Abstract
Plasmodium relictum (lineage pGRW4) causes malaria in birds and is actively transmitted in countries with warm climates and also temperate regions of the New World. In Europe, the lineage pGRW4 has been frequently reported in many species of Afrotropical migrants after their arrival from wintering grounds, but is rare in European resident birds. Obstacles for transmission of this parasite in Europe have not been identified. Culex quinquefasciatus is an effective vector of pGRW4 malaria, but this mosquito is absent from temperate regions of Eurasia. It remains unclear if the lineage pGRW4 completes sporogony in European species of mosquitoes. Here we compare the sporogonic development of P. relictum (pGRW4) in experimentally infected mosquitoes Culex pipiens pipiens form molestus, C. quinquefasciatus, and Ochlerotatus cantans. The pGRW4 parasite was isolated from a garden warbler Sylvia borin, multiplied, and used to infect laboratory-reared Culex spp. and wild-caught Ochlerotatus mosquitoes by allowing them to take blood meals on infected birds. The exposed females were maintained at a mean laboratory temperature of 19 °C, which ranged between 14 °C at night and 24 °C during daytime. They were dissected on intervals to study the development of sporogonic stages. Only ookinetes developed in O. cantans; sporogonic development was abortive. The parasite completed sporogony in both Culex species, with similar patterns of development, and sporozoites were reported in the salivary glands 16 days after infection. The presence of sporogonic stages of the lineage pGRW4 in mosquitoes was confirmed by PCR-based testing of (1) the sporozoites present in salivary glands and (2) the single oocysts, which were obtained by laser microdissection from infected mosquito midguts. This study shows that P. relictum (pGRW4) completes sporogony in C. p. pipiens at relatively low temperatures. We conclude that there are no restrictions for spreading this bird infection in Europe from the point of view of vector availability and temperature necessary for sporogony. Other factors should be considered and were discussed for the explanation of rare reports of this malaria parasite in Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasmodium relictum (Haemosporida, Plasmodiidae) causes malaria in birds and is the first in frequency of the occurrence of avian malaria parasite reported from over 300 species of birds at all continents, except the Antarctic (Garnham 1966; Valkiūnas 2005; Atkinson et al. 2008). Recent polymerase chain reaction (PCR)-based studies identified two P. relictum lineages, which are particularly and widely distributed both by hosts and geographically; these are pSGS1 and pGRW4 (Beadell et al. 2006; Ejiri et al. 2009; Clark et al. 2014; Perkins 2014). Both parasite lineages have been reported in birds all over the world, but areas of their transmission are different (Marzal et al. 2011).
The lineage pSGS1 of P. relictum is cosmopolitan in transmission (Bensch et al. 2009; Marzal et al. 2015) probably because numerous bird and mosquito species are susceptible to this infection (Valkiūnas 2005; Atkinson et al. 2008). Culex pipiens mosquitoes, which are distributed worldwide, are effective vectors of this parasite (Vézilier et al. 2010; Kazlauskienė et al. 2013), and the sporogony completes at a temperature of 12–30 °C (Garnham 1966; Valkiūnas 2005; Žiegytė et al. 2014). That enables transmission of the pSGS1 parasite in countries both with warm and cold climates, for example in northern Norway (Marzal et al. 2011).
Opposite to the pSGS1 parasite, the lineage pGRW4 of P. relictum has been reported to be actively transmitted mainly in countries with warm climates (Ricklefs et al. 2004; Beadell et al. 2006; Bensch et al. 2009; Marzal et al. 2011; Loiseau et al. 2012). Interestingly, there are numerous reports of the pGRW4 parasite in European migrants after their arrival from African wintering grounds, where the birds gain malaria (Bensch et al. 2007; Hellgren et al. 2007; Beadell et al. 2009), but a few studies detected this infection in resident European birds (Ferrer et al. 2012; Ferraguti et al. 2013; Drovetski et al. 2014), suggesting a lack of active transmission in Europe. Factors precluding transmission of the pGRW4 parasite in Europe remain unclear. Culex quinquefasciatus is an effective vector of this parasite (Atkinson et al. 2008; LaPointe et al. 2010; Freed and Cann 2013), but this insect is absent from temperate regions of Europe where C. pipiens mosquitoes act as active vectors of avian malaria (Vézilier et al. 2010; Kazlauskienė et al. 2013; Cornet et al. 2013). It remains unclear if the lineage pGRW4 can complete sporogony in C. pipiens and other widespread European mosquitoes and if that happens at relatively low temperatures. The Hawaiian strain of pGRW4 parasite completed sporogonic development in C. quinquefasciatus at constant laboratory and mean field temperatures between 17 and 30 °C, but development decreased significantly below 21 °C, with a minimum threshold temperature of 13 °C (LaPointe et al. 2010).
The main aim of this study was to compare sporogonic development of P. relictum (pGRW4) in mosquitoes Culex pipiens pipiens form molestus and C. quinquefasciatus at a temperature, which is close to the long-term mean air degrees reported in European temperate regions during the warmest months of the year. Ochlerotatus cantans, a widespread Eurasian bird-biting mosquito, was also exposed. We isolated one strain of the lineage pGRW4 from a naturally infected passeriform bird, infected three species of mosquitoes with this parasite, and followed sporogonic development in the exposed insects. The presence of sporogonic stages of P. relictum (pGRW4) in mosquitoes was confirmed by (1) the microscopic examination of exposed insects and (2) the PCR-based testing of the salivary gland sporozoites and also single oocysts, which were obtained by laser microdissection from infected mosquito midguts.
Materials and methods
Study site, experimental birds, and P. relictum (pGRW4) strain
The experimental research was carried out at the Biological Station of the Zoological Institute of the Russian Academy of Sciences on the Curonian Spit in the Baltic Sea (55° 09′ N, 20° 52′ E) in May–July, 2013 and 2014. Experimental procedures of this study were approved by the International Research Co-operation Agreement between the Biological Station Rybachy of the Zoological Institute of the Russian Academy of Sciences and Institute of Ecology of Nature Research Centre (25-05-2010). All efforts were made to minimize handling time and potential suffering of birds. None of the experimental birds suffered apparent injury during the experiments.
Nine juvenile siskins Carduelis spinus were caught and used for experimental infection (three birds) and control (six birds). The controls were used to monitor the absence of natural transmission in the laboratory. All birds were kept at quarantine in a mosquito-free room approximately for a week (adaptation period) before the experiments. Blood was taken from the birds by puncturing the brachial vein. About 30 μl of whole blood was taken in heparinized microcapillaries and stored in SET buffer (Hellgren et al. 2004) for molecular analysis. Two blood films were prepared from each bird immediately after withdrawal of the blood. They were air dried, fixed in absolute methanol, and stained with Giemsa, as described by Valkiūnas et al. (2008). Bird blood was tested for parasites on days 2 and 7 after their arrival at the laboratory. All birds were uninfected with blood parasites, as revealed by microscopic examination of blood films and PCR-based detection methods (see below).
We used one P. relictum (pGRW4) strain, which was isolated from a naturally infected garden warbler Sylvia borin in May 2013. Parasitemia was light (<0.001 %) in this bird. To multiply the strain, two juvenile siskins were exposed by intramuscular inoculation of approximately 50 μl of blood from the donor bird, as described by Palinauskas et al. (2008). Parasitemia developed in all infected siskins. These birds were maintained in a mosquito-free aviary and were used as donors of gametocytes to infect mosquitoes in 2014, i.e., a year after the initial exposure of the birds. Infected blood of these siskins also was used to induce a fresh infection in one siskin in 2014. In all, three siskins infected with the same strain of P. relictum (pGRW4) were used in this study: two birds were infected with the first passage and one bird contained the second passage of this parasite. To monitor parasitemia, blood for microscopic examination and PCR-based analysis was taken each 3 or 4 days during mosquito infection experiments. Control birds were tested at the same days. All birds were kept indoors in a vector-free room under controlled conditions [20 ± 1 °C, 50–60 % relative humidity (RH), natural light-dark photoperiod (L/D)] and were fed a standard diet for seed-eating or insectivorous bird species. They survived until the end of this study.
Maintenance and experimental infection of laboratory-reared mosquitoes
To establish a colony of C. p. pipiens f. molestus, we used the mosquito larvae, which were obtained from Dr. Roland Kuhn. The colony was originally started from the larvae collected in Hesse region (Germany). A colony of C. quinquefasciatus mosquitoes was established using eggs provided by Dr. Ana Rivero (France). Both mosquito colonies have been in continuous culture for many years. We colonized these insects, as described by Žiegytė et al. (2014). Briefly, mosquitoes were kept in mosquito cages (65 × 65 × 65 cm) under standard conditions (20 ± 1 °C, 60–65 % RH, and natural L/D photoperiod). Adult mosquitoes were fed with 5–10 % saccharose solution. Cotton wool pads moistened with this solution were provided in mosquito cages.
Two days before exposure, approximately 30 unfed females of each species were haphazardly chosen and placed inside separate experimental cages. To increase favor of blood feeding, the experimental mosquitoes were deprived of saccharose. Siskins with pGRW4 gametocytemia of approximately 0.1 % were placed in mosquito cages and exposed, as described by Kazlauskienė et al. (2013). Briefly, infected birds were placed in plastic tubes (length 15 cm, diameter 5 cm) containing a rip, which was used to fix the bird legs. Both tube ends were covered with bolting silk. Only the legs were exposed to mosquito bites. The birds were kept in insect cages for approximately 1 h once per 3–4 days. Both mosquito species willingly took blood meals on bird legs (Fig. 1a). We evaluated parasitemia in all donor siskins immediately after mosquito blood meal on birds. Experiments with C. p. pipiens and C. quinquefasciatus were carried out in parallel. Engorged females were taken from the experimental cages using an aspirator, placed in separate small insect cages (12 × 12 × 12 cm), maintained to allow development of parasites, and dissected in intervals (see below). All experimental mosquitoes were maintained at mean laboratory temperature regime of 19 °C, which ranged between 14 °C at night and 24 °C during daytime. This temperature regime was chosen because it is close to the long-term mean air temperatures, which have been reported in the majority of temperate regions of Europe during the warmest months of a year, i.e., between mid of June and mid of August. The data about mean air degrees during a 30-year observation period (1980–2010) were provided by the Physical Science Division, Earth System Research Laboratory, NOAA, Colorado (Web site at http://www.esrl.noaa.gov/psd/ ).
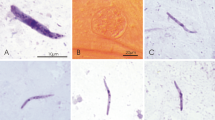
Experimental exposure of Culex quinquefasciatus (a) and Ochlerotatus cantans (b) mosquitoes by allowing them to take blood meals on the legs (a) and head (b) of malaria-infected birds. Short simple arrows—mosquitoes taking blood meals. The bird is held by a hand covered with a rubber glove (triangle arrowhead) and exposed to natural mosquito bites
Infected mosquitoes were kept until 18 days postinfection (dpi). Ookinete preparations were made 1–4 dpi, oocyst preparations 6–18 dpi, and sporozoite preparations 8–18 dpi. In total, we infected and dissected 48 mosquitoes; among them were 23 individuals of C. p. pipiens and 25 of C. quinquefasciatus.
Collection, experimental infection, and maintenance of wild-caught O. cantans
To collect engorged O. cantans females, infected siskins were held by hand and exposed to natural mosquito bites at the study site in the morning each day, as described by Valkiūnas et al. (2013). Briefly, when one to two females began taking a blood meal on a bird’s head (Fig. 1b), the head with feeding insects was carefully placed into an unzipped insect cage. The engorged mosquitoes fly off after the blood meal. The cage with engorged flies was then closed using a zipper. Up to 10 mosquitoes were allowed to take blood meals on each bird. Control mosquitoes were fed on noninfected siskins. Cages with engorged experimental and control females were transported to the laboratory and maintained at the same conditions, as Culex mosquitoes (see above).
To control for the presence of parasites, experimental and control insects were dissected for observation of ookinetes, oocysts, and sporozoites, as described for Culex mosquitoes. In total, we dissected 40 O. cantans mosquitoes; among them were 20 experimental and 20 control insects.
Additionally, to determine the prevalence of possible natural infection with Plasmodium parasites in O. cantans mosquitoes, 120 unfed females were haphazardly collected using an entomological net at the study site. They were tested by PCR-based methods (see below).
Dissection of mosquitoes and making preparations of ookinetes, oocysts, and sporozoites
All experimental and control mosquitoes were processed individually for microscopic and PCR-based detection of parasites. Before dissection, mosquitoes were lightly anesthetized by putting them into a tube closed with a cotton pad wetted in 96 % ethanol for several minutes. Wings and legs of the insects were removed before dissection, which was performed under a binocular stereoscopic microscope. Each mosquito body was carefully separated in three segments: the head, thorax, and abdomen. To eliminate contamination of samples, we either used a new dissecting needle for each dissected insect or disinfected the needles in fire after each dissection.
Permanent preparations of the semidigested contents of the midguts, entire midguts, and salivary glands were prepared in order to observe ookinetes, oocysts, and sporozoites, respectively. All preparations were prepared according to Kazlauskienė et al. (2013). Remnants of salivary gland preparations and thoraxes of the dissected insects were also fixed in 96 % ethyl alcohol for PCR-based screening.
Laser microdissection of single oocysts from mosquito midguts
Single oocysts were dissected from the midguts of experimentally infected C. p. pipiens mosquitoes at 13–14 dpi. Infected midguts were isolated from the insects’ abdomens, placed on membrane slides (Molecular Machines and Industries), cut with a tiny razor along the gut length, and stretched to make a thin gut layer. The midgut preparations were air dried, fixed, and used for the laser microdissection. The fixation was done by the straight application of 40, 50, 70, and finally 96 % ethyl alcohol on midguts, according to traditional histology protocols. The Olympus/MMI CellCut Plus® laser system (Molecular Machines and Industries) equipped with Predefined Target Position software (Molecular Machines and Industries) was used to cut off single oocysts. The parasites were readily visible on the unstained membrane slides (Fig. 2a), and single oocysts were captured on the silicon cap of microtubes (0.5 ml). The success of cell capture was confirmed by microscopic examination of the membrane slides (Fig. 2b) and tube caps. We prepared 100 tubes with single oocysts of Plasmodium parasites, isolated DNA, and determined parasite sequence, as described below.
Laser microdissection of single oocysts of Plasmodium relictum (lineage pGRW4) from midgut of exposed mosquito Culex pipiens pipiens form molestus: a membrane slide with oocysts 13 days postinfection and b membrane slide after dissection of one oocyst. Short simple arrow—oocysts; long simple arrow—a hole in the membrane after dissection of one oocyst. Ethyl alcohol fixed mosquito midgut. Scale bar = 10 μm
DNA extraction, PCR, and sequencing
PCR-based methods were used (1) to determine the presence and identity of parasite lineages in birds, (2) to confirm the identity of parasite lineage of sporozoites reported in salivary glands, and (3) to confirm the identity of parasite lineage of single oocysts dissected from midguts. We applied the same protocols, as described by Palinauskas et al. (2010) and Kazlauskienė et al. (2013). Briefly, we used (1) the standard ammonium-acetate method to extract genomic DNA from birds’ whole blood, the salivary glands, and thoraxes of mosquitoes and (2) the Chelex® extraction methods for DNA extraction from single oocysts. The latter method has been rarely applied in wildlife haemosporidian research; it is described here in more detail. We applied 0.2 g of Chelex suspended in 1000 μl of ddH2O and kept for 1 h in a 56 °C water bath. The small drop of 25 μl of Chelex suspension was attached to the wall of the tube with excised cells, which were on the silicon cap of the tube. Then, 0.7 μl of Proteinase K (10 mg/ml) was added to the Chelex drop and the tube was closed, then turned upside-down, and shaken down once so that the Chelex with Proteinase K was covering all the surface of the cap. The tube was kept in a water bath at 56 °C for 1 h and mixed every 20 min followed by boiling in water for 12 min to inactivate the Proteinase K. The sample was centrifuged briefly to recover the extraction mix at the bottom of the tube. The silicon caps were replaced with standard caps and the tubes were centrifuged at 12,000 rpm for 12 min. The supernatant (about 10–15 μl) was immediately transferred with a sterile micropipette into a new tube and was ready to use for molecular analysis. PCR and sequencing protocols for single oocyst DNA analysis were the same as for blood samples.
For genetic analysis of all samples, a nested PCR protocol was applied (Hellgren et al. 2004). We amplified a segment of parasite mitochondrial cytochrome b gene (cyt b) using two pairs of primers, HaemNFI and HaemNR3, which amplify fragments of cyt b gene of haemosporidians belonging to Haemoproteus, Plasmodium, and Leucocytozoon. For the second PCR, we used primers HAEMF and HAEMR2, which are specific to Haemoproteus and Plasmodium spp. (Bensch et al. 2000). All amplifications were evaluated by running 1.5 μl of the final PCR product on a 2 % agarose gel. For sequencing, we used the procedures described by Bensch et al. (2000). One negative control (nuclease-free water) and one positive control (P. relictum microscopy positive blood sample, in the case of blood testing, and thoraxes of two C. p. pipiens mosquitoes experimentally infected with P. relictum in the case of mosquito testing) were used per 10 samples to control for false amplifications. No case of false amplification was found.
All positive samples were sequenced in order to determine cyt b lineages of the detected parasites. Fragments were sequenced from the 5′ end with the primer HAEMF. Dye terminator cycling sequencing (BigDye) was used and loaded on an ABI PRISMTM 3100 capillary sequencing robot (Applied Biosystems, Foster City, CA). Good quality sequences were obtained; they were edited and aligned using BioEdit (Version 7.0.9.0; Hall 1999) and deposited in the PopSet database of the US National Center for Biotechnology Information (accessions KR139675–KR139678).
Microscopic examination of blood and vector preparations and parasite morphology
An Olympus BX43 light microscope equipped with Olympus SZX2-FOF digital camera and imaging software QCapture Pro 6.0, Image-Pro Plus (Tokyo, Japan) was used to examine preparations, prepare illustrations, and to take measurements. Approximately 100–150 fields were examined in blood films at low magnification (×400), and then at least 100 fields were studied at high magnification (×1000). Intensity of parasitemia was estimated as a percentage by actual counting of the number of parasites per 1000 erythrocytes or per 10,000 erythrocytes if infections were light (<0.1 %). Malaria parasites were identified according to Valkiūnas (2005). All vector preparations were first examined at low magnification (×200, ×600) and then at high magnification (×1000). The morphometric features studied (Table 1) were those defined by Valkiūnas (2005). The analyses were carried out using the “Statistica 7” package. Student’s t test for independent samples was used to determine statistical significance between mean linear parameters of parasites. A P value of 0.05 or less was considered significant. Voucher specimens of ookinetes (accession numbers 48866–48868 NS), oocysts (48869–48872 NS), and sporozoites (48873–48874 NS) of P. relictum (pGRW4) were deposited in the Institute of Ecology, Nature Research Centre, Vilnius, Lithuania.
Results
According to both PCR-based analysis and microscopic examination of blood films, malaria parasites were absent from all siskins prior to infection, and all negative controls remained uninfected throughout the course of the experiment. PCR-based detection did not reveal natural malarial infections both in control and wild-caught O. cantans females, indicating that the wild-caught insects used in our experiments were malaria free.
Parasitemia developed in all experimentally infected siskins (Fig. 4a, b). Siskins with gametocyte parasitemia of approximately 0.1 % were used as donors to infect mosquitoes. Infectivity of the first and the second parasite passages to all mosquito species was the same. Few mature ookinetes were observed in O. cantans (Fig. 3). Oocysts and sporozoites did not develop, indicating the abortive sporogonic development of P. relictum (pGRW4) at ookinete stage in this mosquito species.
All experimental C. p. pipiens and C. quinquefasciatus mosquitoes were susceptible to infection. Ookinetes, oocysts, and sporozoites (Fig. 4c–j) were reported in both species of insects at the same dpi. In other words, the pattern of sporogonic development of this malaria strain was the same in these mosquito species. For both mosquitoes, (1) ookinetes were seen 1 dpi, their number markedly decreases 2 dpi, and the parasites were not seen in the midgut 3 dpi; (2) growing oocysts were numerous in the midgut 6 dpi, and they developed markedly asynchronously; (3) mature oocysts were seen 14 dpi; and (4) sporozoites were first reported in the salivary glands 16 dpi and were seen until the end of the experiment (18 dpi). Thousands of sporozoites were seen in salivary gland preparations of both mosquito species, indicating successful sporogony (Fig. 5). Morphologically similar sporogonic stages developed in C. p. pipiens and C. quinquefasciatus (Fig. 4c–j). There were no morphometric differences discernable among mature ookinetes (Fig. 4c, d) or sporozoites (Fig. 4i, j), which developed in different mosquito species (Table 1, P > 0.2 for all corresponding data). However, significantly larger mature oocysts (Fig. 4e–h) were seen in C. quinquefasciatus (Table 1, P < 0.01 for oocysts’ area).
Gametocytes of Plasmodium relictum (lineage pGRW4) in the circulation of siskins Carduelis spinus (a, b) and ookinetes (c, d), oocysts (e–h), and sporozoites (i, j) of the same parasite in mosquitoes Culex pipiens pipiens form molestus (c, e, g, i) and Culex quinquefasciatus (d, f, h, j): a, b mature macrogametocyte and microgametocyte, respectively; c, d mature ookinetes possessing prominent nuclei and pigment granules; e, f growing oocysts 13 days postinfection (dpi); g, h nearly mature oocysts 16 dpi (note numerous germinal centers and developing elongate sporozoites); i, j salivary gland sporozoites 16 dpi. Methanol-fixed and Giemsa-stained thin films (a–d, i, j). Formalin-fixed whole mounts stained with Erlich’s hematoxylin (e–h). Long simple arrows—nuclei of parasites; simple arrowheads—pigment granules. Scale bars = 10 μm: long bar is for gametocyte, ookinete, and sporozoite images, and short bar is for oocyst images
PCR and sequencing confirmed the presence of the lineage pGRW4 in samples of (1) the salivary glands with sporozoites (Fig. 5) and (2) the single oocysts dissected from the midguts of experimentally infected C. p. pipiens mosquitoes (Fig. 2).
Discussion
The lineage pGRW4 of P. relictum is one of the most well known in avian malariology due to its virulence and markedly invasive nature. This parasite was introduced to the Hawaiian Islands, caused lethal malaria in birds, and even contributed to the extinction of many species of the endemic Hawaiian honeycreepers belonging to the Drepanididae (Freed and Cann 2013; Atkinson et al. 2014). C. quinquefasciatus transmits the pGRW4 parasite (Atkinson et al. 2008; LaPointe et al. 2010). Experimental studies on other possible vectors of this infection are lacking. This study shows that O. cantans mosquitoes are resistant to the pGRW4 infection, which aborts sporogony at the ookinete stage (Fig. 3). In other words, we rule out the involvement of O. cantans in the transmission of P. relictum (pGRW4). This mosquito was formerly a member of the genus Aedes, many species of which transmit closely related avian Plasmodium parasites, including some strains of P. relictum, whose genetic lineages remain unidentified (Valkiūnas 2005; Santiago-Alarcon et al. 2012).
The key result of this study is that P. relictum (pGRW4) readily completed sporogony in C. p. pipiens f. molestus mosquitoes, with massive infection of sporozoites reported in the salivary glands (Fig. 5). The rates of sporogony as well as morphology of sporogonic stages of this parasite lineage were similar in C. p. pipiens and C. quinquefasciatus mosquitoes. However, slightly larger mature oocysts were observed in the latter species (Table 1). That might be related to differences in feeding activity of these insects in our colonies: C. quinquefasciatus mosquitoes usually feed more actively on birds than C. p. pipiens f. molestus (R. Žiegytė, personal observation), resulting in a larger blood meal and potentially greater nutrition resources, which are necessary for development of sporogonic stages. Malaria parasites actively use the host resources during sporogony. For example, oocyst capsular wall is built from materials obtained from invertebrate hosts, and it contains polysaccharides, electron-dense granules, and membrane-bound bubbles of the host origin (Mehlhorn et al. 1980; Valkiūnas 2005). In human Plasmodium spp., the same parasite isolates also produce oocysts of different sizes in different Anopheles mosquitoes and even in different individuals of the same mosquito species (Sherman 1998). This study shows that the same occurs in P. relictum (pGRW4) during sporogony in different species of Culex (Table 1).
Importantly, the sporogony completed and numerous sporozoites appeared in the salivary glands both of C. p. pipiens and C. quinquefasciatus mosquitoes at relatively low temperature conditions, which are close to the long-term mean air degrees reported in the majority of European regions with temperate climates during the warmest months of a year (Earth System Research Laboratory, Colorado; Web site at http://www.esrl.noaa.gov/psd/) when active transmission of malaria parasites occurs (Valkiūnas 2005). Former experimental studies also reported complete sporogony of this parasite in C. quinquefasciatus at the same temperatures (LaPointe et al. 2010). Because (1) C. p. pipiens f. molestus mosquito is widespread in the Holarctic (Vinogradova 2000; Gomes et al. 2009) and (2) sporogony of P. relictum (pGRW4) successfully completes at a mean temperature of 19 °C in this mosquito 16 dpi, we conclude that there are no restrictions for spreading of this avian malaria infection in temperate regions of Europe from the point of view of vector availability and air temperature necessary for sporogony. Reports of this parasite in resident birds at the Iberian Peninsula and Transcaucasia are in accordance with this conclusion (Ferrer et al. 2012; Ferraguti et al. 2013; Drovetski et al. 2014). Culex p. pipiens f. molestus is characterized by broad ecological plasticity and is particularly common in human settlements where it often breeds in sewers, but also occurs in natural sheltered habitats such as caves and other similar ecological niches (Vinogradova 2000; Gomes et al. 2009). We support the hypothesis that this mosquito can act as vector of P. relictum (pGRW4).
It is important to note that the currently used PCR-based diagnostics often do not read malarial coinfections (Martínez et al. 2009; Dimitrov et al. 2015). Additionally, the blood and vector stages of the lineages pSGS1 and pGRW4 of P. relictum are similar morphologically (Palinauskas et al. 2007; Valkiūnas et al. 2007; Kazlauskienė et al. 2013; Žiegytė et al. 2014; Marzal et al. 2015; this study). Morphological characters, which can be used to distinguish these infections both at blood or sporogonic stages, have not been identified. In other words, if a coinfection of the lineages pSGS1 and pGRW4 of P. relictum is present in our donor birds, we might not distinguish this coinfection both by the PCR-based tools and microscopic examination, with possible reported complete sporogony of the pSGS1 lineage, but not of the pGRW4 lineage. To prove that this study deals with complete sporogony of the lineage pGRW4, we determined DNA sequences of (1) the sporozoites present in the salivary glands (Fig. 5) and (2) the single mature oocysts dissected from the midguts of C. p. pipiens mosquitoes (Fig. 2b). All tested samples of the salivary gland sporozoites and the single midgut oocysts belonged to the pGRW4 lineage, indicating that this malaria parasite completes sporogony in C. p. pipiens mosquitoes.
An issue why P. relictum (pGRW4) has been rarely reported in European birds needs additional research, and three possible explanations are worthy of discussion. First, the rare reports of pGRW4 lineage in Europe might be due to mortality caused by this infection in nonadapted local birds, particularly juveniles as is the case in the Hawaiian Island (Atkinson et al. 2008). In spite of numerous reports of the pGRW4 parasite all over the world (Hellgren et al. 2007; Bensch et al. 2007; Beadell et al. 2009; Marzal et al. 2011; Clark et al. 2014), there is no experimental data about virulence of this infection in different species of wild birds, except several experimental studies on the Hawaiian Island (Atkinson et al. 2008; LaPointe et al. 2010) and limited observations in Europe (Dimitrov et al. 2015). Lethal parasite infections are difficult to record in wildlife, particularly due to rapid elimination of weak or dead individuals by predators (Valkiūnas 2005; Møller and Nielsen 2007; Dinhopl et al. 2015). Experimental studies are needed for better understanding the virulence of P. relictum (pGRW4) in different species of birds. We did not observe mortality of birds infected with this parasite; however, the number of exposed host individuals and the experimental design were insufficient to make conclusions on this issue. The level of invasiveness of the lineage pGRW4 of P. relictum in Europe also needs to be identified.
Second, the rare reports of the lineage pGRW4 of P. relictum might be due to shortcomings of the currently used PCR-based diagnostic tools. Mainly, the lineage pGRW4 can be overlooked during its coinfection with the lineages pSGS1, which is a predominant lineage of avian malaria parasites in Europe (Palinauskas et al. 2007; Hellgren et al. 2007; Bensch et al. 2009; Dimitrov et al. 2010; Marzal et al. 2011). That is not unexpected because the PCR-based tools often do not read various haemosporidian coinfections (Martínez et al. 2009; Braga et al. 2011; Clark et al. 2014; Dimitrov et al. 2015; Valkiūnas 2015). It is worth mentioning in this content that the lineage pGRW4 is often found by PCR tools in the New World probably due to the lack of the lineage pSGS1 from many investigated sites; this lineage is rare or is of patchy distribution in the Americas (Marzal et al. 2011, 2015). In other words, the rare cases of this coinfection in the Americas might explain numerous PCR-based records of the lineage pGRW4 due to the lack of preferable amplification of the lineage pSGS1.
Third, rare reports of P. relictum (pGRW4) might be due to low susceptibility of some European bird species to some isolates of pGRW4. The limited experimental evidence shows that the lineage pGRW4, which was isolated from the great reed warblers Acrocephalus arundinaceus, seems to be quite specific. It did not develop in the experimentally exposed house sparrows Passer domesticus, blackcaps Sylvia atricapilla, and chaffinches Fringilla coelebs (Dimitrov et al. 2015). If (1) the vertebrate host specificity of different isolates of the lineage pGRW4 is different and (2) the currently used molecular markers do not recognize these isolates, then future studies should aim testing more variable genes for revealing such isolates (Hellgren et al. 2013). Certainly, further experimental studies are needed for better understanding the sensitivity of the currently used PCR-based diagnostic tools in the detection of different haemosporidian coinfections, particularly in relation to parasite biodiversity research and epidemiology (Jarvi et al. 2013; Valkiūnas et al. 2014; Dimitrov et al. 2015; Valkiūnas 2015). Because haemosporidian coinfections of different malaria parasites are predominant in European birds (Valkiūnas et al. 2008; Martínez et al. 2009; Dimitrov et al. 2010), we call for the experimental testing of sensitivity of different primers in the detection of coinfection of pSGS1 and pGRW4 and other malaria parasites.
Haemosporidians of the genus Plasmodium cause acute, chronic, and debilitating malaria in many species of birds all over the world (Stone et al. 1971; Gabaldon and Ulloa 1980; Valkiūnas 2005; Chagas et al. 2013; Vanstreels et al. 2014; Dinhopl et al. 2015; Palinauskas et al. 2015). The virulence of the same lineages of Plasmodium spp. is markedly different in different species of birds (Palinauskas et al. 2008; Atkinson et al. 2008; Dimitrov et al. 2015). Species of Plasmodium are usually considered to be particularly virulent in nonadapted avian hosts, in which mortality and even devastation epizooties have been reported (Garnham 1966; Bennett et al. 1993; Atkinson et al. 2008; Murata et al. 2008; Braga et al. 2011). However, the application of the in situ hybridization diagnostic tools provided evidence that even widespread Plasmodium lineages can cause lethal diseases in common European wild birds that have been regularly exposed and, potentially, should be adapted to these infections (Dinhopl et al. 2015). In the latter case, malaria parasites caused marked damage of internal organs by exoerythrocytic meronts, which can be found all over the body, including the brain, likely resulting in cerebral paralysis. These findings and the recent field and experimental observations (Valkiūnas et al. 2014; Dimitrov et al. 2015; González et al. 2015) indicate that more research and new approaches are needed for better understanding the patterns of transmission and pathogenicity of avian malaria and related haemosporidian infections in wildlife.
In conclusion, this study adds C. p. pipiens f. molestus to the list of vectors of P. relictum (pGRW4). We show that there are no restrictions for spreading of this parasite lineage in European birds from the point of view of vector availability and air temperature necessary for sporogony. Other factors should be considered for the explanation of rare reports of the lineage pGRW4 in Europe. Both the infectivity of P. relictum (pGRW4) in European birds and the shortcomings of its PCR-based diagnostics during malarial coinfections remain unknown and worth considering first of all.
References
Atkinson CT, Thomas NJ, Hunter DB (2008) Parasitic diseases of wild birds. Wiley-Blackwell, Oxford
Atkinson CT, Utzurrum RB, LaPointe DA, Camp RJ, Crampton LH, Foster JT, Giambelluca TW (2014) Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Glob Chang Biol 20:2426–2436
Beadell JS, Ishtiaq F, Covas R, Melo M, Warren BH, Atkinson CT, Bensch S, Graves GR, Jhala YV, Peirce MA, Rahmani AR, Fonseca DM, Fleischer RC (2006) Global phylogeographic limits of Hawaii’s avian malaria. Proc R Soc B 273:2935–2944
Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins S, Graves GR, Fleischer RC (2009) Host associations and evolutionary relationships of avian blood parasites from West Africa. Int J Parasitol 39:257–266
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenicity. J Nat Hist 27:993–1001
Bensch S, Stjenman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Torres-Pinheiro R (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc B 276:1583–1589
Bensch S, Waldenström J, Jonzén N, Westerdahl H, Hansson B, Sejberg D, Hasselquist D (2007) Temporal dynamics and diversity of avian malaria parasites in a single host species. J Anim Ecol 76:112–122
Bensch S, Hellgren O, Pérez-Tris J (2009) A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358
Braga EM, Silveira P, Belo NO, Valkiūnas G (2011) Recent advances in the study of avian malaria: an overview with an emphasis on the distribution of Plasmodium spp. in Brazil. Mem Inst Oswaldo Cruz 1:3–11
Chagas CRF, Valkiūnas G, Nery CVC, Henrique PC, Gonzalez IHL, Monteiro EF, de Oliveira Guimarães L, Romano CM, Kirchgatter K (2013) Plasmodium (Novyella) nucleophilum from an Egyptian goose in Sao Paulo Zoo, Brazil: microscopic confirmation and molecular characterization. Int J Parasitol Parasites Wildl 2:286–291
Clark NJ, Clegg SM, Lima MR (2014) A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data. Int J Parasitol 44:329–338
Cornet SA, Nicot A, Rivero A, Gandon S (2013) Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar J 12:179
Dimitrov D, Zehtindjiev P, Bensch S (2010) Genetic diversity of avian blood parasites in SE Europe: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitol 55:201–209
Dimitrov D, Palinauskas V, Iezhova TA, Bernotienė R, Ilgūnas M, Bukauskaitė D, Zehtindjiev P, Ilieva M, Shapoval AP, Bolshakov CV, Markovets MY, Bensch S, Valkiūnas G (2015) Plasmodium spp.: an experimental study on vertebrate host susceptibility to avian malaria. Exp Parasitol 148:1–16
Dinhopl N, Nedorost N, Mostegl MM, Weissenbacher-Lang C, Weissenböck H (2015) In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol Res 114:1455–1462
Drovetski SV, Aghayan SA, Mata VA, Lopes RJ, Mode NA, Harvey JA, Voelker G (2014) Does the niche breadth or trade-off hypothesis explain the abundance-occupancy relationship in avian Haemosporidia? Mol Ecol 23:3322–3329
Ejiri H, Sato Y, Sawai R, Sasaki E, Matsumoto R, Ueda M, Higa Y, Tsuda Y, Omori S, Murata K, Yukawa M (2009) Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol Res 105:629–633
Ferraguti M, Martínez-de la Puente J, Ruiz S, Soriguer R, Figuerola J (2013) On the study of the transmission networks of blood parasites from SW Spain: diversity of avian haemosporidians in the biting midge Culicoides circumscriptus and wild birds. Parasit Vectors 6:208. doi:10.1186/1756-3305-6-208
Ferrer ES, García-Navas V, Sanz JJ, Ortego J (2012) Molecular characterization of avian malaria parasites in three Mediterranean blue tit (Cyanistes caeruleus) populations. Parasitol Res 111:2137–2142
Freed LA, Cann RL (2013) Vector movement underlies avian malaria at upper elevation in Hawaii: implications for transmission of human malaria. Parasitol Res 112:3887–3895
Gabaldon A, Ulloa G (1980) Holoendemicity of malaria: an avian model. Trans R Soc Trop Med Hyg 74:501–507
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell, Oxford
Gomes BCA, Sousa MT, Novo FB, Freitas R, Alves AR, Corte-Real P, Salgueiro M, Donnelly AP, Almeida AP, Pinto J (2009) Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol Biol 9:262
González AD, Lotta IA, García LF, Moncada LI, Matta NE (2015) Avian haemosporidians from Neotropical highlands: evidence from morphological and molecular data. Parasitol Int 64:48–59
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser 41:95–98
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Hellgren O, Waldenström J, Peréz-Tris J, Szöll E, Si O, Hasselquist D, Krizanauskienė A, Ottosson U, Bensch S (2007) Detecting shifts of transmission areas in avian blood parasites: a phylogenetic approach. Mol Ecol 16:1281–1290
Hellgren O, Kutzer M, Bensch B, Valkiūnas G, Palinauskas V (2013) Identification and characterization of the merozoite surface protein 1 (msp1) gene in a host-generalist avian malaria parasite, Plasmodium relictum (lineages SGS1 and GRW4) with the use of blood transcriptome. Malar J 12:381
Jarvi SI, Farias ME, LaPointe DA, Belcaid M, Atkinson CT (2013) Next-generation sequencing reveals cryptic mtDNA diversity of Plasmodium relictum in the Hawaiian Islands. Parasitology 140:1741–1750
Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA, Valkiūnas G (2013) Plasmodium relictum (lineages pSGS1 and pGRW11): complete synchronous sporogony in mosquitoes Culex pipiens pipiens. Exp Parasitol 133:454–461
LaPointe DA, Goff ML, Atkinson CT (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J Parasitol 96:318–324
Loiseau C, Harrigan RJ, Robert A, Bowie RC, Thomassen HA, Smith TB, Sehgal RN (2012) Host and habitat specialization of avian malaria in Africa. Mol Ecol 21:431–441
Martínez J, Martínez-De La Puente J, Herrero J, Del Cerro S, Lobato E, Rivero-De Aguilar J, Vásquez RA, Merino S (2009) A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology 136:713–722
Marzal A, Ricklefs RE, Valkiūnas G, Albayrak T, Arriero E, Bonneaud C, Czirják GA, Ewen J, Hellgren O, Hořáková D, Iezhova TA, Jensen H, Križanauskienė A, Lima MR, de Lope F, Magnussen E, Martin LB, Møller AP, Palinauskas V, Pap PL, Pérez-Tris J, Sehgal RN, Soler M, Szöllősi E, Westerdahl H, Zetindjiev P, Bensch S (2011) Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS One 6(7):e21905. doi:10.1371/journal.pone.0021905
Marzal A, Luz García-Longoria L, Cárdenas Callirgos JM, Sehgal RNM (2015) Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol Invasion 17:39–45
Mehlhorn H, Peters W, Haberkorn A (1980) The formation of kinetes and oocyst in Plasmodium gallinaceum (Haemosporidia) and considerations on phylogenetic relationships between Haemosporidia, Piroplasmida and other Coccidia. Protistologica 16:135–154
Møller AP, Nielsen JT (2007) Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881
Murata K, Nii R, Sasaki E, Ishikawa S, Sato Y, Sawabe K, Tsuda Y, Matsumoto R, Suda A, Ueda M (2008) Plasmodium (Bennettinia) juxtanucleare infection in a captive white eared-pheasant (Crossoptilon crossoptilon) at a Japanese zoo. J Vet Med Sci 70:203–205
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b gene lineages and morphospecies of two avian malaria parasites of the subgenera of Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae). Zootaxa 1626:39–50
Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S (2008) Plasmodium relictum (lineage P-SGS1): effects on experimentally infected passerine birds. Exp Parasitol 120:372–380
Palinauskas V, Dolnik OV, Valkiūnas G, Bensch S (2010) Laser microdissection microscopy and single cell PCR of avian hemosporidians. J Parasitol 96:420–424
Palinauskas V, Žiegytė R, Ilgūnas M, Iezhova TA, Bernotienė R, Bolshakov C, Valkiūnas G (2015) Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int J Parasitol 45:51–62
Perkins SL (2014) Malaria’s many mates: past, present and future of the systematics of the order Haemosporida. J Parasitol 100:11–25
Ricklefs RE, Fallon SM, Bermingham E (2004) Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst Biol 53:111–119
Santiago-Alarcon D, Palinauskas V, Schaefer HH (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev 87:928–964
Sherman IW (ed) (1998) Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington
Stone WB, Weber BL, Parks FJ (1971) Morbidity and mortality of birds due to avian malaria. N. Y. Fish Game J 18:62–63
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC, Boca Raton
Valkiūnas G, Zehtindjiev P, Hellgren O, Ilieva M, Iezhova TA, Bensch S (2007) Linkage between mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites, with a description of Plasmodium (Novyella) ashfordi sp. nov. Parasitol Res 100:1311–1322
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401
Valkiūnas G, Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA (2013) Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res 112:2159–2169
Valkiūnas G, Palinauskas V, Ilgūnas M, Bukauskaitė D, Dimitrov D, Bernotienė R, Zehtindjiev P, Ilieva M, Iezhova TA (2014) Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 113:2251–2263
Valkiūnas G (2015) Haemoproteus. In: Mehlhorn H (ed) Encyclopedia of parasitology, 4th edn. Springer, Heidelberg
Vanstreels RE, Kolesnikovas CK, Sandri S, Silveira P, Belo NO, Ferreira Junior FC, Epiphanio S, Steindel M, Braga ÉM, Catão-Dias JL (2014) Outbreak of avian malaria associated to multiple species of Plasmodium in magellanic penguins undergoing rehabilitation in southern Brazil. PLoS One 9:e94994. doi:10.1371/journal.pone.0094994
Vézilier J, Nicot A, Gandon S, Rivero A (2010) Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar J 9:379. doi:10.1186/1475-2875-9-379
Vinogradova EB (2000) Mosquitoes Culex pipiens pipiens: taxonomy, distribution, ecology, physiology, genetics and control. PenSoft, Sofia
Žiegytė R, Bernotienė R, Bukauskaitė D, Palinauskas V, Iezhova TA, Valkiūnas G (2014) Complete sporogony of Plasmodium relictum (lineages pSGS1 and pGRW11) in mosquito Culex pipiens pipiens form molestus, with implications to avian malaria epidemiology. J Parasitol 100:878–882
Acknowledgments
We would like to thank the director of the Biological Station “Rybachy,” Casimir V. Bolshakov, for generously providing the facilities for the experimental research and the staff of the Biological Station “Rybachy” for assistance in the field. The authors are grateful to Dr. Roland Kuhn and Dr. Ana Rivero for providing samples of the C. p. pipiens f. molestus and C. quinquefasciatus mosquitoes, respectively, and Dr. Arūnas Bukantis for consultations on climatology. Data about long-term mean air degrees in Europe were provided by the Physical Science Division, Earth System Research Laboratory, NOAA, Boulder, Colorado, from their Web site at http://www.esrl.noaa.gov/psd/. Care and handling of experimental animals was in accordance with the current laws of Lithuania and Russia. This research was supported by the Open Access to research infrastructure of the Nature Research Centre under Lithuanian open access network initiative. Our sincere thanks to the Department of Molecular and Regenerative Medicine, Hematology, Oncology and Transfusion Medicine Centre of the Vilnius University Hospital Santariškių Klinikos for opportunities to use the laser microdissection microscope. This study was partly supported by the Lithuanian Science Foundation (award no. MIP-15022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valkiūnas, G., Žiegytė, R., Palinauskas, V. et al. Complete sporogony of Plasmodium relictum (lineage pGRW4) in mosquitoes Culex pipiens pipiens, with implications on avian malaria epidemiology. Parasitol Res 114, 3075–3085 (2015). https://doi.org/10.1007/s00436-015-4510-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4510-3