.2009.01831.x
Arnold AP, Breedlove SM (1985) Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19:469–498
Arnold AP, Chen X (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30:1–9. https://doi.org/10.1016/j.yfrne.2008.11.001
Arum O, Boparai RK, Saleh JK, Wang F, Dirks AL, Turner JG, Kopchick JJ, Liu JL, Khardori RK, Bartke A (2014) Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell 13:981–1000. https://doi.org/10.1111/acel.12262
Austad SN (2011) Sex differences in longevity and aging. In: Handbook of the biology of aging, pp 479–495. https://doi.org/10.1016/B978-0-12-378638-8.00023-3
Austad SN, Fischer KE (2016) Sex differences in lifespan. Cell Metab 23:1022–1033. https://doi.org/10.1016/j.cmet.2016.05.019
Baker JM, Al-Nakkash L, Herbst-Kralovetz MM (2017) Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 103:45–53. https://doi.org/10.1016/j.maturitas.2017.06.025
Beebe DC, Holekamp NM, Shui YB (2010) Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res 44:155–165. https://doi.org/10.1159/000316481
Bekris LM, Yu CE, Bird TD, Tsuang DW (2010) Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 23:213–227. https://doi.org/10.1177/0891988710383571
Benayoun BA, Pollina EA, Brunet A (2015) Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol 16:593–610. https://doi.org/10.1038/nrm4048
Benayoun BA, Pollina EA, Singh PP, Mahmoudi S, Harel I, Casey KM, Dulken BW, Kundaje A, Brunet A (2019) Remodeling of epigenome and transcriptome landscapes with aging in mice reveals widespread induction of inflammatory responses. Genome Res. https://doi.org/10.1101/gr.240093.118
Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW (2008) Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci USA 105:15605–15610. https://doi.org/10.1073/pnas.0806883105
Berg BN, Simms HS (1960) Nutrition and longevity in the rat. II. Longevity and onset of disease with different levels of food intake. J Nutr 71:255–263
Berletch JB, Yang F, Xu J, Carrel L, Disteche CM (2011) Genes that escape from X inactivation. Hum Genet 130:237–245. https://doi.org/10.1007/s00439-011-1011-z
Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S (2013) An estimation of the number of cells in the human body. Ann Hum Biol 40:463–471. https://doi.org/10.3109/03014460.2013.807878
Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, DeFronzo RA, Zhang N, Erickson RC, Gelfond J, Hubbard GB, Adamo ML, Richardson A (2011) Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One 6:e26891. https://doi.org/10.1371/journal.pone.0026891
Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A (2006) Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA 103:7901–7905. https://doi.org/10.1073/pnas.0600161103
Bourque G, Leong B, Vega VB, Chen X, Lee YL, Srinivasan KG, Chew J-L, Ruan Y, Wei C-L, Ng HH, Liu ET (2008) Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res 18:1752–1762. https://doi.org/10.1101/gr.080663.108
Brandt JD, Beiser JA, Kass MA, Gordon MO (2001) Central corneal thickness in the ocular hypertension treatment study (OHTS). Ophthalmology 108:1779–1788. https://doi.org/10.1016/S0161-6420(01)00760-6
Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC (2011) Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331:1325–1328. https://doi.org/10.1126/science.1201571
Brown EJ, Bachtrog D (2017) The Y chromosome contributes to sex-specific aging in Drosophila. bioRxiv. https://doi.org/10.1101/156042
Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF (1992) The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71:527–542
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A (1996) Dwarf mice and the ageing process. Nature 384:33. https://doi.org/10.1038/384033a0
Burgoyne PS, Arnold AP (2016) A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ 7:68. https://doi.org/10.1186/s13293-016-0115-5
Buskiewicz IA, Huber SA, Fairweather D (2016) Chapter 4—sex hormone receptor expression in the immune system. In: Neigh GN, Mitzelfelt MM (eds) Sex differences in physiology. Academic Press, Boston, pp 45–60
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022–1030. https://doi.org/10.1038/nn.3743
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. https://doi.org/10.2337/db06-1491
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. https://doi.org/10.2337/db07-1403
Carlson AJ, Hoelzel F (1946) Apparent prolongation of the life span of rats by intermittent fasting. J Nutr 31:363–375. https://doi.org/10.1093/jn/31.3.363
Celojevic D, Petersen A, Karlsson J-O, Behndig A, Zetterberg M (2011) Effects of 17β-estradiol on proliferation, cell viability and intracellular redox status in native human lens epithelial cells. Mol Vis 17:1987–1996
Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P (2010) Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. https://doi.org/10.1186/1471-2415-10-31
Chénais B, Caruso A, Hiard S, Casse N (2012) The impact of transposable elements on eukaryotic genomes: from genome size increase to genetic adaptation to stressful environments. Gene 509:7–15. https://doi.org/10.1016/j.gene.2012.07.042
Chow J, Heard E (2009) X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol 21:359–366. https://doi.org/10.1016/j.ceb.2009.04.012
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184. https://doi.org/10.1038/nature11319
Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW, Walker DW (2015) Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep 12:1656–1667. https://doi.org/10.1016/j.celrep.2015.08.004
Clocchiatti A, Cora E, Zhang Y, Dotto GP (2016) Sexual dimorphism in cancer. Nat Rev Cancer 16:330–339. https://doi.org/10.1038/nrc.2016.30
Clutton-Brock TH, Isvaran K (2007) Sex differences in ageing in natural populations of vertebrates. Proc Biol Sci 274:3097–3104. https://doi.org/10.1098/rspb.2007.1138
Conley MN, Wong CP, Duyck KM, Hord N, Ho E, Sharpton TJ (2016) Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ 4:e1854. https://doi.org/10.7717/peerj.1854
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D (2014) The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 171:P1–P29. https://doi.org/10.1530/eje-14-0253
Cordaux R, Batzer MA (2009) The impact of retrotransposons on human genome evolution. Nat Rev Genet 10:691–703. https://doi.org/10.1038/nrg2640
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923. https://doi.org/10.1126/science.8346443
Davis EJ, Lobach I, Dubal DB (2018) Female XX sex chromosomes increase survival and extend lifespan in aging mice. Aging Cell. https://doi.org/10.1111/acel.12871
De Cecco M, Criscione SW, Peterson AL, Neretti N, Sedivy JM, Kreiling JA (2013) Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging (Albany NY) 5:867–883
De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD, Le O, Beauséjour C, Ambati J, Ambati K, Simon M, Seluanov A, Gorbunova V, Slagboom PE, Helfand SL, Neretti N, Sedivy JM (2019) L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566:73–78. https://doi.org/10.1038/s41586-018-0784-9
Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J (2007) A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177:73–86. https://doi.org/10.1083/jcb.200612094
Della Torre S, Mitro N, Meda C, Lolli F, Pedretti S, Barcella M, Ottobrini L, Metzger D, Caruso D, Maggi A (2018) Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab 28(256–267):e5. https://doi.org/10.1016/j.cmet.2018.05.021
Dennis S, Sheth U, Feldman JL, English KA, Priess JR (2012) C. elegans germ cells show temperature and age-dependent expression of Cer1, a Gypsy/Ty3-related retrotransposon. PLoS Pathogens 8:e1002591. https://doi.org/10.1371/journal.ppat.1002591
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317. https://doi.org/10.1006/exnr.2002.7891
Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P (2010) Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 33:1186–1192. https://doi.org/10.2337/dc09-1649
Dong X, Greven MC, Kundaje A, Djebali S, Brown JB, Cheng C, Gingeras TR, Gerstein M, Guigo R, Birney E, Weng Z (2012) Modeling gene expression using chromatin features in various cellular contexts. Genome Biol 13:R53. https://doi.org/10.1186/gb-2012-13-9-r53
Drange S, Anderson DR, Schulzer M (2001) Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 131:699–708. https://doi.org/10.1016/S0002-9394(01)00964-3
Dulken B, Brunet A (2015) Stem cell aging and sex: are we missing something? Cell Stem Cell 16:588–590. https://doi.org/10.1016/j.stem.2015.05.006
Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis C, Doyle F, Epstein CB, Frietze S, Harrow J, Kaul R, Khatun J, Lajoie BR, Landt SG, Lee BK, Pauli F, Rosenbloom KR, Sabo P, Safi A, Sanyal A, Shoresh N, Simon JM, Song L, Trinklein ND, Altshuler RC, Birney E, Brown JB, Cheng C, Djebali S, Dong XJ, Dunham I, Ernst J, Furey TS, Gerstein M, Giardine B, Greven M, Hardison RC, Harris RS, Herrero J, Hoffman MM, Iyer S, Kellis M, Khatun J, Kheradpour P, Kundaje A, Lassmann T, Li QH, Lin X, Marinov GK, Merkel A, Mortazavi A, Parker SCJ, Reddy TE, Rozowsky J, Schlesinger F, Thurman RE, Wang J, Ward LD, Whitfield TW, Wilder SP, Wu W, Xi HLS, Yip KY, Zhuang JL, Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M, Pazin MJ, Lowdon RF, Dillon LAL, Adams LB, Kelly CJ, Zhang J, Wexler JR, Green ED, Good PJ, Feingold EA, Bernstein BE, Birney E, Crawford GE, Dekker J, Elnitski L, Farnham PJ, Gerstein M, Giddings MC, Gingeras TR, Green ED, Guigo R, Hardison RC, Hubbard TJ, Kellis M, Kent WJ, Lieb JD, Margulies EH, Myers RM, Snyder M, Stamatoyannopoulos JA, Tenenbaum SA et al (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. https://doi.org/10.1038/Nature11247
Dworatzek E, Baczko I, Kararigas G (2016) Effects of aging on cardiac extracellular matrix in men and women. Proteom Clin Appl 10:84–91. https://doi.org/10.1002/prca.201500031
Efe YK, Ugurbas SC, Alpay A, Ugurbas SH (2012) The course of corneal and intraocular pressure changes during pregnancy. Can J Ophthalmol 47:150–154. https://doi.org/10.1016/j.jcjo.2012.01.004
Elbay A, Ercan C, Akbas F, Bulut H, Ozdemir H (2019) Three new circulating microRNAs may be associated with wet age-related macular degeneration. Scand J Clin Lab Investig. https://doi.org/10.1080/00365513.2019.1637931
Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA (2002) Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol 55:25–31. https://doi.org/10.1016/S0895-4356(01)00425-5
Elder J, Pan J, Duncan C, Weissman S (1981) Transcriptional analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res 9:1171–1189
El-Maarri O, Walier M, Behne F, van Üüm J, Singer H, Diaz-Lacava A, Nüsgen N, Niemann B, Watzka M, Reinsberg J, van der Ven H, Wienker T, Stoffel-Wagner B, Schwaab R, Oldenburg J (2011) Methylation at global LINE-1 repeats in human blood are affected by gender but not by age or natural hormone cycles. PLoS One 6:e16252. https://doi.org/10.1371/journal.pone.0016252
El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275:223–228. https://doi.org/10.1074/jbc.275.1.223
Enns LC, Morton JF, Treuting PR, Emond MJ, Wolf NS, Dai D-F, McKnight GS, Rabinovitch PS, Ladiges WC (2009) Disruption of protein kinase A in mice enhances healthy aging. PLoS One 4:e5963. https://doi.org/10.1371/journal.pone.0005963
Estep PW 3rd, Warner JB, Bulyk ML (2009) Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS One 4:e5242. https://doi.org/10.1371/journal.pone.0005242
Fang Y, Hill CM, Darcy J, Reyes-Ordonez A, Arauz E, McFadden S, Zhang C, Osland J, Gao J, Zhang T, Frank SJ, Javors MA, Yuan R, Kopchick JJ, Sun LY, Chen J, Bartke A (2018) Effects of rapamycin on growth hormone receptor knockout mice. Proc Natl Acad Sci USA 115:E1495–E1503. https://doi.org/10.1073/pnas.1717065115
Farrer LA, Adrienne Cupples L, Van Duijn CM, Kurz A, Zimmer R, Müller U, Green RC, Clarke V, Shoffner J, Wallace DC, Chui H, Flanagan SD, Duara R, George-Hyslop PS, Auerbach SA, Volicer L, Wells JM, Van Broeckhoven C, Growdon JH, Haines JL (1995) Apolipoprotein E genotype in patients with alzheimer’s disease: implications for the risk of dementia among relatives. Ann Neurol 38:797–808. https://doi.org/10.1002/ana.410380515
Ferrini RL, Barrett-Connor E (1998) Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol 147:750–754. https://doi.org/10.1093/oxfordjournals.aje.a009519
Finch C (1990) Longevity, senescence, and the genome. University of Chicago Press, Chicago
Fischer KE, Gelfond JAL, Soto VY, Han C, Someya S, Richardson A, Austad SN (2015) Health effects of long-term rapamycin treatment: the impact on mouse health of enteric rapamycin treatment from four months of age throughout life. PLoS One 10:1–18. https://doi.org/10.1371/journal.pone.0126644
Fischer KE, Hoffman JM, Sloane LB, Gelfond JA, Soto VY, Richardson AG, Austad SN (2016) A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging (Albany NY) 8:2370–2391. https://doi.org/10.18632/aging.101059
Fontana L, Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161:106–118. https://doi.org/10.1016/j.cell.2015.02.020
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4–S9. https://doi.org/10.1093/gerona/glu057
Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, Ertl G, Bauersachs J (2005) Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J 19:591–593. https://doi.org/10.1096/fj.04-2459fje
Freeman EE, Munoz B, Schein OD, West SK (2001) Hormone replacement therapy and lens opacities: the Salisbury eye evaluation project. Arch Ophthalmol 119:1687–1692. https://doi.org/10.1001/archopht.119.11.1687
Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC (2013) Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci 33:18531–18539. https://doi.org/10.1523/JNEUROSCI.1278-13.2013
Fuhrman SA, Deininger PL, LaPorte P, Friedmann T, Peter Geiduschek E (1981) Analysis of transcription of the human Alu family ubiquitous repeating element by eukaryotic RNA polymerase III. Nucleic Acids Res 9:6439–6456. https://doi.org/10.1093/nar/9.23.6439
Gambineri A, Pelusi C (2019) Sex hormones, obesity and type 2 diabetes: is there a link? Endocr Connect 8:R1–R9. https://doi.org/10.1530/EC-18-0450
Garratt M, Bower B, Garcia GG, Miller RA (2017) Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 16:1256–1266. https://doi.org/10.1111/acel.12656
Gesing A, Masternak MM, Lewinski A, Karbownik-Lewinska M, Kopchick JJ, Bartke A (2013) Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci 68:639–651. https://doi.org/10.1093/gerona/gls231
Gesing A, Wiesenborn D, Do A, Menon V, Schneider A, Victoria B, Stout MB, Kopchick JJ, Bartke A, Masternak MM (2017) A long-lived mouse lacking both growth hormone and growth hormone receptor: a new animal model for aging studies. J Gerontol A Biol Sci Med Sci 72:1054–1061. https://doi.org/10.1093/gerona/glw193
Geurtsen GJ, Hoogland J, Goldman JG, Schmand BA, Troster AI, Burn DJ, Litvan I, Criteria MDSSGotVoP-M (2014) Parkinson’s disease mild cognitive impairment: application and validation of the criteria. J Parkinsons Dis 4:131–137. https://doi.org/10.3233/JPD-130304
Gibbs VK, Brewer RA, Miyasaki ND, Patki A, Smith DL (2018) Sex-dependent differences in liver and gut metabolomic profiles with acarbose and calorie restriction in C57BL/6 mice. J Gerontol Ser A Biol Sci Med Sci 73:157–165. https://doi.org/10.1093/gerona/glx127
Gillies GE, Pienaar IS, Vohra S, Qamhawi Z (2014) Frontiers in neuroendocrinology sex differences in Parkinson’s disease. Front Neuroendocrinol 35:370–384. https://doi.org/10.1016/j.yfrne.2014.02.002
Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS (2008) Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 149:5747–5757. https://doi.org/10.1210/en.2008-0355
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II, Wilson MR, Kass MA (2002) The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 120:714–720. https://doi.org/10.1001/archopht.120.6.714(discussion 829–30)
Gubbels Bupp MR (2015) Sex, the aging immune system, and chronic disease. Cell Immunol 294:102–110. https://doi.org/10.1016/j.cellimm.2015.02.002
Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Cömert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H, Wolf SA (2018) Transcriptional and translational differences of microglia from male and female brains. Cell Rep 24:2773–2783.e6. https://doi.org/10.1016/j.celrep.2018.08.001
Haaxma CA, Bloem BR, Borm GF, Oyen WJG, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MWIM (2007) Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:819–824. https://doi.org/10.1136/jnnp.2006.103788
Hadad N, Masser DR, Logan S, Wronowski B, Mangold CA, Clark N, Otalora L, Unnikrishnan A, Ford MM, Giles CB, Wren JD, Richardson A, Sonntag WE, Stanford DR, Freeman W (2016) Absence of genomic hypomethylation or regulation of cytosine-modifying enzymes with aging in male and female mice. Epigenetics Chromatin 9:30. https://doi.org/10.1186/s13072-016-0080-6
Haig D (2004) The inexorable rise of gender and the decline of sex: social change in academic titles, 1945–2001. Arch Sex Behav 33:87–96
Hammes SR, Levin ER (2019) Impact of estrogens in males and androgens in females. J Clin Investig 129:1818–1826. https://doi.org/10.1172/JCI125755
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731. https://doi.org/10.1210/jcem.86.2.7219
Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA (2014) Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13:273–282. https://doi.org/10.1111/acel.12170
Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, Flurkey K, Garratt M, Gelfond JAL, Javors MA, Levi M, Lithgow GJ, Macchiarini F, Nelson JF, Sukoff Rizzo SJ, Slaga TJ, Stearns T, Wilkinson JE, Miller RA (2019) Acarbose improves health and lifespan in aging HET3 mice. Aging Cell 18:1–13. https://doi.org/10.1111/acel.12898
Hodgkin J (1992) Genetic sex determination mechanisms and evolution. BioEssays 14:253–261. https://doi.org/10.1002/bies.950140409
Hoffman MM, Ernst J, Wilder SP, Kundaje A, Harris RS, Libbrecht M, Giardine B, Ellenbogen PM, Bilmes JA, Birney E, Hardison RC, Dunham I, Kellis M, Noble WS (2013) Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res 41:827–841. https://doi.org/10.1093/nar/gks1284
Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187. https://doi.org/10.1038/nature01298
Hong K, Choi Y (2018) Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep 51:225–229
Hong JS, Yi SW, Kang HC, Jee SH, Kang HG, Bayasgalan G, Ohrr H (2007) Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas 56:411–419. https://doi.org/10.1016/j.maturitas.2006.11.004
Honjoh S, Ihara A, Kajiwara Y, Yamamoto T, Nishida E (2017) The sexual dimorphism of dietary restriction responsiveness in Caenorhabditis elegans. Cell Rep 21:3646–3652. https://doi.org/10.1016/j.celrep.2017.11.108
Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M (2012) The role of androgens and estrogens on healthy aging and longevity. J Gerontol Ser A Biol Sci Med Sci 67:1140–1152. https://doi.org/10.1093/gerona/gls068
Høvding G, Aasved H (1986) Prognostic factors in the development of manifest open angle glaucoma: a long-term follow-up study of hypertensive and normotensive eyes. Acta Ophthalmol 64:601–608. https://doi.org/10.1111/j.1755-3768.1986.tb00675.x
Hughes JF, Rozen S (2012) Genomics and genetics of human and primate Y chromosomes. Annu Rev Genom Hum Genet 13:83–108. https://doi.org/10.1146/annurev-genom-090711-163855
Hulsman CAA, Westendorp ICD, Ramrattan RS, Wolfs RCW, Witteman JCM, Vingerling JR, Hofman A, De Jong PTVM (2001) Is open-angle glaucoma associated with early menopause? The rotterdam study. Am J Epidemiol 154:138–144. https://doi.org/10.1093/aje/154.2.138
Imtiaz B, Taipale H, Tanskanen A, Tiihonen M, Kivipelto M, Heikkinen AM, Tiihonen J, Soininen H, Hartikainen S, Tolppanen AM (2017) Risk of Alzheimer’s disease among users of postmenopausal hormone therapy: a nationwide case-control study. Maturitas 98:7–13. https://doi.org/10.1016/j.maturitas.2017.01.002
Isensee J, Witt H, Pregla R, Hetzer R, Regitz-Zagrosek V, Noppinger PR (2008) Sexually dimorphic gene expression in the heart of mice and men. J Mol Med (Berl) 86:61–74. https://doi.org/10.1007/s00109-007-0240-z
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080. https://doi.org/10.1126/science.1063127
Jiang Y, Tian W (2017) The effects of progesterones on blood lipids in hormone replacement therapy. Lipids Health Dis 16:1–8. https://doi.org/10.1186/s12944-017-0612-5
Jura M, Kozak LP (2016) Obesity and related consequences to ageing. Age. https://doi.org/10.1007/s11357-016-9884-3
Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, Nickerson RS, Dawber TR (1977) The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol 106:17–32. https://doi.org/10.1093/oxfordjournals.aje.a112428
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, Hao L, Bhan AK, Kang JX (2018) Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6:205. https://doi.org/10.1186/s40168-018-0587-0
Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483:218–221. https://doi.org/10.1038/nature10815
Kidwell MG, Lisch D (1997) Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci 94:7704–7711. https://doi.org/10.1073/pnas.94.15.7704
Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2:a008888. https://doi.org/10.1101/cshperspect.a008888
Klein BEK, Klein R, Linton KLP (1992) Prevalence of age-related lens opacities in a population: the Beaver Dam Eye Study. Ophthalmology 99:546–552. https://doi.org/10.1016/S0161-6420(92)31934-7
Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ (2009) Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 65:650–657. https://doi.org/10.1002/ana.21696
Kozakowski J, Gietka-Czernel M, Leszczyńska D, Majos A (2017) Obesity in menopause—our negligence or an unfortunate inevitability? Prz Menopauzalny 16:61–65. https://doi.org/10.5114/pm.2017.68594
Kua EH, Ho E, Tan HH, Tsoi C, Thng C, Mahendran R (2014) The natural history of dementia. Psychogeriatrics 14:196–201. https://doi.org/10.1111/psyg.12053
Kuk JL, Saunders TJ, Davidson LE, Ross R (2009) Age-related changes in total and regional fat distribution. Ageing Res Rev 8:339–348. https://doi.org/10.1016/j.arr.2009.06.001
Kwa M, Plottel CS, Blaser MJ, Adams S (2016) The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst 108:djw029. https://doi.org/10.1093/jnci/djw029
Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronman H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo S, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ (2017) Sex-specific transcriptional signatures in human depression. Nat Med. https://doi.org/10.1038/nm.4386
Lai RW, Lu R, Danthi PS, Bravo JI, Goumba A, Sampathkumar NK, Benayoun BA (2019) Multi-level remodeling of transcriptional landscapes in aging and longevity. BMB Rep 52:86–108
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335:1638–1643. https://doi.org/10.1126/science.1215135
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng J-F, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M et al (2001) Initial sequencing and analysis of the human genome. Nature 409:860–921. https://doi.org/10.1038/35057062
Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman J-M, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F (2016) Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep 6:35539. https://doi.org/10.1038/srep35539
Lavie L, Maldener E, Brouha B, Meese EU, Mayer J (2004) The human L1 promoter: variable transcription initiation sites and a major impact of upstream flanking sequence on promoter activity. Genome Res 14:2253–2260. https://doi.org/10.1101/gr.2745804
Laws KR, Irvine K, Gale TM (2016) Sex differences in cognitive impairment in Alzheimer's disease. World J Psychiatry 6:54–65. https://doi.org/10.5498/wjp.v6.i1.54
Lee AJ, Mitchell P, Rochtchina E, Healey PR (2003) Female reproductive factors and open angle glaucoma: the Blue Mountains Eye Study. Br J Ophthalmol 87:1324–1328. https://doi.org/10.1136/bjo.87.11.1324
Legro RS (2003) Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev 24:302–312. https://doi.org/10.1210/er.2003-0004
Lemos B, Branco AT, Hartl DL (2010) Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc Natl Acad Sci USA 107:15826–15831. https://doi.org/10.1073/pnas.1010383107
Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, Bandinelli S, Salfati E, Manson JE, Quach A, Kusters CDJ, Kuh D, Wong A, Teschendorff AE, Widschwendter M, Ritz BR, Absher D, Assimes TL, Horvath S (2016) Menopause accelerates biological aging. Proc Natl Acad Sci 113:9327–9332. https://doi.org/10.1073/pnas.1604558113
Li W, Prazak L, Chatterjee N, Gruninger S, Krug L, Theodorou D, Dubnau J (2013) Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci 16:529–531. https://doi.org/10.1038/nn.3368
Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF (2010) Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9:92–95. https://doi.org/10.1111/j.1474-9726.2009.00533.x
Lin FC, Chuang YS, Hsieh HM, Lee TC, Chiu KF, Liu CK, Wu MT (2015) Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine (United States) 94:e2143. https://doi.org/10.1097/MD.0000000000002143
Lombardi P, Goldin B, Boutin E, Gorbach SL (1978) Metabolism of androgens and estrogens by human fecal microorganisms. J Steroid Biochem 9:795–801. https://doi.org/10.1016/0022-4731(78)90203-0
Longo VD (2003) The Ras and Sch9 pathways regulate stress resistance and longevity. Exp Gerontol 38:807–811
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Lu Y, Feng F, Yang Y, Gao X, Cui J, Zhang C, Zhang F, Xu Z, Qv J, Wang C, Zeng Z, Zhu Y, Yang Y (2013) LINE-1 ORF-1p functions as a novel androgen receptor co-activator and promotes the growth of human prostatic carcinoma cells. Cell Signal 25:479–489. https://doi.org/10.1016/j.cellsig.2012.11.004
Lu S, Niu Z, Chen Y, Tu Q, Zhang Y, Chen W, Tong W, Zhang Z (2018) Repetitive element DNA methylation is associated with menopausal age. Aging Dis 9:435–443. https://doi.org/10.14336/AD.2017.0810
Lundström M, Stenevi U, Thorburn W (1999) Gender and cataract surgery in Sweden 1992–1997. Acta Ophthalmol Scand 77:204–208. https://doi.org/10.1034/j.1600-0420.1999.770218.x
Lundström M, Stenevi U, Thorburn W (2002) The swedish national cataract register: a 9-year review. Acta Ophthalmol Scand 80:248–257. https://doi.org/10.1034/j.1600-0420.2002.800304.x
Magwere T, Chapman T, Partridge L (2004) Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol Ser A Biol Sci Med Sci 59:B3–B9. https://doi.org/10.1093/gerona/59.1.b3
Maki PM, Henderson VW (2012) Hormone therapy, dementia, and cognition: the Women’s Health Initiative 10 years on. Climacteric 15:256–262. https://doi.org/10.3109/13697137.2012.660613
Manolopoulos KN, Karpe F, Frayn KN (2010) Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 34:949–959. https://doi.org/10.1038/ijo.2009.286
Marais GAB, Gaillard JM, Vieira C, Plotton I, Sanlaville D, Gueyffier F, Lemaitre JF (2018) Sex gap in aging and longevity: can sex chromosomes play a role? Biol Sex Differ 9:1–14. https://doi.org/10.1186/s13293-018-0181-y
Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088. https://doi.org/10.1126/science.1233521
Martinez-Martin P, Pecurariu CF, Odin P, Van Hilten JJ, Antonini A, Rojo-Abuin JM, Borges V, Trenkwalder C, Aarsland D, Brooks DJ, Chaudhuri KR (2012) Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J Neurol 259:1639–1647. https://doi.org/10.1007/s00415-011-6392-3
Mason CE, Shu F-J, Wang C, Session RM, Kallen RG, Sidell N, Yu T, Liu MH, Cheung E, Kallen CB (2010) Location analysis for the estrogen receptor-alpha reveals binding to diverse ERE sequences and widespread binding within repetitive DNA elements. Nucleic Acids Res 38:2355–2368. https://doi.org/10.1093/nar/gkp1188
Masser DR, Hadad N, Porter HL, Mangold CA, Unnikrishnan A, Ford MM, Giles CB, Georgescu C, Dozmorov MG, Wren JD, Richardson A, Stanford DR, Freeman WM (2017) Sexually divergent DNA methylation patterns with hippocampal aging. Aging Cell 16:1342–1352. https://doi.org/10.1111/acel.12681
Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y, Salmon AB, Hughes LF, Liberati T, Boparai R, Kopchick JJ, Westbrook R (2012) Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell 11:73–81. https://doi.org/10.1111/j.1474-9726.2011.00763.x
Maxwell PH, Burhans WC, Curcio MJ (2011) Retrotransposition is associated with genome instability during chronological aging. Proc Natl Acad Sci USA 108:20376–20381. https://doi.org/10.1073/pnas.1100271108
Mayne BT, Bianco-Miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, Roberts CT (2016) Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet 7:183. https://doi.org/10.3389/fgene.2016.00183
McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr 10:63–79
McClintock B (1953) Induction of instability at selected loci in maize. Genetics 38:579–599
Medzikovic L, Aryan L, Eghbali M (2019) Connecting sex differences, estrogen signaling, and microRNAs in cardiac fibrosis. J Mol Med. https://doi.org/10.1007/s00109-019-01833-6
Menazza S, Murphy E (2016) The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res 118:994–1007. https://doi.org/10.1161/CIRCRESAHA.115.305376
Metchnikoff E (1907) The prolongation of life: optimistic studies, trans. P. Chalmers Mitchell. GP Putnam’s Sons, New York
Miller IN, Cronin-Golomb A (2010) Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord 25:2695–2703. https://doi.org/10.1002/mds.23388
Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66:191–201. https://doi.org/10.1093/gerona/glq178
Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R (2014) Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13:468–477. https://doi.org/10.1111/acel.12194
Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Lee Wetherington C, Makris S, Arnold AP, Einstein G, Miller VM, Sandberg K, Maier S, Cornelison TL, Clayton JA (2017) Considering sex as a biological variable in preclinical research. FASEB J 31:29–34. https://doi.org/10.1096/fj.201600781R
Mink JW (1996) A model for waste processing ? Pergamorr Prog Neurobiol 50:26. https://doi.org/10.1016/S0301-0082(96)00042-1
Mitchell P, Cumming RG, Attebo K, Panchapakesan J (1997) Prevalence of cataract in Australia: the Blue Mountains Eye Study. Ophthalmology 104:581–588. https://doi.org/10.1016/S0161-6420(97)30266-8
Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Buron MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R (2016) Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23:1093–1112. https://doi.org/10.1016/j.cmet.2016.05.027
Miyamura M, Schnell O, Yamashita C, Yoshioka T, Matsumoto C, Mori T, Ukimura A, Kitaura Y, Matsumura Y, Ishizaka N, Hayashi T (2010) Effects of acarbose on the acceleration of postprandial hyperglycemia-induced pathological changes induced by intermittent hypoxia in lean mice. J Pharmacol Sci 114:32–40. https://doi.org/10.1254/jphs.10014fp
Morales JF, Snow ET, Murnane JP (2003) Environmental factors affecting transcription of the human L1 retrotransposon. II. Stressors. Mutagenesis 18:151–158. https://doi.org/10.1093/mutage/18.2.151
Moran LJ, Misso ML, Wild RA, Norman RJ (2010) Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16:347–363. https://doi.org/10.1093/humupd/dmq001
Moreira APB, Texeira TFS, Ferreira AB, do Carmo Gouveia Peluzio M, de Cássia Gonçalves Alfenas R (2012) Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 108:801–809. https://doi.org/10.1017/s0007114512001213
Morgan CL, Jenkins-Jones S, Currie CJ, Rees DA (2012) Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab 97:3251–3260. https://doi.org/10.1210/jc.2012-1690
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H (2018) Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging 4:267–285. https://doi.org/10.3233/NHA-170030
Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ (2014) Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505:555–558. https://doi.org/10.1038/nature12932
Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC (2019) Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. https://doi.org/10.1126/science.aaw7317
Nelson JF, Strong R, Bokov A, Diaz V, Ward W (2012) Probing the relationship between insulin sensitivity and longevity using genetically modified mice. J Gerontol A Biol Sci Med Sci 67:1332–1338. https://doi.org/10.1093/gerona/gls199
Newman-Casey PA, Talwar N, Nan B, Musch DC, Pasquale LR, Stein JD (2014) The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthalmol 132:298–303. https://doi.org/10.1001/jamaophthalmol.2013.7618
Nolen GA (1972) Effect of various restricted dietary regimens on growth, health and longevity of albino-rats. J Nutr 102:1477
Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT (2005) Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 16:556–562
Ostan R, Monti D, Gueresi P, Bussolotto M, Franceschi C, Baggio G (2016) Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin Sci (London, England: 1979) 130:1711–1725. https://doi.org/10.1042/cs20160004
Pal S, Tyler JK (2016) Epigenetics and aging. Sci Adv 2:e1600584. https://doi.org/10.1126/sciadv.1600584
Parker SC, Stitzel ML, Taylor DL, Orozco JM, Erdos MR, Akiyama JA, van Bueren KL, Chines PS, Narisu N, Black BL, Visel A, Pennacchio LA, Collins FS (2013) Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci USA 110:17921–17926. https://doi.org/10.1073/pnas.1317023110
Pasquale LR, Kang JH (2011) Female reproductive factors and primary open-angle glaucoma in the Nurses Health Study. Eye 25:633–641. https://doi.org/10.1038/eye.2011.34
Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F (2014) Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids 90:13–29. https://doi.org/10.1016/j.steroids.2014.06.012
Pattabiraman PP, Toris CB (2016) The exit strategy: pharmacological modulation of extracellular matrix production and deposition for better aqueous humor drainage. Eur J Pharmacol 787:32–42. https://doi.org/10.1016/j.ejphar.2016.04.048
Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM (2009) Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 30:343–357. https://doi.org/10.1016/j.yfrne.2009.03.007
Phillips CI, Gore SM (1985) Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol 69:117–119. https://doi.org/10.1136/bjo.69.2.117
Pike CJ (2017) Sex and the development of Alzheimer’s disease. J Neurosci Res 95:671–680. https://doi.org/10.1002/jnr.23827
Plottel Claudia S, Blaser Martin J (2011) Microbiome and malignancy. Cell Host Microbe 10:324–335. https://doi.org/10.1016/j.chom.2011.10.003
Podcasy JL, Epperson CN (2016) Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 18:437–446
Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S (1993) Polymorphism and Alzheimer’ s disease. Lancet 342:697–699
Pomatto LC, Carney C, Shen B, Wong S, Halaszynski K, Salomon MP, Davies KJ, Tower J (2017) The mitochondrial lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr Biol 27:1–15. https://doi.org/10.1016/j.cub.2016.10.044
Qu K, Zaba LC, Giresi PG, Li R, Longmire M, Kim YH, Greenleaf WJ, Chang HY (2015) Individuality and variation of personal regulomes in primary human T cells. Cell Syst 1:51–61. https://doi.org/10.1016/j.cels.2015.06.003
Quigley H, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. https://doi.org/10.1136/bjo.2005.081224
Quintana-Murci L, Fellous M (2001) The human Y chromosome: the biological role of a “functional wasteland”. J Biomed Biotechnol 2001:18–24. https://doi.org/10.1155/S1110724301000080
Qureshi IA, Mehler MF (2010) Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog Brain Res 186:77–95. https://doi.org/10.1016/B978-0-444-53630-3.00006-3
Reed BG, Carr BR (2000) The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (eds) Endotext. MDText.com, Inc., South Dartmouth
Regan JC, Khericha M, Dobson AJ, Bolukbasi E, Rattanavirotkul N, Partridge L (2016) Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. Elife 5:e10956. https://doi.org/10.7554/eLife.10956
Regitz-Zagrosek V, Kararigas G (2017) Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97:1–37. https://doi.org/10.1152/physrev.00021.2015
Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S (2007) Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med 4(Suppl B):S162–S177
Regitz-Zagrosek V, Jaguszewska K, Preis K (2015) Pregnancy-related spontaneous coronary artery dissection. Eur Heart J 36:2273–2274
Rera M, Clark RI, Walker DW (2012) Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 109:21528–21533. https://doi.org/10.1073/pnas.1215849110
Rochelle TL, Yeung DKY, Bond MH, Li LMW (2015) Predictors of the gender gap in life expectancy across 54 nations. Psychol Health Med 20:129–138. https://doi.org/10.1080/13548506.2014.936884
Sadagurski M, Cady G, Miller RA (2017) Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell 16:652–660. https://doi.org/10.1111/acel.12590
Savage DC (1977) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133. https://doi.org/10.1146/annurev.mi.31.100177.000543
Schachter AS, Davis KL (2000) Alzheimer’s disease. Dialogues Clin Neurosci 2:91–100
Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M (2019) The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genom 13:2. https://doi.org/10.1186/s40246-018-0185-z
Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13:800–812. https://doi.org/10.1038/nrc3610
Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ (2008) Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22:807–818. https://doi.org/10.1096/fj.07-9261com
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144. https://doi.org/10.1126/science.1177221
Sender R, Fuchs S, Milo R (2016a) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164:337–340. https://doi.org/10.1016/j.cell.2016.01.013
Sender R, Fuchs S, Milo R (2016b) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. https://doi.org/10.1371/journal.pbio.1002533
Shadyab AH, Macera CA, Shaffer RA, Jain S, Gallo LC, Gass ML, Waring ME, Stefanick ML, LaCroix AZ (2017) Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s Health Initiative. Menopause 24:35–44. https://doi.org/10.1097/GME.0000000000000710
Shulman LM, Bhat V (2006) Gender disparities in Parkinson’s disease. Expert Rev Neurother 6:407–416. https://doi.org/10.1586/14737175.6.3.407
Simon M, Van Meter M, Ablaeva J, Ke Z, Gonzalez RS, Taguchi T, De Cecco M, Leonova KI, Kogan V, Helfand SL, Neretti N, Roichman A, Cohen HY, Meer MV, Gladyshev VN, Antoch MP, Gudkov AV, Sedivy JM, Seluanov A, Gorbunova V (2019) LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab 29:871–885.e5. https://doi.org/10.1016/j.cmet.2019.02.014
Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC (2003) The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423:825–837. https://doi.org/10.1038/nature01722
Skuse D, Printzlau F, Wolstencroft J (2018) Sex chromosome aneuploidies. Handb Clin Neurol 147:355–376. https://doi.org/10.1016/B978-0-444-63233-3.00024-5
Smith AD (2002) Imaging the progression of Alzheimer pathology through the brain. Proc Natl Acad Sci USA 99:4135–4137. https://doi.org/10.1073/pnas.082107399
Smith KD, Young KE, Talbot CC, Schmeckpeper BJ (1987) Repeated DNA of the human Y chromosome. Development 101:77–92
Snow KK, Cote J, Yang W, Davis NJ, Seddon JM (2002) Association between reproductive and hormonal factors and age-related maculopathy in postmenopausal women. Am J Ophthalmol 134:842–848. https://doi.org/10.1016/S0002-9394(02)01755-5
Sommer A, Tielsch JM (1996) Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol 114:235. https://doi.org/10.1001/archopht.1996.01100130229029
Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MGM, Lendvai A, Verkade HJ, Boekhorst J, Timmerman HM, Plösch T, Müller M (2014) Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ 5:11. https://doi.org/10.1186/s13293-014-0011-9
Stegeman R, Weake VM (2017) Transcriptional signatures of aging. J Mol Biol 429:2427–2437. https://doi.org/10.1016/j.jmb.2017.06.019
Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE (2008) Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7:641–650. https://doi.org/10.1111/j.1474-9726.2008.00414.x
Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS (1997) Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 96:3774–3777. https://doi.org/10.1161/01.cir.96.10.3774
Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A (2013) Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2013:1–24. https://doi.org/10.7554/elife.01098.001
Tanaka M, Nakayama J (2017) Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 66:515–522. https://doi.org/10.1016/j.alit.2017.07.010
Tchkonia T, Morbeck DE (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9:667–684. https://doi.org/10.1111/j.1474-9726.2010.00608.x
Teipel SJ, Bayer W, Alexander GE, Zebuhr Y, Teichberg D, Kulic L, Schapiro MB, Möller HJ, Rapoport SI, Hampel H (2002) Progression of corpus callosum atrophy in Alzheimer disease. Arch Neurol 59:243–248. https://doi.org/10.1001/archneur.59.2.243
Testori A, Caizzi L, Cutrupi S, Friard O, De Bortoli M, Cora D, Caselle M (2012) The role of transposable elements in shaping the combinatorial interaction of transcription factors. BMC Genom 13:400. https://doi.org/10.1186/1471-2164-13-400
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larché MJ, Davidson DJ, Verdú EF, Surette MG, Bowdish DME (2017) Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21:455–466.e4. https://doi.org/10.1016/j.chom.2017.03.002
Toth MJ, Tchernof A, Sites CK, Poehlman ET (2000) Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes 24:226–231. https://doi.org/10.1038/sj.ijo.0801118
Tsai MS, Tangalos EG, Petersen RC, Smith GE, Schaid DJ, Kokmen E, Ivnik RJ, Thibodeau SN (1994) Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet 54:643–649
Tsukamoto I, Kojo S (1990) The sex difference in the regulation of liver regeneration after partial hepatectomy in the rat. Biochim Biophys Acta 1033:287–290. https://doi.org/10.1016/0304-4165(90)90135-j
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. https://doi.org/10.1038/nature05414
Ucar D, Benayoun BA (2018) Chapter 1—aging epigenetics: changes and challenges. In: Moskalev A, Vaiserman AM (eds) Epigenetics of aging and longevity, vol 4. Academic Press, Boston, pp 3–32
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M (2009) Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130–1142. https://doi.org/10.1016/j.cell.2009.11.021
Vajaranant TS, Pasquale LR (2012) Estrogen deficiency accelerates aging of the optic nerve. Menopause 19:942–947. https://doi.org/10.1097/gme.0b013e3182443137
Valle A, Guevara R, García-Palmer FJ, Roca P, Oliver J (2007) Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol 293:1302–1308. https://doi.org/10.1152/ajpcell.00203.2007
Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, Flanagan KL (2019) The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol 41:265–275. https://doi.org/10.1007/s00281-018-0716-7
Vigen R, Maddox TM, Allen LA (2012) Aging of the United States population: impact on heart failure. Curr Heart Fail Rep 9:369–374. https://doi.org/10.1007/s11897-012-0114-8
Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A (2018) Sex-specific features of microglia from adult mice. Cell Rep 23:3501–3511. https://doi.org/10.1016/j.celrep.2018.05.048
Viña J, Lloret A (2010) Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-β peptide. J Alzheimer’s Dis 20:527–533. https://doi.org/10.3233/JAD-2010-100501
Wang X, Simpkins JW, Dykens JA, Cammarata PR (2003) Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability. Investig Ophthalmol Vis Sci 44:2067–2075. https://doi.org/10.1167/iovs.02-0841
Wang YE, Kakigi C, Barbosa D, Porco T, Chen R, Wang S, Li Y, Singh K, Pasquale LR, Lin SC (2016) Oral contraceptive use and prevalence of self-reported glaucoma or ocular hypertension in the United States. Ophthalmology 123:729–736. https://doi.org/10.1016/j.ophtha.2015.11.029
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153:307–319. https://doi.org/10.1016/j.cell.2013.03.035
Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA (2012) Rapamycin slows aging in mice. Aging Cell 11:675–682. https://doi.org/10.1111/j.1474-9726.2012.00832.x
Wilson MR, Hertzmark E, Walker AM, Childs Shaw K, Epstein DL (1987) A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol 105:1066–1071. https://doi.org/10.1001/archopht.1987.01060080068030
Wood JG, Jones BC, Jiang N, Chang C, Hosier S, Wickremesinghe P, Garcia M, Hartnett DA, Burhenn L, Neretti N, Helfand SL (2016) Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc Natl Acad Sci USA 113:11277–11282. https://doi.org/10.1073/pnas.1604621113
Wood-Kaczmar A, Gandhi S, Wood NW (2006) Understanding the molecular causes of Parkinson’s disease. Trends Mol Med 12:521–528. https://doi.org/10.1016/j.molmed.2006.09.007
Xiao L, Sonne SB, Feng Q, Chen N, Xia Z, Li X, Fang Z, Zhang D, Fjære E, Midtbø LK, Derrien M, Hugenholtz F, Tang L, Li J, Zhang J, Liu C, Hao Q, Vogel UB, Mortensen A, Kleerebezem M, Licht TR, Yang H, Wang J, Li Y, Arumugam M, Wang J, Madsen L, Kristiansen K (2017) High-fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome 5:43. https://doi.org/10.1186/s40168-017-0258-6
Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M (2014) Longevity effect of IGF-1R ± mutation depends on genetic background-specific receptor activation. Aging Cell 13:19–28. https://doi.org/10.1111/acel.12145
Xu J, Murphy SL, Kochanek KD, Bastian BA (2016) Deaths: final data for 2013. Natl Vital Stat Rep 64:1–119
Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ (2006) Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16:995–1004. https://doi.org/10.1101/gr.5217506
Yao W, Lay YE, Kot A, Liu R, Zhang H, Chen H, Lam K, Lane NE (2016) Improved mobilization of exogenous mesenchymal stem cells to bone for fracture healing and sex difference. Stem Cells 34:2587–2600. https://doi.org/10.1002/stem.2433
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227. https://doi.org/10.1038/nature11053
Yen A, Kellis M (2015) Systematic chromatin state comparison of epigenomes associated with diverse properties including sex and tissue type. Nat Commun 6:7973. https://doi.org/10.1038/ncomms8973
Younan C, Mitchell P, Cumming RG, Panchapakesan J, Rochtchina E, Hales AM (2002) Hormone replacement therapy, reproductive factors, and the incidence of cataract and cataract surgery: the Blue Mountains Eye Study. Am J Epidemiol 155:997–1006. https://doi.org/10.1093/aje/155.11.997
Yu D, Yang SE, Miller BR, Wisinski JA, Sherman DS, Brinkman JA, Tomasiewicz JL, Cummings NE, Kimple ME, Cryns VL, Lamming DW (2018) Short-Term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J 32:3471–3482. https://doi.org/10.1096/fj.201701211R
Acknowledgements
We apologize to the authors whose work we could not cite due to space constraints. J.I.B.’s work was supported by NIA T32-AG052374 and NSF graduate research fellowship DGE-1842487. L.T.R.’s work was supported by NIA T32-AG052374 and a USC Provost’s Fellowship. B.A.B.’s work was supported by by NIA R00AG049934, NIA R21AG063739, an innovator Grant from the Rose Hills foundation, a seed grant from the NAVIGAGE foundation, and a generous gift from the Hanson-Thorell Family.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sampathkumar, N.K., Bravo, J.I., Chen, Y. et al. Widespread sex dimorphism in aging and age-related diseases. Hum Genet 139, 333–356 (2020). https://doi.org/10.1007/s00439-019-02082-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-019-02082-w