Abstract
Samples of muscle tissue from the diaphragm, oesophagus and/or heart of eight adult red deer (Cervus elaphus hispanicus) from the Quintos de Mora Park in Toledo, Central Spain, were screened for sarcocysts by means of the compression method. From positive samples, individual sarcocysts were excised and examined in wet mounts under a light microscope (LM) in order to study their basic morphology before being preserved for molecular studies. In all red deer examined, only microscopic sarcocysts were found. Those in the diaphragm and oesophagus were spindle-shaped and about 1 × 0.1 mm in size, while those in cardiac muscle were sac-like and 500–800 × 80–180 μm. By LM, the sarcocysts either had densely packed, about 8-μm-long, hair-like protrusions (type 1), sparsely distributed indistinct projections (fuzzy outline; type 2) or no visible protrusions (smooth surface; type 3). In cardiac muscle, only sarcocysts without visible protrusions were found. One of the latter sarcocysts was examined by scanning electron microscopy (SEM) and found to possess thin ribbon-like protrusions. Forty-eight sarcocysts isolated from the diaphragm, oesophagus and heart of one red deer, as well as 55 sarcocysts from the heart of three other red deer, 103 sarcocysts in total, were characterized molecularly through PCR amplification and sequencing of the partial cytochrome c oxidase subunit I gene (cox1) of the mitochondrial genome, revealing the presence of six major cox1 sequence types. Each type comprised either a single sequence (three types) or a collection of several identical or nearly identical sequences. From selected isolates possessing each of these cox1 sequence types, the complete 18S ribosomal RNA (rRNA) gene was amplified and sequenced directly and/or after cloning of the 5′ end half. Supported by the sequence data from the latter gene, as well as the morphology of the sarcocysts from which the sequences originated, the six cox1 sequence types were considered to represent six separate Sarcocystis spp. Two cox1 sequence types were identified as belonging to the previously characterized species Sarcocystis hjorti (one sequence/sarcocyst) and Sarcocystis linearis (38 sequences/sarcocysts), respectively, whereas four sequence types were new. One of the latter types was assigned to the previously named species Sarcocystis cervicanis from red deer, since this sequence type was obtained from 52 sarcocysts from cardiac muscle, which matched the original morphological description (smooth surface) and habitat of this species. The remaining three sequence types were assigned to the three new species Sarcocystis iberica (one sequence/sarcocyst) Sarcocystis venatoria (10 sequences/sarcocysts) and Sarcocystis morae (one sequence/sarcocyst), respectively. The two species S. iberica and S. venatoria shared the same sarcocyst morphology (type 1) and habitat (diaphragm) and had virtually identical 18S rRNA gene sequences, but differed by 4% at cox1, which was considered sufficient to regard them as separate species. The single sarcocyst of S. morae (from the oesophagus) examined by LM had a smooth wall and this species was therefore believed to have the same type of ribbon-like protrusions (ultrastructurally) as sarcocysts of S. cervicanis and S. linearis, which were also the species most closely related to S. morae at cox1. Thus, there seems to be three species with similar ribbon-like cyst wall protrusions in red deer (S. cervicanis, S. linearis, S. morae), as well as three species with similar hair-like protrusions (S. hjorti, S. iberica, S. venatoria). Sarcocysts of S. cervicanis were only identified in cardiac muscle, whereas sarcocysts of S. linearis were found mainly in the diaphragm and oesophagus and rarely in the heart. The relative number of cox1 haplotypes was greater among sequences/isolates of S. linearis (17/38) than among isolates of S. cervicanis (7/52). Four of the species examined (S. cervicanis, S. linearis, S. iberica, S. venatoria) possessed considerable intra-isolate (intra-genomic) sequence variation (insertions/deletions, substitutions) in the 18S rRNA gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protozoan parasites belonging to the genus Sarcocystis have an obligatory two-host life cycle comprising mainly carnivores as definitive hosts and herbivores and omnivores as intermediate hosts. Free-ranging cervids may serve as intermediate host for many Sarcocystis spp. (Gjerde, 2013; Gjerde et al., 2017). Previous molecular and/or morphological studies in several countries have indicated that the European red deer, Cervus elaphus (comprising five subspecies), may act as intermediate host for eight or nine different Sarcocystis spp. forming sarcocysts of five major morphological types (Table 1). Thus, some of these sarcocyst types represent more than one species. On the other hand, the species designated Sarcocystis cf. hofmanni by Wesemeier & Sedlaczek (1995a) seems to comprise two morphological types, corresponding to sarcocysts of Sarcocystis elongata and Sarcocystis truncata, respectively (Dahlgren & Gjerde, 2010a; Gjerde, 2014a). Likewise, it is not clear to which of the latter two morphological types one should assign sarcocysts initially designated Sarcocystis hofmanni-like2 (Prakas, 2011), but later believed to belong to Sarcocystis entzerothi (Prakas et al., 2017). In Table 1, this species has provisionally been grouped with sarcocysts of the S. truncata type.
Two Sarcocystis spp. have so far been reported from the North American elk or wapiti (Cervus elaphus canadensis) in the USA, of which Sarcocystis wapiti (Speer & Dubey, 1982) is morphologically indistinguishable from Sarcocystis cervicanis in European red deer (Table 1). The second named species from wapiti, Sarcocystis sybillensis, was reported by Dubey et al. (1983) to have thick-walled (8 μm) sarcocysts when examined by light microscopy (LM) in histological sections, which suggested the presence of finger-like protrusions. By transmission electron microscopy (TEM), however, sarcocysts of allegedly the same species seemed to have hair-like protrusions like those of Sarcocystis hjorti, but sarcocysts with such protrusions are known to be thin-walled in histological sections. Dahlgren & Gjerde (2010a) therefore believed that S. sybillensis as described by Dubey et al. (1983) represented a mixture of at least two species with different sarcocyst morphology, which is why this taxon has not been included in Table 1.
In a recent study, 61 red deer hunted in the Quintos de Mora Park of the National Wildlife Reserves, Toledo, Central Spain, were examined for sarcocystosis, mainly for the purpose of comparing two diagnostic techniques, that is, compression of fresh muscle tissue and standard histology (Luzón et al., 2015). In addition, samples of the diaphragm and heart of three heavily infected red deer were examined under a stereo microscope, and sarcocysts were isolated and identified to morphological type in wet mounts under a light microscope. The prevalence study revealed that 59 of 61 (97%) red deer examined were infected with sarcocysts, whereas the limited morphological study of isolated sarcocysts, revealed the presence of two morph types. All sarcocysts isolated from cardiac muscle and about 70% of those from the diaphragm, had a thin wall with no visible protrusions, and were thus consistent with sarcocysts assigned to the species S. cervicanis by Hernández-Rodríguez et al. (1981a, b), whereas about 30% of the sarcocysts from the diaphragm had thin hair-like protrusions and were consistent with sarcocysts of S. hjorti as described from red deer and moose in Norway (Dahlgren & Gjerde, 2010a, b).
The abovementioned findings prompted the present study, in which the major aim was to identify which Sarcocystis spp. the different sarcocyst types in red deer from the Quintos de Mora reserve belonged to through the use of molecular methods, that is, sequencing of the complete nuclear 18S ribosomal (r) RNA gene and the partial mitochondrial cytochrome c oxidase subunit 1 gene (cox1). We were particularly interested in examining the S. cervicanis-like sarcocysts from red deer in this region by molecular methods in order to determine their relationship to the previously characterized and named species of this morphological type (Sarcocystis grueneri, Sarcocystis taeniata, Sarcocystis linearis) (Gjerde et al., 2017) through sequence comparisons and phylogenetic analyses. Thus, Hernández-Rodríguez et al. (1981a, b) originally described the species S. cervicanis on the basis of sarcocysts mainly from cardiac muscle of red deer from Sierra Morena, Córdoba, which lies about 200 km to the south of Quintos de Mora.
Materials and methods
Collection of muscle samples and isolation of sarcocysts
Muscle samples were collected from eight adult red deer (Cervus elaphus hispanicus) that were killed during selective hunting in November 2014 in the Quintos de Mora park of the National Wildlife Reserves, Toledo, Central Spain (39° 25′ N, 4° 04′ W). For details about the park, see Santín-Durán et al. (2004). Portions of the heart (inter-ventricular septum), oesophagus and diaphragm (diaphragmatic pillars) of each animal were placed in labelled plastic bags and transported in a cool box to the Animal Health Department at the Veterinary Faculty, Complutense University of Madrid, where the samples were kept at − 20 °C until further examined. Thawed samples were subsequently examined for sarcocysts by the compression technique used for detection of Trichinella larvae as previously described (Luzón et al., 2015). Briefly, sets of 28 oat-grain-sized muscle samples (about 2 mm × 10 mm), which had been cut from the full sample with scissors, were compressed between the two glass plates of a compressorium until they became translucent and examined for sarcocysts using a conventional light microscope at × 100 magnification. Individual samples comprising sarcocysts were released from the glass plates and transferred to separate microscope slides, covered with a drop of distilled water and kept inside a moist chamber at 4 °C for 1–2 h. The moistened muscle samples were then examined under a stereo microscope at × 20–30 magnification and the sarcocysts were excised from the muscle tissue using a fine insulin needle attached to a syringe. Isolated sarcocysts were further examined in a drop of distilled water under a Nomarski microscope at × 100–400 magnification and morphologically classified on the basis of the appearance of the cyst surface and the characteristics of the surface protrusions, if present, following the cyst type descriptions of Hernández-Rodríguez et al. (1981a, b) for S. cervicanis and of Dahlgren & Gjerde (2010a) for the other Sarcocystis species in red deer. Digital photographs were recorded of some sarcocysts at this stage. From all eight red deer, samples of the heart were examined, whereas samples of the diaphragm and oesophagus were examined from five red deer (Table S1 in the Supplementary Material). A total of 212 sarcocysts were excised and classified to morphological type. The cysts were then retrieved from the slides and placed into labelled 1.5-ml micro-centrifuge tubes. Sarcocysts isolated from the first red deer examined (C57/CeS1) were pooled according to morphological type and tissue of origin into nine 1.5-ml micro-centrifuge tubes containing 1 ml of distilled water, which were then stored in a freezer, whereas sarcocysts isolated from the other animals were placed separately into tubes containing 1 ml of 70% ethanol. A first batch of 67 sarcocysts from the oesophagus, diaphragm and heart of red deer CeS1 (C57) were shipped to the first author in Norway for molecular examination in March 2015, following replacement of the distilled water in the tubes with 70% ethanol. A second batch of 67 cysts isolated from the heart of three other red deer (CeS2/C28 = 26 cysts; CeS3/C82 = 18 cysts; CeS4/C85 = 23 cysts) were shipped in May 2016 (Table S1 in the Supplementary Material).
Scanning electron microscopy
A study of the sarcocysts by scanning electron microscopy (SEM) was initially not intended, and hence, no sarcocysts were specifically fixed for this purpose when sarcocysts were isolated from the muscle samples. Only during the course of the study the opportunity to examine some sarcocysts by SEM in Norway arose, and such a study was therefore performed on sarcocysts fixed in 70% ethanol for molecular studies. Two, three and two sarcocysts, isolated from the heart of red deer CeS2, CeS3 and CeS4, respectively, were pooled into one tube, and then further dehydrated in an ascending ethanol series comprising 80–100% ethanol. The ethanol was then gradually replaced by hexamethyldisilazane (HMDS) in several steps until the sarcocysts became immersed in a small volume of 100% HMDS, which was allowed to evaporate at room temperature. The dried sarcocysts were then further processed and examined by SEM as previously described (Gjerde et al., 2017).
Molecular examination of sarcocysts
The molecular examination of sarcocysts was basically as described previously (Gjerde, 2013, 2014a, b, 2016; Gjerde et al., 2017), the major difference being that the sarcocysts had been kept in 70% ethanol rather than in distilled water following isolation from the muscle samples. Moreover, the 67 sarcocysts isolated from red deer CeS1 had been pooled into nine micro-centrifuge tubes. In order to extract DNA from individual sarcocysts, the contents of the latter tubes were pipetted in small portions into an inverted snap cap removed from a micro-centrifuge tube, the fluid was examined under a stereo microscope and individual sarcocysts were pipetted in small volumes of alcohol from the cap and transferred to separate tubes. Some small sarcocysts were apparently lost during this procedure and only 55 of 67 putative sarcocysts were retrieved. The alcohol in all tubes containing individual sarcocysts were allowed to evaporate at room temperature and then about 20 μl of distilled water was added to each tube. Finally, genomic DNA was extracted from about 115 putative sarcocysts using the QIAmp DNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s tissue protocol as previously described (Gjerde, 2013). The resulting DNA samples were kept frozen at − 20 °C in between their use as templates for PCR amplification of the complete 18S rRNA gene and/or the partial cox1. For all available DNA isolates, attempts were made to amplify a 1085-bp-long portion of cox1 with primer pair SF1/SR9 in order to identify the sarcocysts to species and study the variability of this marker. For some isolates, this primer pair resulted in poor amplification and fairly short sequence reads and these isolates were therefore amplified as two overlapping fragments with primer pairs SF1/SR66 and SF4/SR9 as previously described (Gjerde et al., 2017). In addition, two such isolates were amplified with primer pair SF1/SR5. Following the identification of the sarcocyst isolates through sequencing of cox1, the complete 18S rRNA gene (1858–1920 bp) of one or more isolates of each of the six putative species found were amplified as two overlapping fragments using primer pairs ERIB1/S2r and S3f/Primer BSarc. Due to intra-isolate sequence variation detected through initial direct sequencing, amplicons of the 5′ end half of the 18S rRNA gene of five species were subsequently cloned into a plasmid vector before sequencing in order to obtain unambiguous sequences, whereas amplicons of one species (S. hjorti) were only sequenced directly.
The sequences of the primers used are given in the supplementary material of Gjerde et al. (2017), except that of primer SR5 (Gjerde, 2013). The PCR reactions were performed and the PCR products were evaluated and purified as described previously, as were the procedures and the reagents used for cloning and purification of plasmid DNA (Gjerde, 2014b, 2016). Purified amplicons and plasmid DNA (from cloning) were sent to Eurofins Genomics, Germany, for sequencing on both strands as previously described (Gjerde et al., 2017). Forward and reverse sequence reads, as well as overlapping sequences, were assembled into complete consensus sequences using the Alignment Explorer of the MEGA7 software (Kumar et al., 2016). Newly assembled sequences were compared with each other and with related sequences in GenBank using the Nucleotide BLAST program as previously described (Gjerde, 2013). The different sequences obtained by cloning of the 5′ end half of the 18S rRNA gene of five species were also aligned in MEGA7 by ClustalW and carefully examined by eye for the purpose of describing the intraspecific variation. In addition, all new complete 18S rRNA gene sequences were similarly compared with previous sequences of the same or closely related species after multiple alignments had been generated. The software package DNA Sequence Polymorphism (DnaSP) version 5.10.01 (Librado & Rozas, 2009) was used for the analysis of sequence variation and DNA divergence within and between different populations (types) of cox1 sequences found in the present and previous studies, in order to characterize these populations and assign them to different species, if possible.
Phylogenetic analyses
Phylogenetic analyses were conducted separately on nucleotide sequences of cox1 and the 18S rRNA gene, respectively, by means of the MEGA7 software (Kumar et al., 2016). In all analyses, the phylogeny was tested with the bootstrap method, using 1000 bootstrap replications. All sequences included in the analysis of each gene and their GenBank accession numbers are listed in Table S2 in the Supplementary Material.
For cox1, a total of 747 partial sequences from 53 taxa were initially included in the analysis, including 28 new sequences generated from the six Sarcocystis spp. found in the present study. However, in order to reduce computation time, identical superfluous sequences were removed, so that each haplotype (based on nucleotides 1–1020) was represented by a single sequence only. Hence, for the final analysis, a total of 386 sequences (haplotypes) were used. A codon-based multiple alignment of all sequences was obtained by using the ClustalW program integrated in MEGA7 as described previously (Gjerde, 2013). Sequences longer than 1020 bp were truncated at their 3′ end, so that the final alignment comprised 1020 positions with no gaps. The phylogenetic tree was reconstructed using the Neighbour-Joining (NJ) method with the p-distance algorithm. All codon positions were used. The intestinal coccidium Eimeria tenella (family: Eimeriidae) was used as outgroup species to root the tree.
As regards the 18S rRNA gene, a total of 235 near full-length sequences from 57 taxa were used in the main analysis, including 66 new sequences of the six Sarcocystis spp. found in the present study. A multiple sequence alignment was generated with the ClustalW program integrated in MEGA7, using a gap opening penalty of 10 and a gap extension penalty of 0.2 and 0.1 for the pairwise and multiple alignments, respectively. The resulting alignment was checked by eye and slightly corrected for inconsistencies in the treatment of closely related sequences. Most sequences were truncated slightly at both ends, so that most sequences started and ended at the same homologous nucleotide positions, corresponding to positions 75 and 1852, respectively, of GenBank sequence KY973355 of S. linearis. The final alignment comprised 2018 aligned positions, including gaps. The phylogenetic tree was reconstructed using the maximum parsimony (MP) method with the subtree–pruning–regrafting (SPR) algorithm. All sites were used. The intestinal coccidium E. tenella was used as outgroup species to root the tree. An additional analysis was performed on a smaller dataset comprising 28 taxa and 175 nucleotide sequences, of which 171 sequences belonged to taxa with canines as known or presumed definitive hosts. One sequence each of Sarcocystis hardangeri, Sarcocystis ovalis and Sarcocystis oviformis were used as outgroup species to root the tree (Table S2 in the Supplementary Material). A multiple alignment was generated with the same settings as for the larger dataset, resulting in 1942 aligned positions. The phylogenetic tree was reconstructed using the MP method with the SPR algorithm.
Results
Light microscopic appearance of sarcocysts
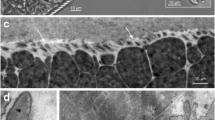
All sarcocysts were microscopic in size and were only detected using the compression method. The light microscopic examination of isolated sarcocysts in wet mounts revealed three types of surface morphology. Type 1 sarcocysts had densely packed, distinct hair-like protrusions (Fig. 1a–c) and resembled sarcocysts of S. hjorti from Norwegian red deer (Dahlgren & Gjerde, 2010a); type 2 sarcocysts displayed a fuzzy outline, which seemed to be due to loosely packed structures resembling short hairs (Fig. 1d); whereas type 3 sarcocysts had a smooth surface with no visible protrusions (Fig. 1 e–h) as reported for S. cervicanis (Hernández-Rodríguez et al., 1981a, b. Type 1 and 2 sarcocysts were found in the diaphragm of one, and in the oesophagus of two of the five red deer from which these tissues were examined, but they were not found in cardiac muscle of any of the eight red deer. Type 3 sarcocysts were found in cardiac muscle of seven of eight red deer, as well as in the diaphragm and oesophagus of three of five animals (Table S1 in the Supplementary Material). In the oesophagus and diaphragm, sarcocysts of all three types were spindle-shaped with pointed ends and about 90-μm wide in the middle. Their full length could not be clearly established, since only a few fairly complete cysts were isolated and measured. Based on the largest cyst fragments and a few intact cysts, the full length was estimated to be 650–1500 μm. In type 1 sarcocysts, the hair-like protrusions were about 8-μm long. Sarcocysts of type 3 in cardiac muscle were sac-like and about 500–800 × 80–180 μm in size (Fig. 1g, h).
Light microscopic appearance of unstained sarcocysts isolated from red deer from Quintos de Mora, Central Spain. a–c Sarcocysts of type 1 with hair-like protrusions (P). a Portion of a spindle-shaped small sarcocyst from the diaphragm of red deer CeS1, probably of either S. iberica or S. venatoria. b Higher magnification of sarcocyst in (a) showing surface with tightly packed delicate hair-like protrusions (P). c Tip of sarcocyst with hair-like protrusions (P) from the oesophagus of red deer CeS1, possibly of S. hjorti. d Sarcocyst of type 2 with a fuzzy outline (F) from the oesophagus of red deer CeS1, possibly of S. linearis. e–h Sarcocysts of type 3 with a smooth surface (S) and no visible protrusions. e Peripheral portion of sarcocyst from the oesophagus of red deer CeS1, possibly of S. linearis. f Middle portion of fusiform sarcocyst from the diaphragm of red deer C233. g Tip of sarcocyst from the heart of red deer CeS1, probably of S. cervicanis. h Peripheral portion of sarcocyst from the heart of red deer C100, probably of S. cervicanis. Scale bars a, g 40 μm and b–f, h 20 μm
Scanning electron microscopy
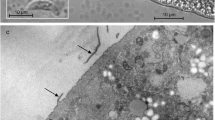
In only one of the seven sarcocysts processed for SEM had portions of the cyst surface become exposed through removal of the surrounding host cell, whereas the other mounted cysts were still completely covered by host cell material. In the partially denuded sarcocyst, regularly distributed thin ribbon-like protrusions were running along the exposed cyst surface (Fig. 2a, b). Many of the protrusions seemed to have become twisted and distorted and to have been torn off at different distances from their point of origin on the cyst surface. Still, their basic configuration was evident. They were band-like and seemed to have a nearly uniform width and thickness throughout their length. They were about 0.3–0.4-μm wide, about 0.02–0.03-μm thick and the longest intact portions were up to 2.5-μm long. The protrusions had a smooth surface, whereas the surface of the cyst itself was provided with regularly distributed small pits, about 0.05 μm in diameter, separated by low ridges. From the bottom of the larger pits, smaller pores seemed to arise and form minute invaginations into the cyst wall. Together, the ridges and pits gave the sarcocyst surface a porous or reticulated appearance (Fig. 2b). Based on the molecular characterization of other sarcocysts from cardiac muscle of the same three red deer (Table S3 in the Supplementary Material), the sarcocyst examined by SEM most likely belonged to S. cervicanis.
Scanning electron micrographs of the surface of a sarcocyst from cardiac muscle of red deer, probably of S. cervicanis. a Numerous ribbon-like protrusions (P) are running along the sarcocyst surface (S) in the same direction, but some protrusions have become twisted and distorted and their distal end have been torn off during removal of the surrounding host cell material. b Higher magnification of cyst in (a), showing that the ribbon-like protrusions (P) have a fairly uniform width throughout their length. The sarcocyst surface (S) in between the bases of the protrusions have a reticulated appearance due to low ridges and invaginations into the sarcocyst wall. Scale bars 3 μm (a) and 1 μm (b)
Molecular characterization of sarcocysts
Partial cox1 gene
A total of 103 sarcocysts were unambiguously characterized through PCR amplification and sequencing of the partial cox1 gene (1038–1053 bp), revealing the presence of six major sequence types, with each type consisting either of a single sequence from a single sarcocyst isolate or a collection of several identical or nearly identical sequences derived from different sarcocysts. On the basis of sequence comparisons using BLAST and the program DnaSP, as well as their phylogenetic placement and the morphological features of the sarcocysts from which they originated, the sequence(s) of different types were considered to represent six different Sarcocystis spp. (Table 2). The molecularly identified sarcocysts comprised 48 sarcocysts from red deer CeS1 (32 from the diaphragm, 13 from the oesophagus, 3 from the heart); and 21, 14 and 20 sarcocysts from cardiac muscle of red deer CeS2, CeS3 and CeS4, respectively (Table S3 in the Supplementary Material).
Of the six major cox1 sequence types found, two types had a high sequence identity to the previously characterized species S. hjorti and S. linearis, respectively, and thus belonged to these species. The four other sequence types differed considerably from previous sequences of known species, as did the corresponding 18S rRNA gene sequences, and they were therefore considered to represent three new species and S. cervicanis, respectively. The species found and the number of sarcocysts/sequences belonging to each of them were: Sarcocystis iberica n. sp. (n = 1), Sarcocystis venatoria n. sp. (n = 10), S. hjorti (n = 1), S. cervicanis (n = 52), S. linearis (n = 38) and Sarcocystis morae n. sp. (n = 1) (Table 2; Tables S3 and S4 in the Supplementary Material). The 103 sequences comprised 28 haplotypes, and 28 sequences representing these haplotypes were deposited in GenBank (KY973286–KY973313).
The 11 cox1 sequences obtained from type 1 sarcocysts (hair-like protrusions) in the diaphragm of red deer CeS1, were of two types, comprising one (isolate CeS1.1) and ten identical sequences (isolates CeS1.2–CeS1.11), respectively (Table S4 in the Supplementary material). The two sequence types differed at 42 of 1038 nucleotide (nt) positions (96% identity) and were considered to belong to two separate species, even though the corresponding 18S rRNA gene sequences could not be separated from each other (see later). Since neither the cox1 nor the 18S rRNA gene sequences from these isolates matched those of any previously examined species, they were considered to represent two new species. The cox1 sequence from isolate CeS1.1 (KY973286) was assigned to S. iberica n. sp., whereas those from isolates CeS1.2–CeS1.11 (KY973287) were assigned to S. venatoria n. sp. Both of these sequence types shared the highest identity (about 91% for S. iberica and about 90% for S. venatoria) with sequences of other species from cervids with hair-like cyst wall protrusions (Sarcocystis capreolicanis, Sarcocystis rangi, S. hjorti, Sarcocystis pilosa).
One sarcocyst (isolate CeS1.48) from the oesophagus of red deer CeS1, which by LM had seemed to have a smooth wall, was found to belong to S. hjorti from its cox1 sequence (KY973288), which were 99.4–99.7% identical with 22 previous cox1 sequences of this species (KC209635–KC209643; KF241342–KF241354) derived from sarcocysts in red deer and moose in Norway (Gjerde, 2013; Gjerde, 2014b). The new cox1 sequence of S. hjorti was 90.7 and 90.1% identical with the abovementioned new sequences of S. iberica and S. venatoria, respectively.
A total of 38 sarcocysts were identified as belonging to S. linearis, including 21 and 11 from the diaphragm and oesophagus, respectively, of red deer CeS1, and two and four from the cardiac muscle of red deer CeS3 and CeS4, respectively (Table 2; Tables S3 and S4 in the Supplementary material). The majority of these cysts (31) had been recorded as being of type 3 when examined by LM, but three and four cysts from the oesophagus had been assigned to type 1 and 2, respectively. The 38 new cox1 sequences of S. linearis comprised 17 haplotypes (KY973296–KY973312), which differed from each other at 1–13 of 1038 nt positions (98.7–99.9% identity), whereas they differed at 6–19 nt positions (98.2–99.4% identity) from the five previous cox1 haplotypes of S. linearis obtained from 19 sarcocyst isolates (KY018969–KY018987) from roe deer in Italy (Gjerde et al., 2017). The 22 haplotypes of S. linearis from both hosts were thus 98.2–99.9% identical with each other, whereas they shared an identity of 95.8–97.1% (on average 96.5%) with the 17 known haplotypes of S. taeniata derived from 25 sarcocyst isolates from moose and sika deer (Gjerde, 2014b; Prakas et al., 2016). There were 18 fixed nucleotide differences between the two populations, that is, positions were all haplotypes of S. linearis differed from all haplotypes of S. taeniata. The 22 haplotypes of S. linearis further shared an identity of 83.5–85.0% with sequences (KC209615–KC209624) of the morphologically similar species S. grueneri from reindeer (Table S5 in the Supplementary material).
From 52 of the 58 type 3 sarcocysts from cardiac muscle of the four red deer examined, a new major cox1 sequence type was obtained, whereas six other sarcocysts of this morphological type belonged to S. linearis as mentioned above (Tables S3 and S4 in the Supplementary material). The 52 sequences of the new major type, comprised three subtypes, which were provisionally designated types A, B and C, respectively. In each red deer, sequences of either type A or B predominated (Table 2; Table S3 in the Supplementary material). The 19 type A sequences comprised four haplotypes (KY973289–KY973292), which differed from each other at 1–3 of 1038 nt positions (99.7–99.9% identity), whereas the 32 type B sequences comprised only two haplotypes (KY973293–KY973294), differing at two positions (99.8% identity). In addition, there was a single sequence/haplotype of type C (KY973295). Type A haplotypes differed from type B haplotypes at 17–21 nt positions (98.0–98.4% identity), including 17 fixed nt differences, and from the single type C haplotype at 17–19 positions (98.2–98.4% identity; 17 fixed nt differences), whereas type B and C haplotypes differed at 10 nt positions (99% identity; nine fixed nt differences). Thus, the single type C sequence seemed to represent an intermediate type between the two other types, but it was more similar to type B than to type A. The finding of this intermediate type suggested that there might have been additional intermediate types between type A and B if more isolated had been examined. Hence, all three subtypes were considered to belong to a single species. Moreover, since this species predominated in cardiac muscle, from which S. cervicanis was originally described (Hernández-Rodríguez et al., 1981a, b), they were attributed to this species. The seven cox1 haplotypes assigned to S. cervicanis thus differed at 1–19 nt positions and were 98.0–99.9% identical. These sequences shared the highest identity (88.4–88.9%) with the new sequence of S. morae (see below) followed by 86.7–87.4% identity with sequences of S. taeniata (Table S5 in the Supplementary material).
From a type 3 sarcocyst (isolate CeS1.47) from the oesophagus of red deer CeS1, still another new cox1 sequence type was obtained. Likewise, sequences of the 18S rRNA gene from this isolate did not match any previous sequences in GenBank. These sequences were therefore attributed to the new species S. morae. The cox1 sequence (KY973313) showed the highest identity with the seven new sequences of S. cervicanis (see above), followed by identities of 86.3–86.9% with all sequences of S. linearis from red deer and roe deer and 86.0–86.8% identity with S. taeniata and S. grueneri (Table S5 in the Supplementary material). The identity was about the same with sequences of S. hjorti (86.0–86.5%) and S. capreolicanis (85.8–86.3%) and slightly less with sequences of several other species, including S. iberica (85.4%) and S. venatoria (85.4%).
Complete 18S rRNA gene
Following the identification of six major cox1 sequence types among the 103 isolates examined, the complete 18S rRNA gene was amplified and sequenced from one or more isolates of each of the six species these types were considered to represent (Table 2). Attempts were initially made to sequence directly the PCR products of the two overlapping gene fragments of all selected isolates. It turned out that the 5′ end half of the gene of S. iberica, S. venatoria, S. linearis and S. cervicanis comprised one or more indels (insertions/deletions), which made the sequence reads ambiguous or unintelligible downstream of these variations. Hence, this portion of the gene of one or two isolates of each of these species, was subsequently cloned before sequencing. The 5′ end half of the gene of S. hjorti and S. morae, on the other hand, was sequenced directly with good results, but amplicons of the latter species were nevertheless cloned and sequenced for comparative purposes. As regards to the 3′ end half of the gene, amplicons of all species, except some isolates of S. cervicanis (see later), were sequenced in both directions with good results. A total of 66 sequences of the (near) complete gene were finally obtained and submitted to GenBank under the accession numbers KY973314–KY973379 (Table 2).
The 18S rRNA gene of the single available isolate of S. iberica and of three isolates of S. venatoria were initially amplified and sequenced directly (Table 2). Subsequently, ten and nine clones were generated and sequenced from the 5′ end half of the gene of S. iberica (isolate CeS1.1) and S. venatoria (isolate CeS1.2), respectively. All clones of S. iberica differed slightly from each other, whereas two clones of S. venatoria were identical. Unique clones of each species were concatenated with the 3′ end half of the gene, resulting in 10 and eight full-length sequences of S. iberica and S. venatoria, respectively (Table 2). The sequences of both species were 1896–1898-bp long; those of S. iberica shared an identity of 99.2–99.9% with each other, while those of S. venatoria were 99.5–99.9% identical. Moreover, sequences of the two species shared an identity of 99.2–100% with each other, that is, one sequence of S. iberica (KY973321) was identical with a sequence of S. venatoria (KY973327). The differences between individual sequences of both species were mainly due to three 2-bp-long indels between positions 745 and 765 in the multiple alignment of all sequences, which was 1902-bp long. In comparison to previously known species, S. iberica and S. venatoria were most similar (97–98% identity) to other species with hair-like cyst wall protrusions (Sarcocystis alceslatrans, S. capreolicanis, Sarcocystis cruzi, S. hjorti, S Sarcocystis levinei, S. pilosa, S. rangi).
The near complete 18S rRNA gene of S. hjorti was PCR amplified and sequenced from the only available isolate of this species (Table 2). The new sequence (KY973332) was 99.8–99.9% identical with previous sequences of S. hjorti from moose (EU282017, JN256128, KF831294–KF831295) and red deer (GQ250990, JN256124) in Norway and Lithuania and it was 97.6–98.1% identical with the abovementioned new sequences of S. iberica and S. venatoria.
Six isolates of S. linearis were examined at the 18S rRNA gene through PCR amplification and direct sequencing of the amplicons. From two of the isolates, the 5′ end half of the gene was cloned, and nine and ten clones were sequenced from isolates CeS1.16 and CeS1.21, respectively (Table 2). Two clones from the first isolate were identical, and hence 18 different clones were submitted to GenBank following concatenation with the 3′ end half of the gene, which was identical in all six isolates examined. There was no indications of any sequence variation in the latter portion of the gene after direct sequencing. The 18 new complete (1878–1887-bp long) 18S rRNA gene sequences of S. linearis were 98.3–99.9% identical with each other and 97.8–99.7% identical with 24 previous sequences of S. linearis (KY019032–KY019055) from roe deer in Italy, which in turn were 98.1–99.9% identical with each other (Gjerde et al., 2017). Hence, the intraspecific identity of all 42 available sequences of S. linearis was 97.8–99.9%. In comparison, these sequences were 97.9–99.7% identical with sequences of S. taeniata from moose and sika deer (KF831277–KF831293; KU753886–KU753890). The differences between individual sequences were in both species due to several indels and substitutions (single nucleotide polymorphisms, SNPs). When sequences of S. linearis and S. taeniata were compared in a multiple alignment, both species seemed to have a similar pattern of sequence variation with no consistent differences in the cloned 5′ end half of the gene as described before (Gjerde et al., 2017). Near the 3′ end of the gene, on the other hand, all the new sequences of S. linearis from red deer differed from previous sequences from roe deer in having only three versus five or six consecutive thymine bases (Ts) from nucleotide position 108 onwards from the 3′ end of the gene (Fig. S1 in the Supplementary Material). Moreover, there seemed to be no intra-isolate variation with respect to the number of Ts in the six isolates from Spanish red deer, since both forward and reverse sequence reads were fine, whereas all roe deer isolates examined seemed to possess this indel, since the reverse sequence reads became illegible beyond this position (Gjerde et al., 2017). In addition, the new sequences of S. linearis from red deer differed from all available sequences of S. taeniata from moose and sika deer published in GenBank (KF831277–KF831293; KU753886–KU753890), which also possess six consecutive Ts. However, a re-examination of the sequencing chromatograms from the study of S. taeniata from Canadian moose (Gjerde, 2014b), revealed that there was actually an indel in this region, and thus five or six consecutive Ts as in the roe deer isolates of S. linearis. The illegible reverse sequence reads beyond this position had at that time been interpreted as being due to polymerase slippage during amplification or sequencing as a result of the presence of six consecutive Ts. The new sequences of S. linearis displayed two polymorphic sites in positions 95 and 96 from the 3′ end of the gene. At both positions, the possible character states were T or C, as evidenced by double peaks in the chromatograms, but submitted sequences of S. linearis have either TT or CC, since either T or C predominated at both positions. In published sequences of S. taeniata, on the other hand, there are either TC or TT. However, the re-examination of the sequencing chromatograms of S. taeniata from moose, revealed that both positions were polymorphic with T and C as possible character states even in this species. Thus, there seems to be no consistent differences between the 18S rRNA gene sequences of S. linearis and S. taeniata, except perhaps between isolates of S. linearis from red deer and S. taeniata. Interestingly, the sequences from red deer in Lithuania and Argentina (JN256126–JN256127; KT626602) tentatively attributed to S. taeniata (Reissig et al., 2016; Prakas et al., 2016) also possessed only three Ts like the present isolates of S. linearis from red deer and may therefore belong to the latter species (Fig. S1 in the Supplementary material).
Six isolates of S. cervicanis were examined at the 18S rRNA gene through PCR amplification and direct sequencing of the amplicons (Table 2), but portions of the 5′ end half of the gene could not be properly estimated from most sequence reads due to superimposed sequences caused by indels. A total of 23 clones were therefore generated and sequenced from isolate CeS1.55, from which cox1 sequences of subtype A had been obtained. Two of these clones differed considerably (10–20%) from the others through a 59-bp-long deletion and several short stretches of atypical sequence composition. These clones were therefore considered to be aberrant and were not deposited in GenBank or included in the sequence comparisons. The 21 other clones were of two major types, with the major differences between them being found in the region spanning nucleotide positions 765 to 830 in the final sequences. In this region, there were a series of substitutions and six 1–3-bp-long indels, making the total length of this stretch of sequence differ by five nucleotides between the two types (e.g. KY973333 versus KY973335). In the clones, there were about 40 polymorphic sites in addition to the aforementioned differences, but at 35 of these sites, only one clone had an atypical nucleotide, suggesting that these deviations were not true SNPs, but a result of polymerase errors during PCR amplification prior to cloning. Near the 3′ end of the gene, there were three additional polymorphic sites (true SNPs), as well as an indel, in three (CeS1.54, CeS1.55, CeS4.12) of four isolates having type A cox1 sequences. In these isolates, there were both A and G (double peaks in chromatograms) in position 82 from the 3′ end, both C and T at position 103, five or six consecutive Ts from position 107 onwards, and both C and T at position 114 or 115 (depending on number of Ts) from the 3′ end (Fig. S2 in the Supplementary material). Thus, the reverse sequence reads became illegible beyond the indel as described for sequences of S. linearis from roe deer (Gjerde et al., 2017) and for sequences of S. taeniata from moose (see above), which had five or six consecutive Ts in the homologous region. In the fourth type A isolate (CeS4.4), as well as in the three type B isolates (CeS2.4, CeS2.15, CeS3.8) and the single type C isolate (CeS4.11) examined, there were no indications of any sequence variation at these sites (Fig. S2 in the Supplementary material). Due to the sequence variations described above, the complete 18S rRNA gene of S. cervicanis was 1913–1920 bp long in different clones and the alignment comprising all sequences was 1926 bp long. The 22 new sequences (KY973333–KY973354) shared an identity of 98.1–99.9% with each other. They were most similar (97.6–98.6% identity) to a sequence of the unnamed Sarcocystis sp. T18 from sika deer in Japan (AB698065), from which they mainly differed in the same variable region (nucleotide positions 765–830) that separated the clones from each other. The new sequences of S. cervicanis were up to 95.8% identical with sequences of S. linearis, S. taeniata and S. grueneri.
From the only available isolate of S. morae, the complete 18S rRNA gene was initially amplified and sequenced directly with good results, but the 5′ end half of the gene was nevertheless cloned in order to explore whether there might be some undisclosed sequence variation. Ten clones were processed and sequenced, resulting in four identical clones and six other clones that were slightly different from these. The seven different clones were concatenated with the 3′ end half of the gene and the full-length sequences were submitted to GenBank (Table 2). The four identical clones, represented by sequence KY973374, were also identical with the sequence obtained by direct sequencing. The complete gene was 1858-bp long and the seven sequences differed from each other at 1–10 nucleotide positions (99.5–99.9% identity). These differences were due to various combinations of substitutions occurring at 17 polymorphic sites in the cloned region of the gene. However, at 16 of these sites, only a single sequence deviated from the majority, whereas at one site two sequences deviated from the five other sequences. Hence, most of these substitutions might be due to polymerase errors during the PCR amplification before cloning, and sequence KY973374, which was supported by four clones, might be regarded as the most accurate representation of the 18S rRNA gene of the examined isolate of S. morae. In comparison to sequences of previously characterized species available in GenBank, the most similar sequences were those of S. cruzi and S. levinei with up to 97.5 and 97.3% identity, respectively. With respect to the sequences of other species in the present study, the new sequences of S. morae shared identities of up to 96.7% with those of S. linearis, 96.4% with that of S. hjorti, 96.2% with those of S. iberica and S. venatoria, and up to 95.6% with those of S. cervicanis.
Phylogeny
Cox1
The phylogenetic analysis using NJ placed all Sarcocystis spp. with ruminant intermediate hosts into three major clades according to their known or presumed definitive hosts, that is, canines, felids and corvid birds (Fig. 3). Species with non-ruminant intermediate hosts formed a clade basal to these three clades. Sequences of the six species found in this study were placed within the canine clade. Those of S. linearis were placed with previous sequences of this species from roe deer into a monophyletic cluster, which with maximum bootstrap support was sister to all sequences of S. taeniata. Sequences of S. cervicanis were with high support sister to the single sequence of S. morae, and these taxa were sister to sequences of S. grueneri and Sarcocystis hircicanis. The latter four taxa in turn were a sister group to S. linearis and S. taeniata. However, the relationships between the different pairs of species were not well resolved. The sequences of S. iberica and S. venatoria were with maximum support sister to each other and both were sister to sequences of S. rangi, but the latter relationship was not well supported. The single new sequence of S. hjorti clustered with previous sequences of this species. Sequences of S. iberica, S. venatoria and S. hjorti were placed within a subclade comprising species with hair-like cyst wall protrusions, whereas those of S. cervicanis, S. morae and S. taeniata were placed within a subclade comprising species with ribbon-like protrusions, except S. hircicanis, which has hair-like protrusions (Hu et al., 2016).
Phylogenetic tree for members of the Sarcocystidae based on 386 partial sequences of cox1 of 53 taxa and inferred using the neighbour-joining method and with evolutionary distances computed using the p-distance method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Subtrees formed by two or more sequences/haplotypes of the same species have been collapsed, but the number of sequences/haplotypes included is given in parentheses behind the taxon names, which are in boldface when comprising sequences from the present study. The GenBank accession numbers of all sequences are listed in Table S2 in the Supplementary material
18S rRNA gene
The phylogenetic analysis using MP and the largest dataset placed all Sarcocystis spp. with ruminant intermediate hosts into three major clades according to their known or presumed definitive hosts (Fig. S3 in the Supplementary Material), as in the analysis using cox1 sequences. Sequences of the six species found in the present study were placed within the canine clade. However, sequences of several sister species pairs (assignment based on cox1 sequences), were not separated from each other, but rather formed paraphyletic clusters. Thus, within the canine clade, sequences of S. iberica were interleaved with those of S. venatoria, sequences of S. cervicanis were interleaved with the single available sequence of the unnamed Sarcocystis sp. T18 from sika deer in Japan (AB698065), and sequences of S. linearis were interleaved with those of S. taeniata. In this analysis, sequences of S. morae were placed within a subclade comprising species with hair-like protrusions. However, in the subsequent analysis based on the smaller dataset comprising taxa of the canine clade, sequences of S. morae were, albeit with low support, sister to the single sequence of S. grueneri, and both species were sister to the paraphyletic cluster comprising sequences of S. linearis and S. taeniata (Fig. 4). Moreover, these four taxa were sister to the paraphyletic cluster comprising sequences of S. cervicanis and the unnamed Sarcocystis sp. T18 from sika deer. The relationships between the species with hair-like cyst wall protrusions were largely the same in both analyses.
Phylogenetic tree for selected members of the Sarcocystidae (mainly species known or believed to be transmitted by canids) based on near full-length sequences of the 18S rRNA gene and inferred using the maximum parsimony method with the Subtree–Pruning–Regrafting algorithm. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Subtrees formed by two or more sequences of the same or closely related species have been collapsed, but the number of sequences included are given behind the taxon names, which are in boldface when comprising sequences from the present study. The GenBank accession numbers for all sequences are given in Table S2 in the Supplementary material. In the collapsed subtree formed by sequences of S. linearis and S. taeniata, sequences of the two species were interleaved. The same was true for sequences of S. iberica and S. venatoria. Note also that the single sequence of Sarcocystis sp. T18 from sika deer (AB698065) clusters in between sequences of S. cervicanis. A phylogenetic tree reconstructed from a larger dataset, comprising Sarcocystis spp. with different intermediate hosts, is shown in Fig. S3 in the Supplementary Material
Taxonomic summary of the three new species and Sarcocystis cervicanis
Important taxonomic features of the three new species found in this study have been summarized in Table 3. Corresponding data for the previously named species S. cervicanis have also been included for comparative purposes since this study has re-defined this species through the provision of molecular data. Two of the new species have been named after the geographical location of the species, S. iberica from the Iberian peninsula, which includes Spain and the study area, and S. morae from the locality Mora and the wildlife reserve Quintos de Mora, from which the red deer examined originated. The name of the third species, S. venatoria, is related to hunting (ars venatoria). Venatoria is the Latin nominative of the feminine form of the adjective venatorius. In addition, the word ‘venatus’ (animal hunted) is related to the Spanish word venado, which is a common name for all deer (Cervidae), but mainly used about red deer.
Discussion
The molecular characterization at cox1 of 103 sarcocyst isolates from four red deer revealed that these sequences belonged to six major sequence types, which on the basis of their characteristics were considered to represent six separate Sarcocystis species. In addition, the corresponding 18S rRNA gene sequences and the morphology of the sarcocysts from which they originated were taken into consideration, but the latter features are not sufficient to discriminate closely related Sarcocystis spp. in ruminants from each other (Gjerde, 2014a, b, 2016; Gjerde et al., 2017). Hence, the identification and assignment of sarcocysts to species were based mainly on the new cox1 sequences. In this manner, some sarcocysts were found to belong to the previously characterized species S. hjorti and S. linearis, whereas the remaining sarcocysts seemed to belong to species not previously known and/or characterized molecularly, since neither the cox1 nor the 18S rRNA gene sequences derived from these sarcocysts showed a high identity to published sequences. Therefore, one of the four new cox1 sequence types was attributed to the previously named species S. cervicanis, while the remaining new sequence types were assigned to the three new species S. morae, S. iberica and S. venatoria, respectively. In addition, the associated 18S rRNA gene sequences obtained from certain isolates of the latter four species, were also attributed to them. The rationale for these assignments will be discussed in the following.
Based on the sarcocyst morphology in wet mounts, there seemed to be only two or three species present in the red deer examined. The majority of the sarcocysts, including all cysts from cardiac muscle, seemed to have a smooth cyst wall with no visible protrusions (type 3), and were thus consistent with sarcocysts of S. cervicanis as described by Hernández-Rodríguez et al. (1981a, b) from red deer from Sierra Morena, Córdoba, which lies about 200 km to the south of Quintos de Mora. In addition, some sarcocysts from the diaphragm and oesophagus possessed hair-like protrusions (type 1) and were consistent with sarcocysts of S. hjorti as described from red deer and moose in Norway (Dahlgren & Gjerde, 2010a, b), and a few sarcocysts had an intermediate type of surface morphology (type 2; fuzzy surface). The latter appearance could have been due to aggregations of barely visible ribbon-like protrusions and host cell material, since a fuzzy surface was also noticed on sarcocysts of S. linearis from roe deer (Gjerde et al., 2017), or it could have been caused by partial removal of hair-like protrusions during isolation of the sarcocysts. In the preceding study of red deer from the Quintos de Mora reserve (Luzón et al., 2015), only S. cervicanis-like (type 3) and S. hjorti-like (type 1) sarcocysts were found.
However, the initial molecular characterisation of 48 sarcocysts from red deer CeS1, revealed that there were three major cox1 sequence types associated with type 3 sarcocysts, and one of these types were nearly identical with sequences of a species found in Italian roe deer in a concurrent study, which were subsequently named S. linearis (Gjerde et al., 2017). The latter sequence type were found in 32 sarcocyst isolates from the diaphragm and oesophagus of red deer CeS1, but not in any of the three available sarcocyst isolates from cardiac muscle, which indicated that the two species had different tissue preferences. Moreover, Hernández-Rodríguez et al. (1981a, b) described and named S. cervicanis mainly on the basis of sarcocysts from cardiac muscle. Unfortunately, the type material deposited at the Faculty of Veterinary Medicine, University of Córdoba, in connection with the description of that species (Hernández-Rodríguez et al., 1981a), could no longer be retrieved (Hernández-Rodríguez, personal communication 2016) and subjected to a molecular examination. We therefore focussed our efforts on identifying sarcocysts from cardiac muscle of three additional red deer (CeS2–CeS4). Of the 55 sarcocysts examined from these animals, 49 sarcocyst shared the same major sequence type as the three sarcocysts from cardiac muscle of red deer CeS1, while six sarcocysts shared the sequence type derived from sarcocysts in the diaphragm and oesophagus of that animal, as well as from sarcocysts in Italian roe deer (Table S3 in the Supplementary material). Hence, we attributed the sequence type predominating in sarcocysts from cardiac muscle to S. cervicanis in accordance with the original description of that species.
The sequence comparison and the phylogeny based on 18S rRNA gene sequences, however, suggested that an unnamed species from sika deer in Japan designated Sarcocystis sp. T18 (AB698065) might be identical with or a sister species to S. cervicanis, since this sequence clustered among sequences of the latter species. It will therefore be necessary to examine this species in sika deer at cox1 to determine its precise relationship to S. cervicanis. Likewise, the relationship of S. wapiti-like sarcocysts in wapiti in North-America to the morphologically similar species in red deer in Europe and elsewhere (Table 1) might only be determined through extensive molecular studies of such sarcocysts.
Only one of the seven sarcocysts from cardiac muscle processed for SEM in this study had an exposed surface, which allowed an examination of its surface structure. The sarcocyst was found to possess ribbon-like protrusions (Fig. 2) similar to those revealed in previous SEM studies of sarcocysts of S. grueneri from reindeer (Gjerde, 1986), S. taeniata in moose (Gjerde, 2014b) and S. linearis in roe deer (Gjerde et al., 2017). Since sarcocysts of S. cervicanis predominated in the heart of the three red deer from which sarcocysts were processed for SEM, it is likely that this sarcocyst also belonged to S. cervicanis. In the recent study of S. linearis in roe deer, intact protrusions of this type could be examined, revealing that they were ribbon-like in their proximal portion before terminating in a plate-like structure with an elliptic outline, together forming a seesaw-like structure (Gjerde et al., 2017). In the single sarcocyst examined in this study, only the ribbon-like proximal portion of each protrusion was present, probably because the delicate terminal plates had been torn off when the surrounding host cell was removed, as suggested by the distorted appearance of many protrusions. Thus, by TEM (Fig. 5 in Hernández-Rodríguez et al., 1981b), some of the protrusions of S. cervicanis are seen to have bifurcated (T-shaped) profiles, which represent sectioned terminal plates (Gjerde et al., 2017).
Although sarcocysts of S. cervicanis predominated in cardiac muscle of the red deer examined, a few sarcocysts of S. linearis were also identified. Likewise, three sarcocysts of S. linearis were identified in cardiac muscle of an Italian roe deer, whereas 16 sarcocysts were found in the diaphragm of another roe deer (Gjerde et al., 2017). Reissig et al. (2016) examined sarcocysts from both cardiac and skeletal muscle of red deer in Argentina and characterized a few of them at the 18S rRNA gene (KT626599–KT626602). The resulting sequences were assigned to S. taeniata, but as demonstrated in the present study and in the previous comparison of S. linearis with S. taeniata (Gjerde et al., 2017), these species cannot be separated on the basis of their 18S rRNA gene sequences. Hence, the abovementioned four sequences from Argentinian red deer and the two similar sequences from Lithuanian red deer (JN356126, JN356127) might belong to S. linearis rather than to S. taeniata as assumed by Reissig et al. (2016) and Prakas et al. (2016), respectively. This question might be resolved by examining sarcocyst isolates from red deer in these countries at cox1, since cox1 sequences seem to clearly separate the two species from each other. In addition, this study indicated that there might be consistent differences between sequences of S. linearis from red deer and sequences of S. taeniata from moose and sika deer near the 3′ end of the 18S rRNA gene (three versus five or six consecutive T’s). At cox1, the difference between the two lineages were slightly smaller after the addition of the new sequences from red deer, but there were still no overlap between the intra- and interspecific sequence variation, and sequences assigned to each species formed separate monophyletic clusters in the phylogenetic tree. Hence, we consider S. linearis in roe deer and red deer to be different from S. taeniata as reported from moose and sika deer (Gjerde, 2014b; Prakas et al., 2016).
From a type 3 sarcocysts from the oesophagus of red deer CeS1, unique sequences were obtained both at cox1 and the 18S rRNA gene, and these sequences were assigned to the new species S. morae. Since only a single sarcocyst had been examined by LM, we could not be absolutely sure whether this sarcocyst actually lacked visible protrusions or had possessed hair-like protrusions that were hidden by host cell material. Simple sequence comparisons indicated that this species was most closely related to species with ribbon-like cyst wall protrusions (S. cervicanis, S. taeniata) at cox1, whereas at the 18S rRNA gene, S. morae showed a similar identity to species with either hair-like or ribbon-like protrusions. However, in the phylogenetic analysis based on sequences of the latter gene, S. morae clustered with species having ribbon-like protrusions when the dataset comprising mainly species transmitted by canids was used (Fig. 4), but not when a larger dataset was used (Fig. S3 in the Supplementary material). We believe that the phylogeny based on the smaller dataset better reflected the relationship among the species included, since the sequences probably were more correctly aligned than in the larger dataset. Thus, in the phylogeny based on cox1 sequences (Fig. 3), S. morae were placed within a cluster comprising all species with ribbon-like protrusions, but also S. hircicanis. Based on these molecular findings, we believe that S. morae also possess ribbon-like cyst wall protrusions, and that its sarcocysts therefore might be indistinguishable by TEM and SEM from sarcocysts of S. cervicanis and S. linearis in red deer. Clearly, additional studies are necessary to characterize S. morae better morphologically, but the molecular data provided in this study, particularly the cox1 sequence, should be sufficient to delimit this species from other species with similar sarcocyst morphology in red deer or other hosts.
Three species with hair-like cyst wall protrusions were found in red deer CeS1. Of these, only S. hjorti was known from before, having been reported from both red deer and moose (Dahlgren & Gjerde, 2010a, b; Prakas, 2011; Stephan et al., 2012). However, when the single cyst of this species was examined by LM, no cyst wall protrusions were visible, which was also the case with several small sarcocysts of S. hjorti from cardiac muscle of red deer and moose in Norway (Dahlgren & Gjerde, 2010a). This was probably due to incomplete removal of the surrounding host cell material during cyst isolation. In the sarcocysts assigned to the two new species S. iberica and S. venatoria, on the other hand, hair-like protrusions were clearly seen by LM. Moreover, these species clustered with S. hjorti and other species with hair-like protrusions in the phylogenetic analysis. The cox1 sequence obtained from one of the sarcocysts with hair-like protrusions differed by 4% from the 10 identical sequences obtained from the 10 other sarcocysts of this type. This difference was considered to be sufficient to attribute the two sequence types to separate species, that is, S. iberica and S. venatoria. In comparison, the difference between all cox1 sequences assigned to S. linearis and those of S. taeniata was 2.9–4.2% and the difference between sequences of S. elongata in red deer and Sarcocystis tarandi in reindeer was 2.4–3.1% (Gjerde, 2014a). Moreover, the intraspecific divergence of cox1 sequences of similar length of various Sarcocystis spp. is less than 2%, and frequently less than 1.5% (Gjerde, 2013, 2014a, b, 2016; Gjerde et al., 2017). Hence, it is unlikely that the cox1 sequence attributed to S. iberica should represent an atypical sequence of S. venatoria, and even more so, since the 10 cox1 sequences of the latter species were identical. Based on the 18S rRNA gene sequences, on the other hand, neither S. iberica and S. venatoria, nor S. linearis and S. taeniata, or S. elongata and S. tarandi could be separated from each other. The same is true with this marker for several other such sister species in ruminants as reviewed by Gjerde et al. (2017). It is also a characteristic of such closely related sister species that they have considerable intra-isolate (intra-genomic) sequence variation in the 18S rRNA gene, which makes attempts to separate them on the basis of this marker even more difficult.
The assignment of isolates with indistinguishable 18S rRNA gene sequences to separate species on the basis of their cox1 sequences is based on the premise that differences at this marker above a certain level signify separate species. However, we still do not know the exact boundaries of each species at the cox1 marker, and with respect to S. iberica and S. venatoria, additional isolates ought to be examined in order to ascertain that they represent distinct species. The low bootstrap support for many of the nodes in the phylogenetic tree based on cox1 sequences (Fig. 3) seems to have been partially due to the large number of sequences (386) included in the analysis. Thus, a reduction of the dataset to 80 sequences through inclusion of a single sequence of each species only, except those found in the present study, resulted in much higher support values, but also changed the relationship between some taxa (data not shown). Other studies have also shown that bootstrap support decreases when the number of sequences included in the analysis is increased, whereas the phylogenetic accuracy increases with increased taxon sample size (Soltis & Soltis, 2003).
Molecular identification of sarcocysts in recent years have revealed that many Sarcocystis spp. are not as intermediate host specific as once believed. Some Sarcocystis spp. may also recently have become adapted to new intermediate host due to confinement of their hosts to smaller areas and new opportunities for cross-transmission as a result of human activity. Hence, it is possible that some of the species found in red deer CeS1 in this study may use other cervids as their principal intermediate hosts. Thus, S. morae, S. iberica and S. venatoria could possibly use fallow deer (Dama dama) as their principal host, since this cervid is sympatric with red deer in Quintos de Mora (Santín-Durán et al. 2004), but less numerous. Given that the sarcocysts of S. morae have band-like protrusions, they would be similar to sarcocysts of an unnamed Sarcocystis sp. reported from fallow deer in Germany and Italy (Entzeroth et al., 1985; Poli et al., 1988; Wesemeier & Sedlaczek, 1995b). No species with hair-like cyst wall protrusions have so far been reported from fallow deer, and none of the Sarcocystis spp. in fallow deer have so far been examined by molecular methods.
References
Dahlgren SS, Gjerde B (2010a) Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitology 137:815–840. https://doi.org/10.1017/S0031182009991569
Dahlgren SS, Gjerde B (2010b) The red fox (Vulpes vulpes) and the arctic fox (Vulpes lagopus) are definitive hosts of Sarcocystis alces and Sarcocystis hjorti from moose (Alces alces). Parasitology 137:1547–1557. https://doi.org/10.1017/S0031182010000399
Dubey JP, Jolley WR, Thorne ET (1983) Sarcocystis sybillensis sp. nov. from the north American elk (Cervus elaphus). Can J Zool 61:737–742. https://doi.org/10.1139/z83-098
Entzeroth R, Neméseri L, Scholtyseck E (1983) Prevalence and ultrastructure of Sarcocystis sp. from the red deer (Cervus elaphus L.) in Hungary. Parasitol Hung 16:47–52
Entzeroth R, Chobotar B, Scholtyseck E, Nemeseri L (1985) Light and electron microscope study of Sarcocystis sp. from the fallow deer (Cervus dama). Z Parasitenkd 71:33–39. https://doi.org/10.1007/bf00932916
Gjerde B (1986) Scanning electron microscopy of the sarcocysts of six species of Sarcocystis from reindeer (Rangifer tarandus tarandus). Acta Pathol Microbiol Immunol Scand [B] 94:309–317. https://doi.org/10.1111/j.1699-0463.1986.tb03058.x
Gjerde B (2013) Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitol 43:579–591. https://doi.org/10.1016/j.ijpara.2013.02.004
Gjerde B (2014a) Sarcocystis species in red deer revisited: with a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 141:441–452. https://doi.org/10.1017/S0031182013001819
Gjerde B (2014b) Morphological and molecular characteristics of four Sarcocystis spp. in Canadian moose (Alces alces), including Sarcocystis taeniata n. sp. Parasitol Res 113:1591–1604. https://doi.org/10.1007/s00436-014-3806-z
Gjerde B (2016) Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res 115:1473–1492. https://doi.org/10.1007/s00436-015-4881-5
Gjerde B, Giacomelli S, Bianchi A, Bertoletti I, Mondani H, Gibelli LR (2017) Morphological and molecular characterization of four Sarcocystis spp., including Sarcocystis linearis n. sp., from roe deer (Capreolus capreolus) in Italy. Parasitol Res 116:1317–1338. https://doi.org/10.1007/s00436-017-5410-5
Hernández-Rodríguez S, Navarrete I, Martínez-Gómez F (1981a) Sarcocystis cervicanis, nueva especie parásita del ciervo (Cervus elaphus). Rev Ibér Parasitol 41:43–51
Hernández-Rodríguez S, Martínez-Gómez F, Navarrete I, Acosta-García I (1981b) Estudio al microscopio óptico y electrónico del quiste de Sarcocystis cervicanis. Rev Ibér Parasitol 41:351–361
Hu JJ, Liu TT, Liu Q, Esch GW, Chen JQ, Huang S, Wen T (2016) Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming, China. Parasitol Res 115:3973–3981. https://doi.org/10.1007/s00436-016-5163-6
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kutkienė L (2003) Investigations of red deer (Cervus elaphus) Sarcocystis species composition in Lithuania. Acta Zool Lituanica 13:390–395. https://doi.org/10.1080/13921657.2003.10512311
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Luzón M, Domínguez-González J, Soto-Carrión AM, Alunda JM (2015) Sarcocystosis in Cervus elaphus: comparison of diagnostic methods. Int J Parasitol Parasites Wildl 4:396–400. https://doi.org/10.1016/j.ijppaw.2015.11.001
Poli A, Mancianti F, Marconcini A, Nigro M, Colagreco R (1988) Prevalence, ultrastructure of the cyst wall and infectivity for the dog and cat of Sarcocystis sp. from fallow deer (Cervus dama). J Wildl Dis 24:97–104. https://doi.org/10.7589/0090-3558-24.1.97
Prakas P (2011) Diversity and ecology of Sarcocystis in Lithuanian game fauna. PhD thesis, Vilnius University, Vilnius, Lithuania
Prakas P, Butkauskas D, Rudaityte E, Kutkiene L, Sruoga A, Puraite I (2016) Morphological and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus nippon) in Lithuania. Parasitol Res 115:3021–3032. https://doi.org/10.1007/s00436-016-5057-7
Prakas P, Rudaityte E, Butkauskas D, Kutkiene L (2017) Sarcocystis entzerothi n. sp. from the European roe deer (Capreolus capreolus). Parasitol Res 116:271–279. https://doi.org/10.1007/s00436-016-5288-7
Reissig EC, Moré G, Massone A, Uzal FA (2016) Sarcocystosis in wild red deer (Cervus elaphus) in Patagonia, Argentina. Parasitol Res 115:1773–1778. https://doi.org/10.1007/s00436-016-4915-7
Santín-Durán M, Alunda JM, Hoberg EP, de la Fuente C (2004) Abomasal parasites in wild sympatric cervids, red deer, Cervus elaphus and fallow deer, Dama dama, from three localities across central and western Spain: relationship to host density and park management. J Parasitol 90:1378–1386. https://doi.org/10.1645/GE-3376
Soltis PS, Soltis DE (2003) Applying the bootstrap in phylogeny reconstruction. Stat Sci 18:256–267. https://doi.org/10.1214/ss/1063994980
Son H-Y, Kim N-S, Ryu S-Y, Kim H-C, Rhee J-H, Cho J-G, Park B-K (2009) Ultrastructure of Sarcocystis grueneri-like sarcocysts from cardiac muscle of red deer (Cervus elaphus) in Korea. J Vet Clin 26:595–599
Speer CA, Dubey JP (1982) Sarcocystis wapiti sp. nov. from the north American wapiti (Cervus elaphus). Can J Zool 60:881–888. https://doi.org/10.1139/z82-120
Stephan R, Loretz M, Eggenberger E, Grest P, Basso W, Grimm F (2012) Erster Nachweis von Sarcocystis hjorti bei der Fleischuntersuchung von Rothirschen in der Schweiz. Schweiz Arch Tierheilkd 154:539–542. https://doi.org/10.1024/0036-7281/a000403
Wesemeier HH, Sedlaczek J (1995a) One known Sarcocystis species and two found for the first time in red deer and wapiti (Cervus elaphus) in Europe. Appl Parasitol 36:245–251
Wesemeier HH, Sedlaczek J (1995b) One known Sarcocystis species and one found for the first time in fallow deer (Dama dama). Appl Parasitol 36:299–302
Acknowledgements
The authors would like to thank Ms. Elena González Sánchez for her appreciated help in the collection of muscular samples from the animals selected for this study and Mr. Ángel Moreno Gómez, Director of Quintos de Mora, for allowing us access to the park in order to collect biological samples from hunted animals. We would also like to extend our gratitude to Ms. Michaela Salajkova at the EM lab, Department of Biosciences, University of Oslo, for her skilful operation of the scanning electron microscope.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 1096 kb)
Rights and permissions
About this article
Cite this article
Gjerde, B., Luzón, M., Alunda, J.M. et al. Morphological and molecular characteristics of six Sarcocystis spp. from red deer (Cervus elaphus) in Spain, including Sarcocystis cervicanis and three new species. Parasitol Res 116, 2795–2811 (2017). https://doi.org/10.1007/s00436-017-5590-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5590-z