Abstract
The major aim of the present study was to determine by molecular methods whether the wide and narrow types of macroscopic sarcocysts in Spanish sheep belonged to different species, that is, Sarcocystis gigantea and Sarcocystis medusiformis, respectively. Additionally, we wanted to identify and characterize molecularly the species forming microscopic sarcocysts and determine the phylogenetic placement of all species found. Portions of the oesophagus, diaphragm and hind legs containing macroscopic sarcocysts were collected from slaughtered culled ewes at an abattoir in the Province of Madrid, Central Spain, but both macroscopic and microscopic sarcocysts were isolated for molecular examination. Genomic DNA from 63 sarcocysts (21 macroscopic, 42 microscopic) were examined at the cytochrome c oxidase subunit I gene (cox1), while selected isolates of each species found were further examined at the 18S and 28S ribosomal RNA (rRNA) genes. The 63 sarcocysts comprised five cox1 sequence types, each corresponding to a particular sarcocyst type, and thus represented five Sarcocystis spp. The slender fusiform and thick macrocysts belonged to S. medusiformis and S. gigantea, respectively. The microscopic sarcocysts belonged to Sarcocystis arieticanis, Sarcocystis tenella and a Sarcocystis mihoensis-like species with slanting thorn-like cyst wall protrusions, which was characterised molecularly for the first time. Based on its phylogenetic position, the S. mihoensis-like species probably uses corvids as definitive hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protozoan parasites belonging to the genus Sarcocystis have an obligatory two-host life cycle comprising sexual development and formation of oocysts in the intestinal mucosa of their definitive hosts (carnivores and omnivores), and asexual multiplication in vascular endothelial and striated muscle cells, respectively, of their intermediate hosts (herbivores, omnivores and carnivores). The multiplication within striated muscle cells leads to the formation of mature sarcocysts, which have a characteristic morphology (size, shape and surface structure) for each species, and by which, definitive hosts become infected through ingestion of infected muscle tissue. The sarcocyst morphology of a given Sarcocystis sp. is fairly fixed, but it may be shared among two or more closely related species, which usually employ different, but phylogenetically related, intermediate hosts. Prior to the molecular era, morphologically indistinguishable sarcocysts in different intermediate hosts could only be assigned to different species on the basis of transmission experiments, which required that the definitive hosts were also known. Currently, molecular methods may be used to characterise Sarcocystis spp., identify their sarcocysts and determine their phylogenetic relationships.
Six named Sarcocystis spp. have hitherto been reported to form sarcocysts in domestic sheep, i.e. Sarcocystis tenella, Sarcocystis gigantea, Sarcocystis medusiformis, Sarcocystis arieticanis, Sarcocystis microps and Sarcocystis mihoensis. In addition, an unidentified species, differing from the aforementioned six species in sarcocyst ultrastructure, has been found in a single sheep in Sicily, Italy (Giannetto et al. 2005). Prior to the elucidation of the two-host life cycle of Sarcocystis spp. in the 1970s, it was believed that only a single species, mostly designated S. tenella, occurred in sheep, and that the sarcocysts of this species varied in size and surface morphology according to their age and developmental stage. As a result of transmission experiments, it was initially established that sheep acted as intermediate hosts for two species, one with macroscopic sarcocysts cycling via cats, and one with microscopic sarcocysts cycling via dogs (Rommel et al. 1972, 1974; Munday and Rickard 1974; Ford 1974, 1975; Gestrich et al. 1974, 1975). Heydorn et al. (1975) proposed to name these species Sarcocystis ovifelis and Sarcocystis ovicanis, respectively, in connection with their proposal for a new nomenclature of Sarcocystis spp. based on the names of their intermediate and definitive hosts. The rationale for using such names was further explained in a subsequent paper (Frenkel et al. 1979), but other scientists believed that previously named taxa should retain their old names, since these names had priority according to the rules of the Code of Zoological Nomenclature. Moreover, if an old name had been applied to more than one of the newly recognised taxa, the usage of this name should be restricted to a particular and well-defined taxon (Levine and Tadros 1980; Melville 1980). Levine and Tadros (1980) synonymized the newly proposed names S. ovicanis and S. ovifelis with the old names S. tenella and Sarcocystis gigantea, respectively, and the latter names are currently used for these species in sheep by most authors.
The third species to be named from sheep was S. medusiformis, which was initially reported from New Zealand (Collins et al. 1976, 1979) and Australia (Obendorf and Munday 1987), and found to use cats as definitive hosts. Its sarcocysts were macroscopic, but thinner and more elongate than those of S. gigantea, and their surface protrusions were different. A second dog-transmitted species with microscopic sarcocysts and hair-like protrusions was named S. arieticanis by Heydorn (1985), but it had previously been reported as an unnamed species by Bergmann and Kinder (1975) and Boch et al. (1979) and as S. tenella by Erber (1982). Dogs have also been claimed to act as definitive hosts for S. microps (Wang et al. 1988) and S. mihoensis (Saito et al. 1997), but each of these species have been found and studied only once in China and Japan, respectively. The species S. tenella, S. arieticanis and S. gigantea, on the other hand, are common and globally distributed in sheep, whereas S. medusiformis seems to have a limited distribution, having been identified with certainty in sheep only in New Zealand and Australia, as mentioned above, and in Iran (Farhang-Pajuh et al. 2014) and Sardinia, Italy (Pipia et al. 2016). The common occurrence of S. tenella, S. arieticanis and S. gigantea has facilitated a fairly extensive molecular characterisation of these species at several genetic loci in recent years, resulting in many nucleotide sequences having been deposited in public nucleotide databases, whereas only a single short sequence of S. medusiformis and no sequences of S. microps and S. mihoensis have been available.
As regards Sarcocystis infections in sheep in Spain, these parasites have been found to be widespread and to be an important cause of carcass condemnation. Thus, a high prevalence of sarcosporidiosis in the oesophagus has been reported in different surveys, either based on recovering bradyzoites by peptic digestion (Pérez-Garro and González-Castro 1970) or by complementing that method with the detection of microscopic sarcocysts by the compression technique (Díez-Baños 1978; Sánchez-Acedo et al. 1983; Pereira and Bermejo 1988), but neither of those methods allowed the sarcocysts to be identified to species. Microscopic cysts of S. tenella and S. arieticanis have, however, been identified either by histological examination of different muscles of sheep (Martínez-Moreno et al. 1989; Babín-Vich 1991), or by transmission electron microscopic (TEM) examination of sarcocysts from skeletal muscles (Simón-Vicente and Ramajo-Martín 1984). With respect to macroscopic sarcocysts, most authors have reported the presence of S. gigantea in the oesophagus (Díez-Baños 1978; Sánchez-Acedo et al. 1983; Martínez-Moreno et al. 1989; Babín-Vich 1991), but there have also been mentions of macroscopic cysts in the diaphragm that were morphologically different from S. gigantea and considered to be possible cysts of S. medusiformis (Martínez-Moreno et al. 1987; Babín-Vich 1991). In a recent comprehensive study, Martínez-Navalón et al. (2012) examined the prevalence of macroscopic sarcocysts and associated carcass condemnation in 5720 slaughtered sheep. At the post-mortem examination, macroscopic sarcocysts were found in 712 sheep (12%), of which 148 sheep (3%) harboured sarcocysts at a single location, causing partial carcass condemnation, whereas 564 sheep (10%) harboured macroscopic sarcocysts in two or more locations, causing total carcass condemnation. In addition, the size of all 141 macroscopic sarcocysts in 32 infected carcasses were measured, revealing the presence of three cyst types: a narrow filiform type (2–10 × ≤ 1 mm) in the diaphragm, abdominal muscles and hind legs, and two wider types (2–20 × 2–6 mm), including short and wide cysts in the oesophagus and more elongated cysts in other muscles. These cyst types matched those described by Collins et al. (1976, 1979) from sheep in New Zealand, and Martínez-Navalón et al. (2012) therefore suggested that the filiform type might represent sarcocysts of S. medusiformis, while the thicker cysts might belong to S. gigantea.
The primary aim of the present study was to obtain macroscopic sarcocysts from older sheep and characterise them by molecular methods in order to determine whether both S. gigantea and S. medusiformis might be present in Spain. However, since there were no previous molecular studies of any Sarcocystis sp. from sheep in Spain, we also took the opportunity to collect and examine microscopic sarcocysts in order to identify and characterise molecularly S. tenella and S. arieticanis from this country. Unexpectedly, a fifth species, similar in sarcocyst morphology to S. mihoensis in Japanese sheep (Saito et al. 1997), was also encountered, and it then became an important aim to characterise this species molecularly for the first time. The overriding aim of the molecular characterisation was to use the sequence data in phylogenetic analyses in order to determine the phylogenetic placement of all Sarcocystis spp. in sheep, particularly that of S. medusiformis and the S. mihoensis-like species.
Materials and methods
Collection of muscle samples: detection, isolation and microscopic examination of sarcocysts
Since the major objective was to collect and study macroscopic sarcocysts that might belong to S. gigantea and S. medusiformis, which are predominantly found in older sheep due to their slow development (Collins et al. 1979; Munday and Obendorf 1984; Obendorf and Munday 1987; Martínez-Navalón et al. 2012), a slaughterhouse that usually receives culled ewes was selected. This slaughterhouse was located in Villarejo de Salvanés, Province of Madrid, Central Spain. In September 2017, muscle samples found to harbour macroscopic sarcocysts at the general carcass inspection were collected from several ewes. For practical reasons and time constraints, the collected samples were pooled by the inspectors and not individually labelled with respect to which animal and anatomic location they originated from. Hence, the exact number of sampled ewes was unfortunately not recorded. Most of the samples were from the diaphragm and oesophagus, and a few were from the leg muscles. The samples were put in a freezer shortly after collection and kept frozen until studied at the Department of Animal Health, Complutense University of Madrid. At the laboratory, the muscle pieces were first sorted according to their anatomic origin, subdivided into smaller pieces (about 7 × 7 cm for diaphragm samples; about 7 cm in length for oesophagus) and refrozen inside labelled plastic bags at − 20 °C until further examined.
Thawed samples of each muscle region were subsequently examined for sarcocysts. Macroscopic cysts (grossly visible) were photographed and measured in situ with a ruler and classified according to their size and shape. Those intended for molecular identification were then isolated with scissors together with a small amount of the surrounding muscle tissue. After the macroscopic cysts had been excised, small portions of muscle were examined for the presence of microscopic sarcocysts by the compression technique used for detection of Trichinella larvae, as previously described (Luzón et al. 2015; Gjerde et al. 2017b). Briefly, sets of 28 oat-grain-sized muscle samples (about 2 × 10 mm) were compressed until they became translucent and then examined for sarcocysts using a light microscope at × 100 magnification. Individual samples containing sarcocysts were released from the glass plates of the compressorium and transferred to separate microscope slides, covered with a drop of distilled water and kept inside a moist chamber at 4 °C for 1–2 h. The moistened muscle samples were then examined under a stereo microscope at × 20–30 magnification, and the sarcocysts were excised from the muscle tissue using a fine insulin needle. The longest slender sarcocysts detected by means of the compression method (1–2 mm long) were designated large microscopic, since once detected, they were found to be barely visible even without magnification. Some of the microscopic sarcocysts, including the larger ones, were further examined in wet mounts under a Nomarski microscope at × 100 and × 400 magnification. Those completely or partially excised from the host cell (about 20 sarcocysts) were tentatively classified on the basis of the presence and appearance of surface protrusions according to previous cyst type descriptions of Sarcocystis spp. in sheep (Boch et al. 1979; Heydorn 1985; Saito et al. 1997; Hu et al. 2017). Digital photographs with a calibrated scale bar were taken of some of the sarcocysts at this stage. All isolated sarcocysts intended for molecular identification were placed individually into labelled 1.5-mL micro-centrifuge tubes containing 70% ethanol. In addition, some microscopic and macroscopic sarcocysts were fixed in glutaraldehyde in case a separate morphological study by electron microscopy would become possible. The majority of the collected sarcocysts were from diaphragm samples (4–18 macroscopic and 26–62 microscopic cysts per sample); seven macrocysts and 10 microcysts were from samples of oesophagus and two macrocysts were collected from leg muscles (Table 1). The isolated sarcocysts were shipped to the first author in Norway for molecular studies in two batches: the first batch, comprising 16 sarcocysts, was shipped in October 2017 and the second batch, comprising 87 sarcocysts, was shipped in February 2018. A total of 63 sarcocysts were subsequently examined by molecular methods, including all 16 sarcocysts from the first batch (OS1–OS16) and 47 sarcocysts (OS17–OS53; OS101–OS110) from the second batch (Table 1).
Molecular examination of sarcocysts
The molecular examination of sarcocysts was basically as described previously (Gjerde 2013, 2014b, 2016; Gjerde et al. 2017a, b). The alcohol in the tubes containing sarcocysts was allowed to evaporate at room temperature and then about 20 μL of distilled water was added to each tube. Finally, genomic DNA was extracted from 63 tubes using the QIAmp DNA Mini Kit (Qiagen, Germany) according to the manufacturer's tissue protocol as previously described (Gjerde 2013). The resulting DNA samples were kept frozen at − 20 °C in between their use as templates for PCR amplification of the complete 18S ribosomal (r) RNA gene, the partial 28S rRNA gene and/or the partial mitochondrial cytochrome c oxidase subunit I gene (cox1). From all available DNA isolates, attempts were made to amplify a 1085-bp-long portion of cox1 with primer pair SF1y/SR9 in order to identify the sarcocysts to species, or rather confirm the preliminary identification based on sarcocyst morphology. Four isolates of one species could not be amplified with reverse primer SR9, but were successfully amplified with reverse primer SR10, yielding 1149-bp-long amplicons. Primers SF77 and SR77 were designed and used as additional sequencing primers for the latter isolates, while primers SF6 and SR66 were used as additional sequencing primers for a few isolates of the other species. Following the identification of the sarcocyst isolates through sequencing of cox1, the complete 18S rRNA gene (1878–1930 bp) of 1–4 isolates of each of the five species found were amplified as two overlapping fragments using primer pairs ERIB1/S2r and S3f/Primer BSarc. Likewise, the partial 28S rRNA gene (about 1850 bp from the 5' end) was amplified from 1–3 isolates of each species using primer pair KL0/LS3R, while primers LS2F and LS2R were used as additional sequencing primers. The sequences of all primers used are given in Table S1 of the Supplementary material.
The PCR reactions were performed and the PCR products were evaluated and purified as described previously (Gjerde 2016). Purified amplicons were sent to Eurofins Genomics, Germany, for sequencing on both strands. Forward and reverse sequence reads, as well as overlapping sequences, were manually assembled into complete consensus sequences using the Alignment Explorer of the MEGA7 software (Kumar et al. 2016). Newly assembled sequences were compared with each other and with related sequences in GenBank using the Nucleotide BLAST program as previously described (Gjerde 2013). The software package DnaSP (DNA Sequence Polymorphism) version 5.10.01 (Librado and Rozas 2009) was used for the analysis of sequence variation and DNA divergence within and between different populations of cox1 sequences found in the present and previous studies, in order to characterize these populations and assign them to different species.
Phylogeny
Phylogenetic analyses were conducted separately on nucleotide sequences of cox1 and the 18S rRNA gene, respectively, by means of the MEGA7 software (Kumar et al. 2016). In both analyses, the phylogeny was tested with the bootstrap method, using 1000 bootstrap replications, and Toxoplasma gondii was used as outgroup species to root each tree. The GenBank accession numbers of all sequences included in the analyses are given behind the taxon names in the phylogenetic trees.
As regards cox1, a total of 97 partial sequences from 56 taxa were included in the final analysis, including 42 new sequences/haplotypes generated from the five Sarcocystis spp. found in the present study. Sequences longer than 1020 bp were truncated at their 3′ end, so that the final codon-based multiple alignment comprised 1020 positions with no gaps. The phylogenetic tree was reconstructed using the neighbour joining method, in which evolutionary distances were computed using the Kimura 2-parameter method. All codon positions were used. Gaps due to missing data (in a few sequences shorter than 1020 bp) were treated with the pair-wise deletion option. An additional analysis using 17 more sequences of S. tenella downloaded from GenBank was also run in order to determine the placement of these sequences relative to the new sequences of S. tenella from this study. They included 10 sequences from domestic sheep in Norway (KC209723–32), six sequences from Tatra chamois in Poland (KP263744–46, KP263749, KP263750–51) and one sequence (MH561854) from wild sheep in China.
Concerning the 18S rRNA gene, a total of 63 near full-length sequences from 55 taxa were used in the analysis, including seven new sequences of the five Sarcocystis spp. found in the present study. A multiple sequence alignment was generated with the ClustalW program integrated in MEGA7, using a gap opening penalty of 10 and a gap extension penalty of 0.2 for both the pair-wise and multiple alignments. The resulting alignment was checked by eye and slightly corrected for inconsistencies in the treatment of closely related taxa. Most sequences were truncated slightly at both ends, so that the majority of sequences could start and end at the same homologous nucleotide positions, corresponding to positions 75 and 1854, respectively, of GenBank sequence KY019032 of Sarcocystis linearis. The final alignment comprised 2118 aligned positions, including gaps. The phylogenetic tree was reconstructed using the maximum parsimony method with the subtree–pruning–regrafting algorithm. All sites were used.
Results
Sarcocyst morphology
Based on their size and visibility, three types of sarcocysts were found: macroscopic (grossly visible), large microscopic (barely visible grossly; 1–2 mm long) and microscopic. Based on their shape, the macroscopic sarcocysts comprised two types, which were designated thick and thin sarcocysts, respectively (Table 1). The thick sarcocysts were ovoid to sac-like with blunt-rounded ends, whereas the thin sarcocysts were slender and elongate with pointed ends (fusiform). The thick sarcocysts were found in all three muscle regions examined (Fig. 1), whereas the thin cysts were detected in the diaphragm and leg muscles, but not in the oesophagus (Fig. 2). The thick sarcocysts varied in size from 3.0 × 1.5 to 12.5 × 6.5 mm in the oesophagus, from 9.0 × 3.0 to 22.0 × 7.5 mm in the diaphragm and from 14.0 × 5.5 to 15.0 × 5.9 mm in the leg muscles. The thin sarcocysts varied in size from 5.0 × 0.6 mm to 7.0 × 0.9 mm in the diaphragm, while the single thin cyst found in the leg muscles (not included in the molecular study) measured 5.0 × 0.7 mm. Thus, thick sarcocysts in the oesophagus (Fig. 1a) were rounder than those in the diaphragm (Fig. 1b) and leg (Fig. 1c), whereas the shape of thin cysts was similar in the diaphragm and leg muscle (Fig. 2a,b). Thick and thin macroscopic cysts were tentatively assigned to S. gigantea (Fig. 1) and S. medusiformis (Fig. 2), respectively.
The type designated large microscopic was found in the diaphragm (Table 1), and comprised fairly thin cysts detected after compression of the muscle samples, but once detected by this method, they were found to be barely visible even without magnification. Wet mounts of four of these cysts, isolated from the same diaphragm sample, revealed slanting thorn-like surface protrusions, about 15 μm long (Fig. 3), which were similar to those of S. mihoensis (Fig. 2 in Saito et al. 1997). This species will therefore be referred to as S. mihoensis-like or S. cf. mihoensis in the following. The microscopic cysts were found in the oesophagus and diaphragm (Table 1). Wet mounts of some of those in the diaphragm revealed two morph types according to their surface, that is, cysts with short erect finger-like protrusions, giving the surface a fuzzy appearance (Fig. 4a), and cysts with delicate hair-like protrusions (Fig. 4b). These morph types were tentatively assigned to S. tenella and S. arieticanis, respectively. In addition, some microcysts had no clearly discernible protrusions, but this was likely due to the cysts still being surrounded by a thin layer of host cell material.
Light microscopic appearance of a sarcocyst of S. cf. mihoensis in a wet mount. a Portion of a sarcocyst that measured about 2 × 0.45 mm, showing slanting thorn-like surface protrusions (arrows). Scale bar = 50 μm. b Higher magnification of the protrusions (arrow) on the same cyst. Scale bar = 10 μm
Light microscopic appearance of sarcocysts of S. tenella and S. arieticanis in wet mounts. a Portion of a slender sarcocyst of S. tenella with a 2–4-μm thick cyst wall (arrows) consisting of densely packed finger-like protrusions. b Portion of a damaged sarcocyst of S. arieticanis with delicate hair-like protrusions (arrows). Scale bars = 20 μm
Molecular characterisation
A total of 63 sarcocysts were examined by molecular methods through PCR amplification and direct sequencing of the PCR products. All 63 isolates were initially examined at cox1, while 16 and nine of the isolates were further examined at the 18S rRNA gene and the 28S rRNA gene, respectively (Table 2). The molecular characterisation revealed that the 63 sarcocysts belonged to five Sarcocystis spp., that is, S. arieticanis (N = 2), S. tenella (N = 36), S. gigantea (N = 10), a species presumed to be S. medusiformis (N = 11), and a species (N = 4) that had not been characterised molecularly before, but which was S. mihoensis-like based on its sarcocyst morphology (Tables 1 and 2). The molecular identification in most instances corroborated the preliminary identification based on sarcocyst morphology. The 21 microscopic sarcocysts from the diaphragm that had not been examined in wet mounts following isolation, all belonged to S. tenella (Table 1). A total of 54 different nucleotide sequences were submitted to GenBank and were assigned accession numbers MK419975–MK420028 (Table 2). Details about which GenBank sequence each sarcocyst isolate was associated with is given in Table S2 in Supplementary material as regards the cox1 sequences and in Table S3 in Supplementary material with respect to the 18S and 28S rRNA gene sequences. The characteristics of the five species at each of the three DNA regions examined will be described in the following.
Cox1 gene
The partial cox1 of 57 isolates belonging to S. arieticanis, S. tenella, S. gigantea and S. medusiformis were successfully amplified with primer pair SF1y/SR9, yielding 1038-bp-long sequences exclusive of primers. Two isolates of S. tenella, both with low concentrations of Sarcocystis DNA, were poorly amplified with this primer pair, and were therefore amplified and sequenced with primer pair SF1y/SR66 (656- and 644-bp long). The four isolates of S. cf. mihoensis could not be amplified with primer pair SF1y/SR9, but were successfully amplified with primer pair SF1y/SR10, yielding 1092-bp-long sequences exclusive of primers.
Two microscopic sarcocysts could be assigned to S. arieticanis on the basis of their cox1 sequences. These sequences (MK419975–76) differed from each other at three of 1038 nucleotide positions (99.7% identity), whereas they differed at 10 and 13 positions (98.8–99.0 % identity) from sequence MF039324 of S. arieticanis from domestic sheep in China (Hu et al. 2017). With respect to other species, they shared the highest identity (92.4–92.6%) with sequences (KU820975–76) of Sarcocystis hircicanis from domestic goats in China. Thirty-six microscopic sarcocysts were identified as belonging to S. tenella from the generated cox1 sequences. The 34 sequences amplified with primer pair SF1y/SR9 comprised 34 haplotypes, which differed from each other at 1–20 of 1038 nucleotide positions (98.1–99.9% identity; on average 99.2%). The two shorter sequences obtained from two other isolates represented two additional haplotypes, but these sequences were not submitted to GenBank and were not included in the sequence comparisons due to their short length. In the 1038-bp-long sequences, 52 nucleotide positions were variable (polymorphic); at 25 of these sites, only a single sequence differed from the others, while at 27 polymorphic sites, two or more sequences differed from the majority (i.e. parsimony informative sites). At 44 variable sites, there were two possible character states (nucleotides), and at eight sites, there were three possible states. Most of the variation represented silent mutations, and thus 26 of the 34 haplotypes encoded the same amino acid sequence. The 34 submitted sequences of S. tenella (MK419977–MK420019) differed from all 21 previous cox1 sequences of this species in GenBank. They were most similar to 10 sequences/haplotypes (KC209723–32; 98.2–99.8% identity) from domestic sheep in Norway (Gjerde 2013), to eight sequences comprising six haplotypes (KP263744–51; 98.1–99.9% identity) from Tatra chamois (Rupicapra rupicapra tatrica) in Poland (Kolenda et al. 2015), and to a sequence (MH561854; 98.5–99.5% identity) from a wild sheep (Ovis ammon) in China, whereas they differed considerably from two sequences/haplotypes (MF039322–23; 96.6–97.5% identity) from domestic sheep in China (Hu et al. 2017). Most of the latter nucleotide differences were, however, due to silent mutations. Among the 968 nucleotide positions covered by all 55 available sequences of S. tenella, there were 81 variable sites, of which 47 were parsimony informative. In comparison to other species, the 34 new sequences of S. tenella were most similar to sequences of Sarcocystis capracanis (KU820974, KU820977) from goats in China, having an identity of about 93%, whereas they shared an identity of 81.5–82.6% with the two new sequences of S. arieticanis.
Ten thick macroscopic sarcocysts could be assigned to S. gigantea on the basis of cox1. The 10 new sequences represented three haplotypes; each type comprising five, two and three sequences, respectively (MK420011–13). These haplotypes differed from each other at 1–3 of 1038 nucleotide positions (99.7–99.9% identity). The most common haplotype (MK420011) was identical in the overlapping region with the single haplotype of S. gigantea found in four isolates from sheep in Norway (KC209601–04) (Gjerde 2013), whereas the two other new haplotypes differed at one or two positions from the previous cox1 sequences of S. gigantea (99.8–99.9% identity).
The cox1 sequences obtained from 11 thin fusiform macroscopic sarcocysts comprised two haplotypes. Ten sequences were identical (represented by GenBank sequence MK420014) and differed at only one nucleotide position (99.9% identity) from the remaining sequence (MK420015). These sequences did not match any previous cox1 sequences in GenBank, and shared the highest identity (87.8%) with the new (MK420011–13) and the previous (KC209601–04) sequences of S. gigantea. Since the gross appearance of these sarcocysts matched the original descriptions of S. medusiformis (Collins et al. 1979; Obendorf and Munday 1987), these sequences were assigned to this species (see Discussion).
The four cox1 sequences obtained from the four S. mihoensis-like sarcocysts were identical (MK420016), and differed considerably from all previous cox1 sequences in GenBank. They shared the highest identity with those of Sarcocystis dehongensis (80%) from water buffaloes and those of Sarcocystis frondea (78%), Sarcocystis oviformis (77–78%), Sarcocystis ovalis (77–78%) and Sarcocystis hardangeri (76–77%) from various cervid intermediate hosts (sika deer, roe deer, moose, red deer, reindeer). They shared an identity of only 59.3–62.7% with the new cox1 sequences of the four other species in sheep.
18S rRNA gene
The complete 18S rRNA gene was amplified and sequenced from one isolate of S. arieticanis. The resulting sequence (MK420017) was 1882-bp long and shared an identity of 99.8–99.9% with two slightly shorter sequences (MF039330–31) of this species from domestic sheep in China (Hu et al. 2017). The complete gene (1878 bp) was amplified and sequenced from four isolates of S. tenella. Three of the sequences were identical (MK420018) and differed from the forth sequence (MK420019) at only one nucleotide position. These haplotypes shared an identity of 99.9–100% with four sequences (KC209734–37) of S. tenella from domestic sheep in Norway (MK420019 being identical with KC209737), and had a slightly lower identity (99.7–99.9%) with sequences of this species from domestic sheep in China (MF039329) and from Tatra chamois in Poland (KP263752–59).
The complete 18S rRNA gene (1910 bp) was amplified and sequenced from three isolates of S. gigantea and all sequences turned out to be identical (MK420020). This haplotype was also identical with sequence KC209733 of S. gigantea obtained from domestic sheep in Norway (Gjerde 2013), but differed by about 1% from sequence L24384 of S. gigantea from sheep in Australia, and by 1.2% from sequence L76473 of Sarcocystis moulei from a goat in Germany. Similarly, the complete 18S rRNA gene (1928 bp) was amplified and sequenced from four putative isolates of S. medusiformis, yielding four identical sequences (MK420021). These sequences shared an identity of 96.8% with the new sequence of S. gigantea (MK420020) and an identity of 96.9% with sequence L76473 of S. moulei, whereas there were no previous sequences of similar length in GenBank completely matching the new sequence of S. medusiformis. However, a 467-bp-long sequence (KX223754) from sheep in Sardinia attributed to S. medusiformis (Pipia et al. 2016) was identical in the overlapping region except at the first three nucleotides and a missing nucleotide near the 3′ end, but these differences seemed to be due to sequencing errors in that sequence. Moreover, our sequences and sequence KX223754 included an ~ 50-bp-long variable region of the gene, which was clearly different from the homologous region of the 18S rRNA gene of S. gigantea and S. moulei, and we therefore assigned the four sequences of this type and the corresponding sarcocysts to S. medusiformis. Sequence similarity searches using BLAST further identified a 944-bp-long sequence (KP053891) derived from sheep in Iran, which had been assigned to S. moulei (Kalantari et al. 2016), but which shared an identity of 99.8% with our sequences of S. medusiformis. That sequence also comprised the abovementioned variable region, as well as an additional variable region separating S. medusiformis from S. gigantea and S. moulei. The complete 18S rRNA gene was amplified and sequenced from four isolates of S. cf. mihoensis. In the 3′ end half of the gene of all isolates, there was a 1-bp-long indel, which caused the forward and reverse sequences to become mixed from that point onwards (two juxtaposed sequence variants being superimposed). The indel was located within a homo-polymer consisting of either 10 or 11 consecutive T’s (thymine bases) followed by a second homo-polymer of eight consecutive A’s (adenine bases). Hence, the complete gene was either 1929- or 1930-bp long and these variants are represented by sequences MK420022 and MK420023, respectively (extra T at position 1475 in MK420023). The new sequences of S. cf. mihoensis shared the highest identity (92.8–93.3%) with sequences of S. dehongensis from water buffalos in China (KY711373–75), and a slightly lower identity with sequences of S. ovalis, S. oviformis and S. hardangeri from various cervid intermediate hosts.
28S rRNA gene
One isolate of S. arieticanis was amplified and sequenced, but all sequences were of poor quality, apparently because of co-amplification of an organism different from Sarcocystis even though the reverse primer used (LS3R) was believed to target only this genus. Two isolates of S. tenella were amplified and sequenced. The resulting sequences were of variable quality due to indels. Those from one isolate comprised several indels, causing highly mixed sequences, and only portions of these sequences could be estimated. Those from the other isolate were of good quality except some noise caused by two indels in a reverse sequence, but the entire amplified region (1855 bp) could be estimated and the consensus sequence submitted to GenBank (MK420024). The new sequence shared the highest identity (99.2%) with a sequence of a German strain of S. tenella (AF076899); part of the difference being due to two insertions in the region where two indels had been recognised in our isolate. Our sequence differed by an additional indel and several substitutions from sequences of S. tenella from sheep in China (MF039326–27; 98.3–98.6% identity) and Egypt (MH413037–39; 97.7–97.9% identity).
Two isolates of S. gigantea were amplified and sequenced. The resulting sequences were identical and the longest one (1820 bp) was submitted to GenBank (MK420025). This sequence differed at only three of 1818 overlapping nucleotides (99.8% identity) from sequences U85706 and AF044250 of S. gigantea. The latter sequence is actually attributed to S. arieticanis, but is the reverse complement of sequence U85706 of S. gigantea. The new sequence was 97.9% identical with the overlapping portion of sequence AF012884 of S. moulei from goats. Three isolates of S. medusiformis were amplified and sequenced, yielding three identical sequences, which are represented by sequence MK420026 (1847 bp). There were no previous sequences of the 28S rRNA gene of S. medusiformis in GenBank, but the new sequence was 95.4% identical with the new sequence of S. gigantea and 95.0% identical with sequence AF012884 of S. moulei.
Two isolates of S. cf. mihoensis were amplified and sequenced. The forward and reverse sequence chromatograms showed two double peaks at the same sites in both isolates. At these polymorphic sites, there were both G (guanine) and A, but since G was more prominent than A in isolate OS25, and vice versa in isolate OS26, sequence MK420027 from the former isolate has G at both positions (nucleotides 536 and 571), whereas sequence MK420028 from the other isolate has A at both positions. Thus, the two sequences differ at two of 1837 nucleotide positions (99.9% identity). The two new sequences shared the highest identity with S. oviformis (KJ396592; 88.5%), S. ovalis (KJ396588; 88.0%) and S. hardangeri (KJ396589; 86.8%).
Phylogeny
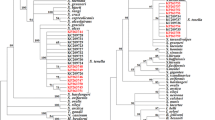
The phylogenetic analysis based on cox1 sequences (Fig. 5) placed sequences of various Sarcocystis spp. with ruminant intermediate hosts into three major clades according to their known or presumed definitive hosts, that is, corvids, felids/humans/unknown and canids. Basal to these clades was a clade comprising species with carnivores or birds as intermediate hosts. The new sequences of S. arieticanis and S. tenella were placed within the canid clade and clustered with previous sequences of the respective species. Those of S. arieticanis were sister to S. hircicanis, but these taxa did not cluster together with Sarcocystis spp. of cervids with similar hair-like cyst wall protrusions. The new sequences of S. tenella were clearly separated from two sequences of this species from domestic sheep in China, which was also true in the expanded analysis comprising 17 additional sequences of S. tenella, but in the latter analysis, the new sequences were interspersed with the additional sequences from domestic sheep in Norway, the Tatra chamois in Poland and wild sheep in China (data not shown). The two new sequences of S. gigantea clustered with the previous sequence of this species within the felid clade, and these sequences were sister to the two new sequences of S. medusiformis. The new sequence of S. cf. mihoensis was placed within a clade also comprising S. dehongensis from water buffaloes and S. frondea, S. hardangeri, S. ovalis and S. oviformis from cervid intermediate hosts. In this clade, S. cf. mihoensis was a sister taxon to the five other species.
Phylogenetic tree for selected Sarcocystis spp. based on 97 partial cox1 sequences from 56 taxa and inferred using the neighbour joining method with evolutionary distances computed employing the Kimura 2-parameter method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Subtrees formed by two or more sequences of some species have been collapsed, but the number of sequences included may be inferred from the GenBank accession numbers given behind the taxon names. Sequences from the five Sarcocystis spp. found in the present study are in boldface
The phylogenetic analysis based on sequences of the 18S rRNA gene (Fig. 6) showed a similar placement of and relationship between the species as that based on cox1. Thus, Sarcocystis spp. of ruminants were placed into three major clades, and the new sequences of S. arieticanis, S. tenella and S. gigantea clustered with previous sequences of the respective species. The new sequence of S. medusiformis was sister to sequences of both S. gigantea and S. moulei. The new sequences of S. cf. mihoensis were placed in a clade comprising the same six species as in the cox1-based analysis, but S. cf. mihoensis was now a sister taxon to S. dehongensis only.
Phylogenetic tree for selected Sarcocystis spp. based on 63 near complete sequences of the 18S rRNA gene from 55 taxa and inferred using the maximum parsimony method with the subtree–pruning–regrafting algorithm. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The GenBank accession numbers of the sequences are given behind the taxon names. Sequences from the present study are in boldface
Discussion
The present study identified by gross examination and by light microscopy of sarcocysts in wet mounts five sarcocyst types, which by the subsequent molecular examination at three loci were found to represent five distinct Sarcocystis spp. Sequences from three of these species could be unambiguously assigned to the species S. arieticanis, S. tenella and S. gigantea, respectively, since they closely matched many previous sequences in GenBank of these species at the three markers used. Moreover, the morphology of the respective sarcocysts also matched previous descriptions of these species. The molecular identification of S. arieticanis, S. tenella and S. gigantea in this study confirms their presence in sheep in Spain as suggested in previous studies using sarcocyst morphology for identification (Díez-Baños 1978; Sánchez-Acedo et al. 1983; Simón-Vicente and Ramajo-Martín 1984; Martínez-Moreno et al. 1989; Babín-Vich 1991; Martínez-Navalón et al. 2012).
The size, shape and macroscopic appearance of the thin fusiform type of macroscopic sarcocysts were consistent with the original descriptions of S. medusiformis from sheep in New Zealand and Australia (Collins et al. 1976, 1979; Obendorf and Munday 1987), but a definitive identification of S. medusiformis on the basis of sarcocyst morphology would have required an examination of some sarcocysts of this type by TEM. Thus, while sarcocysts of S. gigantea have highly branched cauliflower- or papilloma-like protrusions, those of S. medusiformis seem to have protrusions with a more compact (less branched) main body, but from which numerous thin filaments seem to project when viewed by TEM (Collins et al. 1979; Obendorf and Munday 1987). Another distinguishing feature between S. gigantea and S. medusiformis is that the muscle cell harbouring sarcocysts of the former species become encapsulated by host-derived connective tissue (inaccurately termed a secondary cyst wall), whereas the host cells of S. medusiformis sarcocysts do not become encapsulated. The presence of a secondary cyst wall may be ascertained through Periodic acid–Schiff (PAS) staining of sarcocysts in histological sections or through TEM of sarcocysts excised and fixed along with a small amount of the adjacent tissue (Moore 1980; Munday and Obendorf 1984; Obendorf and Munday 1987). Although we could not confirm by TEM that the fusiform macroscopic cysts actually belonged to S. medusiformis, we are fairly confident that they did so on the basis of their gross appearance, molecular characteristics and phylogenetic placement, and since they definitely did not belong to S. gigantea. Thus, these sarcocysts were genetically different at all three loci examined from the thick/fat sarcocysts with blunt ends that belonged to S. gigantea. Specifically, the sequences attributed to S. medusiformis shared an identity of 87.8%, 96.8% and 95.4% with sequences of S. gigantea at cox1, the 18S and the 28S rRNA gene, respectively. In addition, the new 18S rRNA gene sequences assigned to S. medusiformis were nearly identical in the overlapping region with a 467-bp-long sequence (KX223754) derived from similar slender, fusiform sarcocysts from sheep in Sardinia, Italy, which Pipia et al. (2016) likewise believed belonged to S. medusiformis, since they differed both morphologically and at the partial 18S rRNA gene from S. gigantea. In both of our phylogenetic analyses (Figs. 5 and 6), sequences assigned to S. medusiformis were sister to sequences of S. gigantea within a major clade comprising species with felids as known or presumed definitive hosts. This placement supports our contention that these sequences indeed belong to S. medusiformis, which have been shown to use cats as definitive hosts (Collins et al. 1976, 1979; Obendorf and Munday 1987). Still, it would be of interest to examine some slender fusiform sarcocysts in sheep both by TEM and molecular methods (either different portions of individual cysts or different cysts from the same sheep) in order to link data on sarcocyst ultrastructure directly to molecular data and thus presumably confirm that the sequence types assigned to S. medusiformis in this study originates from a species with the ultrastructural features of S. medusiformis as described by Collins et al. (1979).
The putative identification of S. medusiformis in this study confirms previous suspicions based solely on the gross appearance of sarcocysts that this species is present in sheep in Spain (Martínez-Moreno et al. 1987; Babín-Vich 1991; Martínez-Navalón et al. 2012). Thus, the slender type of macroscopic sarcocysts, 2–10 × ≤ 1 mm in size, described by Martínez-Navalón et al. (2012) probably belonged to S. medusiformis. It is possible that S. medusiformis may have a wider distribution in Europe than in Spain and Sardinia, but its slender sarcocysts might have escaped detection in many studies, possibly partly due to the concurrent presence of the more prominent sarcocysts of S. gigantea. In addition to its occurrence in Spain, Italy, New Zealand and Australia, S. medusiformis has been reported from sheep in Iran based on gross observations of slender fusiform sarcocysts consistent with this species (Oryan et al. 1996; Farhang-Pajuh et al. 2014), and allegedly also by the PCR-RFLP method applied to the 18S rRNA gene (Farhang-Pajuh et al. 2014), but the latter results were not confirmed through sequencing of PCR products. Our sequence similarity searches identified, however, a partial 18S rRNA gene sequence (KP053891) derived from a macroscopic sarcocyst in sheep (Kalantari et al. 2016), which had been assigned to S. moulei, but which clearly belonged to the species identified as S. medusiformis in our study. Thus, sequence KP053891 shared an identity of 99.8% with our new sequence assigned to S. medusiformis (MK420021), but an identity of only 95.0% with GenBank sequence L76473 of S. moulei from goats.
In one sample of the diaphragm, we found four fusiform sarcocysts, 1–2 mm long, which possessed slanting, thorn-like, about 15-μm long surface protrusions (Fig. 3). Thus, they resembled sarcocysts of S. mihoensis reported from sheep in Japan (Saito et al. 1997), and they have therefore been referred to as S. mihoensis-like in this paper. However, several other Sarcocystis spp. with similar surface protrusions have been described from various ruminant intermediate hosts belonging to the families Bovidae, Cervidae and Tragulidae as summarised in Table 3. These species have been examined by various methods including light microscopy of fresh sarcocysts in wet mounts (as in this study), TEM, scanning electron microscopy (SEM) and molecular characterisation at one or more loci. The studies listed in Table 3 have revealed that the species S. hardangeri, S. ovalis and S. oviformis from cervid intermediate hosts have macroscopic oval sarcocysts, and their host cells become encapsulated by fibrillar material, whereas the other species have smaller fusiform sarcocysts, which do not seem to induce an encapsulation of their host cells. In all species, the slanting protrusions are thorn-like or tongue-like in shape, that is, they have a broad/thick base and become gradually attenuated towards their tips. These protrusions seem to be more densely packed on the surface of the smaller fusiform sarcocysts than on the larger oval ones. Moreover, in species examined by TEM, the protrusions have been shown to possess a central thick bundle of microtubules. The four species of this morphological type found in cervid intermediate hosts (S. hardangeri, S. ovalis, S. oviformis, S. frondea), as well as S. dehongensis from the water buffalo, have been well characterised molecularly, and may thus be easily identified by molecular methods. The application of such methods has revealed that some of these species are not intermediate host specific; S. hardangeri having been found in reindeer and red deer, and S. ovalis in moose, red deer and sika deer (Table 3).
Both our phylogenetic analyses (Figs. 5 and 6) placed the S. mihoensis-like species in a clade comprising the abovementioned four species from cervids plus S. dehongensis from water buffalos. Hence, it might be expected that the sarcocyst ultrastructure of the S. mihoensis-like species from this study is closely similar to that of the other species in this clade, as well as to that of the other species listed in Table 3, including S. mihoensis. In addition, the S. mihoensis-like species might be expected to use the same definitive hosts as the five other species in this clade. So far, the definitive hosts of only S. ovalis have been determined and found to be corvids (Gjerde and Dahlgren 2010; Irie et al. 2017), but it is likely that the same applies to the other species, including the S. mihoensis-like species. Saito et al. (1997) claimed, however, that they had successfully infected two dogs with sarcocysts of S. mihoensis from sheep. In the ‘Material and Methods’ section, they stated that the two dogs ‘were fed with 15 fresh cysts together with a small amount of food’, but under the section ‘Results’, they stated that the dogs had been ‘orally inoculated with muscle infected with cysts’. Hence, it seems that the dogs were actually fed muscle tissue containing sarcocysts, rather than excised and isolated sarcocysts. The muscle tissue fed to these dogs may therefore have contained undetected microscopic sarcocysts of S. tenella and/or S. arieticanis, which are species cycling via dogs, in addition to the larger sarcocysts of S. mihoensis, and the sporocysts shed by these dogs may have belonged to one or both of the former species. Foreyt (1989) found a Sarcocystis sp. with the same sarcocyst type as S. mihoensis in mountain goats (Oreamnos americanus) in Washington, USA, and tried to establish its definitive host through feeding of fresh infected muscle tissue (tongue) to four coyotes (Canis latrans), eight domestic dogs, four domestic cats, three black bears (Ursus americanus), two raccoons (Procyon lotor), two martens (Martes americana), two fishers (Martes pennanti), three skunks (Mephitis mephitis), five mink (Mustela vison), five ferrets (Mustela putorius), one pig-tail macaque (Macaca nemestrina), two red-tailed hawks (Buteo jamaicensis) and one great horned owl (Buto virginianus), but oocysts or sporocysts were not detected in the faeces of any of them. The failure to infect any of these animals, including dogs and coyotes, strongly suggests that dogs do not act as definitive host of S. mihoensis as claimed by Saito et al. (1997), but that species with this sarcocyst type indeed may cycle via corvids as shown for S. ovalis (Gjerde and Dahlgren 2010). The use of corvids as definitive hosts may explain why species having this sarcocyst type are so rare in domestic ruminants like sheep (this study; Saito et al. 1997), cattle (Novak et al. 1987; Saito et al. 2000) and water buffalos (Chen et al. 2017), but more prevalent in free-ranging wild ruminants, whose carcasses are frequently scavenged upon by corvids and whose environment may become more easily contaminated with sporocysts shed in the faeces of such birds.
In the present paper, we have used the designation S. mihoensis-like for the species resembling S. mihoensis as described by Saito et al. (1997) because S. mihoensis is the only species with this type of sarcocysts previously found in sheep. However, a species with the same sarcocyst ultrastructure as S. mihoensis has been found in chamois (Rupicapra rupicapra) and the alpine ibex (Capra hircus) in Europe. This species was named Sarcocystis cornagliai by Odening et al. (1996) based on examination of a few small sarcocysts found in the oesophagus and diaphragm of chamois from Germany and Austria, but the authors considered this species to be identical with an unnamed Sarcocystis sp. (type 2) found in the diaphragm of chamois in the north-western Italian Alps by Cornaglia et al. (1980). Cornaglia et al. (1998) later assigned the same name to a morphologically indistinguishable species in the alpine ibex, since they believed that the same species might infect both intermediate hosts. That might be the case, since, as noted above, the related species S. ovalis and S. hardangeri have been found in three and two different intermediate hosts, respectively (Table 3). Moreover, it is likely that sheep may act as an additional intermediate host of S. cornagliai, since the chamois has already been found by molecular methods to be an intermediate host of S. tenella (Kolenda et al. 2015). This question can only be resolved through molecular characterisation of S. cornagliai in chamois and the similar species in the alpine ibex. If such studies should show that S. cornagliai in chamois is identical with the S. mihoensis-like species in sheep from this study, S. cornagliai would become the valid name of this species. That would be the case even if future molecular studies of S. mihoensis-like sarcocysts from sheep in Japan should show that they are genetically identical with S. cornagliai, since the latter name was published some months before the name S. mihoensis (Odening et al. 1996; Saito et al. 1997), and therefore has priority.
As regards the possible occurrence of a S. mihoensis-like species in sheep in other European countries, it should be noted that Bratberg et al. (1982) found a few thick-walled sarcocysts in histological sections of the diaphragm of three 4–5-month-old lambs slaughtered at an abattoir in south-western Norway. The sarcocyst wall was 5–6-μm thick with prominent radial striations (see Fig. 10 in their paper). This appearance is similar to that of sectioned sarcocysts of S. mihoensis in sheep (Fig. 1 in Saito et al. 1997), of S. cornagliai in chamois (Fig. 5 in Odening et al. 1996), and of an unnamed Sarcocystis sp. in cattle (Fig. 1 in Saito et al. 2000) and mountain goats (Fig. 2 in Foreyt 1989). In all of these species, the thick sarcocyst wall seen in histological section was shown by TEM to represent thorn-like or tongue-like protrusions. The thick-walled sarcocysts found in a single sheep from Sicily, Italy, by Giannetto et al. (2005), on the other hand, possessed erect finger-like (columnar) protrusions with loosely packed microtubules, and thus clearly belong to a different species. The authors believed those sarcocysts were Sarcocystis gracilis-like since they resembled sarcocysts in roe deer attributed to this species by Entzeroth (1985). However, as pointed out previously (Dahlgren and Gjerde 2008; Gjerde 2012; Gjerde et al. 2017a), Entzeroth (1985) actually described two species, of which the one with finger-like protrusions examined by light microscopy and TEM was not S. gracilis, but probably the species later named Sarcocystis silva by Gjerde (2012). The sarcocysts found by Giannetto et al. (2005) are morphologically similar not only to S. silva from roe deer, but also to Sarcocystis hominis and Sarcocystis bovifelis in cattle (Gjerde 2016); hence, the infection of this sheep may have originated from cattle (via sporocysts from humans or cats) rather than from roe deer.
The 34 isolates of S. tenella successfully characterised at cox1 comprised 34 different haplotypes, which differed from each other at 1–20 of 1038 nucleotide positions (98.1–99.9% identity). These haplotypes differed from all 21 previous cox1 sequences of this species in GenBank. Similarly, in the first study of cox1 of Sarcocystis spp., the 10 isolates of S. tenella from Norwegian sheep represented 10 haplotypes (Gjerde 2013), whereas Kolenda et al. (2015) found six haplotypes among eight isolates of S. tenella from Tatra chamois in Poland. Additionally, the new sequences shared an identity of only 96.6–97.5% with two haplotypes from domestic sheep in China (Hu et al. 2017). Thus, there seems to be considerable variation in cox1 of S. tenella, resulting in a high number of haplotypes and a fairly large intraspecific variation. A detailed comparison of the sequences showed that there were many variable sites, and that some of these could have three possible character states. Hence, there were many possible nucleotide combinations. However, most of the substitutions represented silent mutations causing no changes in the inferred amino acid sequences. Still, several other Sarcocystis species from which many sarcocyst isolates from different host animals have been characterised, have shown a much lower variation at this gene. For instance, the ratio between the number of isolates examined and the number of haplotypes found (i/h) were 22/1 and 17/2 for S. ovalis from Norwegian moose and red deer, respectively (Gjerde 2014a); 22/10 and 24/15 for Sarcocystis gracilis in Norwegian and Italian roe deer, respectively (Gjerde 2014a; Gjerde et al. 2017a); and 45/25, 24/16 and 56/13 for Sarcocystis bovifelis, Sarcocystis bovini and Sarcocystis hirsuta, respectively, in cattle (Gjerde 2016). In the present study, there seemed to be little variation at cox1 of S. gigantea (i/h = 10/3), S. medusiformis (i/h = 11/2) and the Sarcocystis mihoensis-like species (i/h = 4/1), but since the sarcocysts of the latter species were obtained from a single sample, the lack of variation found may not be representative of this species. However, it appears that some Sarcocystis spp. in ruminants are more variable at cox1 than others, both with respect to the magnitude of the intraspecific variation (sometimes exceeding 2%) and the number of possible haplotypes. This should be kept in mind when trying to assign a given sequence to a particular species.
The phylogenetic analyses (Figs. 5 and 6) placed sequences of Sarcocystis tragulusi from Williamson’s mouse deer (Tragulus williamsoni; Tragulidae) in the clade comprising species with felids as known or presumed definitive hosts in spite of the fact that the sarcocysts attributed to this species were S. mihoensis-like when viewed by TEM (Fig. 1b, c in Hu et al. 2016). Those authors obtained the same placement and therefore suggested that felids might be the definitive hosts of this species. However, in phylogenetic analyses, Sarcocystis spp. of ruminants tend to cluster first according to their definitive host and then according to their sarcocyst morphology within one of the major clades. Moreover, a particular type of sarcocyst morphology seems to be confined to a particular group of definitive hosts (felids, canids, corvids), that is, a particular sarcocyst type is not shared by Sarcocystis spp. using different groups of definitive hosts. We therefore suspect that the unexpected placement of S. tragulusi in the felid rather than the corvid clade given its reported sarcocyst ultrastructure, is due to two different species having been examined molecularly and by TEM, respectively, that is sarcocysts of a S. mihoensis-like species might have been processed for TEM, while similar sarcocysts from a different species were processed for molecular studies, and possibly also for light microscopy (Fig. 1a in Hu et al. 2016).
In summary, the present study has identified five Sarcocystis spp. through sampling of a few sheep from a small geographic area of Spain, which was quite unexpected, but fortunate. The study has confirmed the presence of S. arieticanis, S. tenella, S. gigantea and S. medusiformis in sheep in Spain, and has provided additional molecular data on the former three species and the first comprehensive molecular characterisation of the latter species. Further, the study has detected for the first time in sheep in Europe, a species that was morphologically similar to S. mihoensis from sheep in Japan, but also to S. cornagliai from chamois in Europe, neither of which has been characterised molecularly. Hence, the S. mihoensis-like species from the present study cannot be definitely assigned to any of these species until S. cornagliai from chamois has been characterised molecularly in a similar manner. The phylogenetic analyses based on the 18S rRNA gene and cox1 both suggested that corvids act as definitive hosts for the S. mihoensis-like species from this study, which may explain the rare occurrence of this species in domestic sheep worldwide compared to S. arieticanis and S. tenella, which are transmitted by dogs and other canids, and S. gigantea and S. medusiformis, which are transmitted by cats and other felids.
References
Babín-Vich M (1991) Epidemiología y patogenia de la sarcocystosis ovina (Epidemiology and pathogenesis of ovine sarcocystosis) [In Spanish]. Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Doctoral Thesis Available at https://eprints.ucm.es/3395/
Bergmann V, Kinder E (1975) Unterschiede in der Struktur der Zystenwand bei Sarkozysten des Schafes. Monatsh Veterinarmed 31:772–774
Boch J, Bierschenk A, Erber M, Weiland G (1979) Sarcocystis- und Toxoplasma-Infektionen bei Schlactschafen in Bayern. Berl Münch Tierärztl Wschr 92:137–141
Bratberg B, Helle O, Hilali M (1982) Sarcocystis infection in sheep from south-western Norway. Acta Vet Scand 23:221–234
Chen X, Wen T, Hu J, Liu T, Esch GW, Liang Y, Li H, Huang S (2017) Sarcocystis dehongensis n. sp. (Apicomplexa: Sarcocystidae) from water buffalo (Bubalus bubalis) in China. Parasitol Res 116:2145–2150. https://doi.org/10.1007/s00436-017-5515-x
Collins GH, Charleston WAG, Moriarty KM (1976) Sarcocystis species in sheep [Letters to the editor]. N Z Vet J 24:123–124. https://doi.org/10.1080/00480169.1976.34299
Collins GH, Atkinson E, Charleston WAG (1979) Studies on Sarcocystis species. III: the macrocystic species of sheep. N Z Vet J 27:204–206. https://doi.org/10.1080/00480169.1979.34651
Colwell DD, Mahrt JL (1981) Ultrastructure of the cyst wall and merozoites of Sarcocystis from moose (Alces alces) in Alberta, Canada. Z Parasitenkd 65:317–329. https://doi.org/10.1007/BF00926727
Cornaglia E, Guarda F, Guarda F, Misciattelli ME (1980) Ricerca, frequenza, diagnosi, morfologia istopatologica ed ultrastrutturale della sarcosposporidiosi cardiaca nei camosci e stambecci. Ann Fac Med Vet Torino 27:279–296
Cornaglia E, Giaccherino AR, Peracino V (1998) Ultrastructural morphology of sarcosporidiosis in alpine ibex (Capra ibex). Vet Parasitol 75:21–32. https://doi.org/10.1016/S0304-4017(97)00185-4
Dahlgren SS, Gjerde B (2007) Genetic characterisation of six Sarcocystis species from reindeer (Rangifer tarandus tarandus) in Norway based on the small subunit rRNA gene. Vet Parasitol 146:204–213. https://doi.org/10.1016/j.vetpar.2007.02.023
Dahlgren SS, Gjerde B (2008) Sarcocystis in moose (Alces alces): molecular identification and phylogeny of six Sarcocystis species in moose, and a morphological description of three new species. Parasitol Res 103:93–110. https://doi.org/10.1007/s00436-008-0936-1
Dahlgren SS, Gjerde B (2009) Sarcocystis in Norwegian roe deer (Capreolus capreolus): molecular and morphological identification of Sarcocystis oviformis n. sp. and Sarcocystis gracilis and their phylogenetic relationship with other Sarcocystis species. Parasitol Res 104:993–1003. https://doi.org/10.1007/s00436-008-1281-0
Dahlgren SS, Gjerde B (2010) Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitology 137:815–840. https://doi.org/10.1017/S0031182009991569
Díez-Baños P (1978) Sobre la prevalencia de la sarcosporidiosis ovina en la provincia de León, con un estudio comparativo de diversos métodos diagnósticos [In Spanish]. An Fac Vet León 24:195–199
Entzeroth R (1985) Light-, scanning-, and transmission electron microscope study of the cyst wall of Sarcocystis gracilis Rátz, 1909 (Sporozoa, Coccidia) from the roe deer (Capreolus capreolus L.). Arch Protistenkd 129:183–186 https://doi.org/1016/s0003-9365(85)80020-8
Erber M (1982) Life cycle of Sarcocystis tenella in sheep and dog. Z Parasitenkd 68:171–180. https://doi.org/10.1007/BF00935058
Farhang-Pajuh F, Yakhchali M, Mardani K (2014) Molecular determination of abundance of infection with Sarcocystis species in slaughtered sheep of Urmia, Iran. Vet Res Forum 5:181–186
Ford GE (1974) Prey-predator transmission in the epizootiology of sarcosporidios. Aust Vet J 50:38–39
Ford GE (1975) Transmission of sarcosporidiosis from dogs to sheep maintained specific pathogen free. Aust Vet J 51:407–408
Foreyt WJ (1989) Sarcocystis sp. in mountain goats (Oreamnos americanus) in Washington: prevalence and search for the definitive host. J Wildl Dis 25:619–622. https://doi.org/10.7589/0090-3558-25.4.619
Frenkel JK, Heydorn AO, Mehlhorn H, Rommel M (1979) Sarcocystinae: Nomina dubia and available names. Z Parasitenkd 58:115–139. https://doi.org/10.1007/bf01951337
Gestrich R, Schmitt M, Heydorn A-O (1974) Pathogenität von Sarcocystis tenella-Sporozysten aus den Fäzes von Hunden für Lämmer. Berl Münch tierärztl Wschr 87:362–363
Gestrich R, Heydorn A-O, Baysu N (1975) Beiträge zum Lebenszyklus der Sarkosporidien. VI Untersuchungen zur Artendifferenzierung bei Sarcocystis fusiformis und Sarcocystis tenella. Berl Münch Tierärztl Wschr 88:191–197
Giannetto S, Poglayen G, Brianti E, Gaglio G, Scala A (2005) Sarcocystis gracilis-like sarcocysts in a sheep. Vet Rec 156:322–323. https://doi.org/10.1136/vr.156.10.322
Gjerde B (1984a) Sarcocystis infection in wild reindeer (Rangifer tarandus) from Hardangervidda in southern Norway: with a description of the cysts of Sarcocystis hardangeri n. sp. Acta Vet Scand 25:205–212
Gjerde B (1984b) Sarcocystis hardangeri and Sarcocystis rangi n. sp. from the domestic reindeer (Rangifer tarandus) in northern Norway. Acta Vet Scand 25:411–418
Gjerde B (1985) Ultrastructure of the cysts of Sarcocystis hardangeri from skeletal muscle of reindeer (Rangifer tarandus tarandus). Can J Zool 63:2676–2683. https://doi.org/10.1139/z85-400
Gjerde B (1986) Scanning electron microscopy of the sarcocysts of six species of Sarcocystis from reindeer (Rangifer tarandus tarandus). Acta Pathol Microbiol Immunol Scand [B] 94:309–317. https://doi.org/10.1111/j.1699-0463.1986.tb03058.x
Gjerde B (2012) Morphological and molecular characterization and phylogenetic placement of Sarcocystis capreolicanis and Sarcocystis silva n. sp. from roe deer (Capreolus capreolus) in Norway. Parasitol Res 110:1225–1237. https://doi.org/10.1007/s00436-011-2619-6
Gjerde B (2013) Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitol 43:579–591. https://doi.org/10.1016/j.ijpara.2013.02.004
Gjerde B (2014a) Sarcocystis species in red deer revisited: with a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 141:441–452. https://doi.org/10.1017/S0031182013001819
Gjerde B (2014b) Morphological and molecular characteristics of four Sarcocystis spp. in Canadian moose (Alces alces), including Sarcocystis taeniata n. sp. Parasitol Res 113:1591–1604. https://doi.org/10.1007/s00436-014-3806-z
Gjerde B (2016) Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res 115:1473–1492. https://doi.org/10.1007/s00436-015-4881-5
Gjerde B, Dahlgren SS (2010) Corvid birds (Corvidae) act as definitive hosts for Sarcocystis ovalis in moose (Alces alces). Parasitol Res 107:1445–1453. https://doi.org/10.1007/s00436-010-2017-5
Gjerde B, Giacomelli S, Bianchi A, Bertoletti I, Mondani H, Gibelli LR (2017a) Morphological and molecular characterization of four Sarcocystis spp., including Sarcocystis linearis n. sp., from roe deer (Capreolus capreolus) in Italy. Parasitol Res 116:1317–1338. https://doi.org/10.1007/s00436-017-5410-5
Gjerde B, Luzón M, Alunda JM, de la Fuente C (2017b) Morphological and molecular characteristics of six Sarcocystis spp. from red deer (Cervus elaphus) in Spain, including Sarcocystis cervicanis and three new species. Parasitol Res 116:2795–2811. https://doi.org/10.1007/s00436-017-5590-z
Heydorn AO (1985) Zur Entwicklung von Sarcocystis arieticanis n. sp. Berl Münch Tierärztl Wschr 98:231–241
Heydorn AO, Gestrich R, Mehlhorn H, Rommel M (1975) Proposal for a new nomenclature of the Sarcosporidia. Z Parasitenkd 48:73–82. https://doi.org/10.1007/bf00389639
Hu JJ, Huang S, Chen MY, Wen T, Esch GW, Liu Q, Liu TT (2016) Sarcocystis tuagulusi, n.sp. (Apicomplexa: Sarcocystidae) from Williamson’s mouse deer (Tuagulus williamsoni) (Artiodactyla:Tragulidae). Parasitol Res 115:1325–1330. https://doi.org/10.1007/s00436-015-4869-1
Hu JJ, Huang S, Wen T, Esch GW, Liang Y, Li HL (2017) Sarcocystis spp. in domestic sheep in Kunming City, China: prevalence, morphology, and molecular characteristics. Parasite 24:30. https://doi.org/10.1051/parasite/2017025
Irie T, Ikeda T, Nakamura T, Ichii O, Yamada N, Ito T, Yamazaki A, Takai S, Yagi K (2017) First molecular detection of Sarcocystis ovalis in the intestinal mucosa of a Japanese jungle crow (Corvus macrorhynchos) in Hokkaido, Japan. Vet Parasitol Region Stud Rep 10:54–57. https://doi.org/10.1016/j.vprsr.2017.08.005
Kalantari N, Khaksar M, Ghaffari S, Hamidekish SM (2016) Molecular analysis of Sarcocystis spp. isolated from sheep (Ovis aries) in Babol area, Mazandaran Province, Northern Iran. Iran J Parasitol 11:73–80
Kolenda R, Schierack P, Zieba F, Zwijacz-Kozica T, Bednarski M (2015) First molecular characterization of Sarcocystis tenella in Tatra chamois (Rupicapra rupicapra tatrica) in Poland. Parasitol Res 114:3885–3892. https://doi.org/10.1007/s00436-015-4619-4
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Levine ND, Tadros W (1980) Named species and hosts of Sarcocystis (Protozoa: Apicomplexa: Sarcocystidae). Syst Parasitol 2:41–59. https://doi.org/10.1007/BF00015094
Librado P, Rozas J (2009) DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Luzón M, Domínguez-González J, Soto-Carrión AM, Alunda JM (2015) Sarcocystosis in Cervus elaphus: comparison of diagnostic methods. Int J Parasitol Parasites Wildl 4:396–400. https://doi.org/10.1016/j.ijppaw.2015.11.001
Martínez-Moreno A, Martínez-Cruz MS, Becerra C, Guerra-Zamora MJ (1987) Sarcosporidiosis ovina en la provincia de Córdoba (Ovine sarcocystosis in Córdoba province) [In Spanish]. V Congreso Nacional de Parasitología, Salamanca (España). Libro de resúmenes, 119–120
Martínez-Moreno A, Moreno-Montañez T, Martínez-Gómez F, Hernández-Rodríguez S, Martínez-Cruz S (1989) Prevalence of ovine Sarcocystis in Córdoba. Rev Ibér Parasitol 49:283–285
Martínez-Navalón B, Anastasio-Giner B, Cano-Fructuoso M, Sánchez-Martínez P, Llopis-Morant A, Pérez-Castarlenas B, Goyena E, Berriatua E (2012) Short communication. Sarcocystis infection: a major cause of carcass condemnation in adult sheep in Spain. Span J Agric Res 10:388–392. https://doi.org/10.5424/sjar/2012102-523-11
Melville RV (1980) Nomina dubia and available names. Z Parasitenkd 62:105–109. https://doi.org/10.1007/bf01951337
Moore S (1980) Two types of ovine Sarcocystis macrocysts distinguished by periodic acid-Schiff staining of the cyst walls. N Z Vet J 28:101–102. https://doi.org/10.1080/00480169.1980.34710
Munday BL, Obendorf DL (1984) Morphology of Sarcocystis gigantea in experimentally-infected sheep. Vet Parasitol 16:193–199. https://doi.org/10.1016/0304-4017(84)90036-0
Munday BL, Rickard MD (1974) Is Sarcocystis tenella two species? Aust Vet J 50:558–559. https://doi.org/10.1111/j.1751-0813.1974.tb14076.x
Novak MD, Fedoseenko VM, Orazalinova VA (1987) The ultrastructure of the cyst of Sarcocystis sp. from cattle [in Russian]. Izv Akad Nauk Kaz SSR. Ser Biol 2:46–49
Obendorf DL, Munday BL (1987) Experimental infection with Sarcocystis medusiformis in sheep. Vet Parasitol 24:59–65. https://doi.org/10.1016/0304-4017(87)90130-0
Odening K, Stolte M, Bockhardt I (1996) On the diagnostics of Sarcocystis in chamois (Rupicapra rupicapra). Appl Parasitol 37:153–160
Oryan A, Moghaddar N, Gaur SNS (1996) The distribution pattern of Sarcocystis species, their transmission and pathogenesis in sheep in Fars Province of Iran. Vet Res Commun 20:243–253. https://doi.org/10.1007/BF00366922
Pereira A, Bermejo M (1988) Prevalence of Sarcocystis in pigs and sheep in Spain. Vet Parasitol 27:353–355. https://doi.org/10.1016/0304-4017(88)90049-0
Pérez-Garro MC, González-Castro J (1970) Sarcocystis tenella, Raillet, 1886: Recientes adquisiciones sobre su morfología y estructura. Frecuencia de la sarcosporidiosis en el ganado ovino de Granada [In Spanish]. Rev Ibér Parasitol 30:719–723
Pipia AP, Varcasia A, Zidda A, Dessì G, Panzalis R, Tamponi C, Marrosu R, Tosciri G, Sanna G, Dore F, Chiesa F, Scala A (2016) Cross-sectional investigation on sheep sarcosporidiosis in Sardinia, Italy. Vet Parasitol Reg Stud Rep 3–4:13–17. https://doi.org/10.1016/j.vprsr.2016.05.004
Prakas P, Kirillova V, Calero-Bernal R, Kirjušina M, Rudaityte-Lukošiene E, Habela MÁ, Gavarane I, Butkauskas D (2019) Sarcocystis species identification in the moose (Alces alces) from the Baltic States. Parasitol Res 118:1601–1608. https://doi.org/10.1007/s00436-019-06291-0
Rommel M, Heydorn AO, Gruber F (1972) Beiträge zum Lebenszyklus der Sarkosporidien I. Sporozyste von S. tenella in der Fäzes der Katze. Berl Münch Tierärztl Wschr 85:101–105
Rommel M, Heydorn AO, Fischle B, Gestrich R (1974) Beiträge zum Lebenszyklus der Sarkosporidien. V. Weitere Endwirte der Sarkosporidien von Rind, Schaf und Schwein und die Bedeutung des Zwischenwirtes für die Verbreitung dieser Parasitose. Berl Münch Tierärztl Wschr 87:392–396
Rudaityte-Lukošiene E, Prakas P, Butkauskas D, Kutkiene L, Vepštaite-Monstavice I, Serviene E (2018) Morphological and molecular identification of Sarcocystis spp. from the sika deer (Cervus nippon), including two new species Sarcocystis frondea and Sarcocystis nipponi. Parasitol Res 117:1305–1315. https://doi.org/10.1007/s00436-018-5816-8
Saito M, Shibata Y, Kubo M, Itagaki H (1997) Sarcocystis mihoensis n. sp. from sheep in Japan. J Vet Med Sci 59:103–106. https://doi.org/10.1292/jvms.59.103
Saito M, Kubo M, Itagaki H (2000) Sarcocystis sp. from cattle slaughtered in Japan. J Vet Med Sci 62:1209–1211
Sánchez-Acedo C, Lucientes-Curdi J, Gutiérrez-Galindo J, Castillo-Hernández JA, Estrada-Peña A, García-Pérez A (1983) Incidencia de la sarcosporidiosis en animales de abasto del matadero de Zaragoza (Incidence of sarcocystosis in food-producing animals slaughtered in the slaughterhouse of Zaragoza) [In Spanish]. Rev Ibér Parasitol 43:341–346
Simón-Vicente F, Ramajo-Martín V (1984) Sarcocystosis natural en ovinos y caprinos (Natural sarcocystosis in sheep and goats) [In Spanish]. Rev Ibér Parasitol 44:367–377
Stolte M, Bockhardt I, Odening K (1997) First report of Sarcocystis rangiferi and a second Sarcocystis species with parasite-induced encapsulation in cervids from Central Europe. Acta Protozool 36:131–135
Takano K, Hamada K, Ogiwara Y, Yagi K (2006) Phylogenetic analysis of Sarcocystis sp. isolated from muscle of sika deer in Hokkaido by partial 18S rRNA gene sequence. Rep Hokkaido Inst Pub Health 56:41–44
Wang G, Wei T, Wang X, Li W, Zhang P, Dong M, Xiao H (1988) The morphology and life cycle of Sarcocystis microps n. sp. in sheep of Qinghai in China [in Chinese]. China Vet Technol 6:9–11
Acknowledgments
We are indebted to the veterinary services and staff of the abattoir in Villarejo de Salvanés (Madrid). Their kind collaboration made possible the sampling of sheep carcasses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The muscle samples used in the present study were collected from the carcasses of sheep slaughtered according to EU regulations at an abattoir in Villarejo de Salvanés, Province of Madrid.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Daniel K. Howe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 691 kb)
Rights and permissions
About this article
Cite this article
Gjerde, B., de la Fuente, C., Alunda, J.M. et al. Molecular characterisation of five Sarcocystis species in domestic sheep (Ovis aries) from Spain. Parasitol Res 119, 215–231 (2020). https://doi.org/10.1007/s00436-019-06504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06504-6