Abstract
Several species of captive birds at zoological gardens of Japan were found to be infected with avian Plasmodium. However, incriminated vector mosquito species have not been identified yet. To indicate the competent vectors of avian malaria parasite, we collected mosquitoes at a zoological garden in Japan and examined for the avian malaria parasite DNA. Totally, 1,361 mosquitoes of 11 species were collected in the zoological garden of Kanagawa, the south of Tokyo in Japan in 2005. Captured mosquitoes were pooled by each species, date collected, and location and used for DNA extraction. Eight out of 169 DNA samples were positive for the nested PCR of avian Plasmodium cyt b gene. Estimated minimum infection rates of mosquitoes were 5.9 per 1,000. The PCR positive mosquito species were Culex pipiens group and Lutzia vorax. Some DNA sequences amplified from collected mosquitoes were identical to avian Plasmodium lineages detected from captive birds in the same zoological garden studied. Our results suggest that C. pipiens group and L. vorax could be incriminated vectors of avian malaria parasite transmitting in captive birds kept in the zoological garden in Japan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, several species of wild and captive birds were found to be infected with various avian blood protozoa including Plasmodium, Haemoproteus, and Leucocytozoon as well as in other countries (Murata 2002; Hagihara et al. 2004; Nagata 2006; Murata et al. 2008a, b). Some avian malaria parasite infections of the captive birds caused serious clinical condition and induced fatal results to the host birds in USA, Brazil, New Zealand, and Asia (Fleischman et al. 1968; Bennett et al. 1993; Clarke and Kerry 1993; Jones and Shellam 1999; Murata 2002; Grim et al. 2004; Alley et al. 2008; Belo et al. 2009); however, pathogenesis of captive birds in Japan have not been investigated in detail. Furthermore, pathology of these avian Plasmodium in wild birds were still unclear yet especially in the endangered species of Japan. For the prevention and control of the avian hematozoa infections in Japan, the transmission cycles of these blood protozoa should be investigated for the host birds and vectors. Blood sucking arthropods such as mosquitoes (Diptera; Culicidae) of Culex spp. and Aedes spp. play an important role as the vectors of avian Plasmodium spp. (Bennett 1987; Valkiūnas 2005). Prevalence of vector-borne diseases is principally determined by biting density and infection rate of vector population (Stokstad 2004). Thus, surveillance of the incidence of avian Plasmodium spp. in vector mosquitoes is important to establish preventive measures for avian malaria infection not only in wild birds but also in captive birds.
Recently, suspected arthropod vectors of both avian Plasmodium (Ejiri et al. 2008) and of Leucocytozoon (Sato et al. 2009) have been suggested for Japanese wild birds, respectively. However, prevalence of avian malaria in mosquitoes inhabiting at artificial environments such as zoological gardens in Japan has not been reported so far. Several cases of avian malaria parasite infections have been found from captive birds in a zoological garden of Japan (Murata et al. 2008a) suggesting the occurrence of infective vector arthropods. In this study, to indicate the competent vectors of avian malaria parasite whose host might be captive birds in a zoological garden of Japan, we collected mosquitoes and carried out the detection of avian malaria parasite DNA from the mosquitoes.
Materials and methods
Mosquitoes were collected monthly in a zoological garden of Kanagawa, Japan from May to August 2005. The zoo is 40.2 ha in area and located in the south of Tokyo, 35.49′ N, 139.52′ E and surrounded by secondary forests apart from human residential areas. A total of 41 mammal species, 22 bird species, and three reptile species are kept and exhibited in the zoo. CDC traps, gravid traps, and a sweeping net were used for mosquito collection. Captured mosquitoes were identified to species following morphological keys (Tanaka 1979; Toma and Miyagi 1986). All the collected mosquitoes were kept at −20°C until DNA extraction.
DNA extraction from mosquitoes and a nested PCR amplification of partial cyt b gene of avian malaria mitochondrial genome were implemented as described previously (Ejiri et al. 2008). Briefly, after DNA extraction, we used the DW2 and DW4 primers (Perkins and Schall 2002) for the first PCR, APFN (5′-CTT ATG GAA TTA TGG ATT TCT TTT AGG-3′) and APRN (5′-ATA ATA AAG CAT AGA ATG AAC ATA TAA ACC-3′) primers for the second. PCR products were subsequently sequenced in both directions using BigDye™ terminator mix (Applied Biosystems, Foster City, CA, USA). Phylogenetic analyses using about 433-bp sequences were performed as described previously (Ramsey et al. 1986). Briefly, using the neighbor-joining (NJ) method by PAUP program, the Kimura two-parameter model was utilized to estimate the evolutionary distances. Bootstrap re-sampling (1,000 cycles) was performed for each method to assess tree topology. Parasite lineages used for the phylogenetic comparisons included four known avian malaria species, Plasmodium cathemerium, Plasmodium gallinaceum, Plasmodium relictum, and Plasmodium juxtanucleare with other avian Plasmodium from GenBank and other avian Plasmodium lineages were obtained from personally communicated DNA sequence data of captive birds kept in the zoological garden. A lineage of lizard malaria parasite, Plasmodium. floridense, was used as out group in the tree.
To estimate infection rate of the mosquitoes examined, the minimum infection rate (MIR) of each mosquito species was calculated as previously described (White et al. 2006). MIR has been utilized to estimate an infection rate by presuming that at least one individual of the pooled sample could be infected. The formula of MIR is as follows; MIR = number of PCR positive/number of collected mosquitoes × 1,000.
Results
Prevalence of parasites in mosquitoes
Total of 1,361 mosquitoes of 11 species in six genera including Aedes albopictus, Aedes japonicus, Armigeres subalbatus, Culex bitaeniorhynchus, Culex pipiens group, Culex sasai, Culex tritaeniorhynchus, Culex (Culiciomyia) sp., Lutzia vorax, Tripteroides bambusa, and Uranotaenia novobscura (Table 1). Among them C. pipiens group was the most dominant species throughout the study. Totally, 169 DNA samples were obtained and eight out of 169 samples were positive for avian malaria parasite cyt b gene. Over all infection rate of avian Plasmodium parasite was estimated as MIR of 5.9 per 1,000. Samples of two mosquito species, C. pipiens group (four DNA samples) and L. vorax (four DNA samples) were PCR positive with MIRs of 5.2 and 51.3 per 1,000, respectively (Table 1).
Molecular phylogenetic analyses
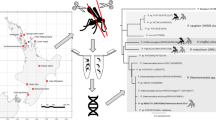
A phylogenetic tree was successfully obtained from the amplified sequences of mosquito samples, including at least five lineages of Plasmodium closely related to avian malaria protozoa in the collected mosquitoes (Fig. 1). Two lineages from both C. pipiens group and L. vorax were completely identical to the avian Plasmodium sp. lineage amplified from captive birds kept in the same zoological garden, namely, white-eared pheasant (Crossoptilon crossoptilon) and common crane (Grus grus). Other two lineages detected from C. pipiens group and L. vorax were completely identical to P. relictum lineage (DQ659544) amplified from carrion crow (Corvus corone) in Japan. One lineage detected from L. vorax was identical to avian Plasmodium sp. lineage amplified from captive birds, brown-eared bullbul (Hypsipetes amaurotis), Himalayan Monal Pheasant (Lophophorus impejanus), Humboldt Penguin (Spheniscus humboldti), and Temminck’s tragopan (Tragopan temmineckii). While another lineage was also detected from L. vorax (AB474379) with a few base substitutions to other lineages from birds in the same location.
Phylogenetic status of detected lineages from mosquitoes collected in a zoological garden of Japan: phylogenetic relationship among amplified avian Plasmodium lineages from the mosquitoes collected in zoological garden of Kanagawa using NJ method with cyt b sequences. Numbers in branches indicate bootstrap values on 1,000 replicates. Avian malaria lineages detected from the mosquitoes in this study are indicated by species names within boxes and with black mosquito silhouette in the tree. And species names with white mosquito silhouette and accession number in the tree indicate the lineages detected from the mosquitoes in the previous study of Minami Daito Island (Ejiri et al. 2008). Plasmodium sp. with bird silhouette indicate avian Plasmodium lineages from personally communicated DNA sequence data of captive birds (1 Crossoptilon crossoptilon, 2 Chrysolophus amherstiae, 3 Grus grus, 4 Hypsipetes amaurotis, 5 Lophophorus impeyanus, 6 Spheniscus humboldti, 7 Tragopan temmineckii). Operational taxonomic units with gray background indicate the lineages detected in the same zoological garden of Kanagawa. Cx Culex, Lt Lutzia
All the obtained sequences from the mosquitoes were deposited to GenBank, having serial accession numbers from AB474376 to AB474379, AB4743781, and AB474382.
Discussion
In the present study, we detected avian Plasmodium DNA sequences from the mosquitoes collected in zoological garden of Japan. Detection of avian Plasmodium from mosquitoes was described on an oceanic island of Minami Daito Island in Japan (Ejiri et al. 2008). However, the island is located far from the main land of Japan with different mosquito fauna (Miyagi 1977). There are almost no concrete studies on the prevalence of avian malaria parasite in mosquitoes in the main land of Japan. The present study is the first one which suggests that mosquitoes inhabiting at the zoological garden located in urban area could be vectors of avian malaria in Japan. Moreover, we observed firstly the mosquito fauna among zoological gardens of Japan, showing that C. pipiens group and A. albopictus were dominant in studied area as well as previous report in urban areas surrounding Tokyo, Japan (Tsuda et al. 2006).
Among the mosquitoes examined in this study, MIR of avian malaria parasite was estimated as 5.9 per 1,000. Recently, we have reported MIR of collected mosquitoes in natural environment, Minami Daito Island of Japan was 12 per 1,000 (Ejiri et al. 2008). Another study in a secondary forest near residential area in the same prefecture as the present study showed that MIR was seven per 1,000 (Shirotani et al. 2009). No significant difference in MIR was found among the three study areas. Comparing to human malaria vectors, vector species of avian malaria in Japan have not been clarified in detail. Although six species have been incriminated as avian malaria vectors so far in Japan (Ejiri et al. 2008; Shirotani et al. 2009), intensive field studies on avian malaria vectors will be required to understand the transmission cycle and prevalence of this vector-borne protozoa infection throughout Japan.
Amplified DNA sequences from two mosquito species (L. vorax and C. pipiens group) found in this study corresponded to those of avian Plasmodium lineages previously detected from the captive birds of the same zoological garden (Fig. 1). Therefore, it is suggested that transmission of avian Plasmodium could occur between those two mosquito species and host bird species within the zoological garden. In addition to previously incriminated four vector species of A. albopictus, Lutzia fuscanus, Culex quinquefasciatus, and Mansonia sp. on Minami Daito Island, the present study indicated C. pipiens group and L. vorax as vectors of avian Plasmodium in main land of Japan. Further examinations if these mosquitoes harbor infective sporozoites of Plasmodium and which host bird species could be the blood meal of the vector mosquitoes are necessary to demonstrate studied mosquitoes as vectors of the targeted pathogens. Detection of avian Plasmodium from bird hosts and vectors in other places may be also needed to reveal the present status of the prevalence of avian malaria in Japan.
In this study, we suggested that C. pipiens group could transmit pathogens such as avian malaria protozoa to host bird species. Both humans and birds have been known as blood source of this mosquito species (Magnarelli 1977; Karoji et al. 1980), suggesting that C. pipiens group may serve as bridge vectors of the pathogens from birds to human such as West Nile virus (WNV) (Andreadis et al. 2001; Anderson et al. 2004). Therefore, to demonstrate the whole transmission cycle of avian malaria protozoa may be important to evaluate the risk of WNV infection in Japan. Moreover, risk assessment of vector-borne diseases in zoological garden might be significant in public health aspect, namely, if captive birds are infected with WNV, those mosquitoes in the facilities could be vectors and/or bridge vectors to humans.
References
Alley MR, Fairley RA, Martin DG, Howe L, Atkinson T (2008) An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala). N Z Vet J 56:247–251
Anderson JF, Andreadis TG, Main AJ, Kline DL (2004) Prevalence of West Nile virus in tree canopy-inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg 71:112–119
Andreadis TG, Anderson JF, Vossbrinck CR (2001) Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis 7:670–674
Belo NO, Passos LF, Júnior LMC, Goulart CE, Sherlock TM, Braga EM (2009) Avian malaria in captive psittacine birds: Detection by microscopy and 18S rRNA gene amplification. Prev Vet Med 88:220–224
Bennett GF (1987) Hematozoa. In: Burr EW (ed) Companion Bird Medicine. Ames Iowa, The Iowa State University Press, pp 120–128
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenecity. J Nat History 27:993–1001
Clarke JR, Kerry KR (1993) Diseases and parasites of penguins. Korean J Polar Res 4:79–96
Ejiri H, Sato Y, Sasaki E, Sumiyama D, Tsuda Y, Sawabe K, Matsui S, Horie S, Akatani K, Takagi M, Omori S, Murata K, Yukawa M (2008) Detection of avian Plasmodium spp. DNA sequences from mosquitoes captured in Minami Daito Island of Japan. J Vet Med Sci 70:1205–1210
Fleischman RW, Squire RA, Sladen WJ, Melby EC Jr (1968) Malaria (Plasmodium elongatum) in captive African penguins (Spheniscus demersus). J Am Vet Med Assoc 153:928–935
Grim KC, McCutchan T, Li J, Sullivan M, Graczyk TK, McConkey G, Cranfield M (2004) Preliminary results of an anticircumsporozoite DNA vaccine trial for protection against avian malaria in captive African black-footed penguins (Spheniscus demersus). J Zoo Wildl Med 35:154–161
Hagihara M, Yamaguchi T, Kitahara M, Hirai K, Murata K (2004) Leucocytozoon lovati infections in wild rock ptarmigan (Lagopus mutus) in Japan. J Wildlife Dis 40:804–807
Jones HI, Shellam GR (1999) Blood parasites in penguins, and their potential impact on conservation. Marine Ornithol 27:181–184
Karoji Y, Sasaki O, Kuroda O, Karaki T (1980) Host-feeding patterns of Japanese mosquitoes II: A host-blood identification study daytime-resting Culex pipiens pallens in Kyoto, Japan. Jap J Sanit Zool 31:289–295
Magnarelli LA (1977) Host feeding patterns of Connecticut mosquitoes (Diptera: Culicidae). Am J Trop Med Hyg 26:547–552
Miyagi I (1977) On the mosquitoes of Minami and Kita Daito Islands of the Ryukyu Archipelago. Med Entomol Zool 28:245–247 (in Japanese with English summary)
Murata K (2002) Prevalence of blood parasites in Japanese wild birds. J Vet Med Sci 64:785–790
Murata K, Nii R, Sasaki E, Ishikawa S, Sato Y, Sawabe K, Tsuda Y, Matsumoto R, Suda A, Ueda M (2008a) Plasmodium (Bennettia) juxtanucleare infection in captive white eared-pheasant (Crossoption crossoptilon) at a Japanese Zoo. J Vet Med Sci 70:203–205
Murata K, Nii R, Yui S, Sasaki E, Ishikawa S, Sato Y, Matsui S, Horie S, Akatani K, Takagi M, Sawabe K, Tsuda Y (2008b) Avian haemosporidian parasites infection in wild birds inhabiting Minami-Daito Island of the Northwest Pacific, Japan. J Vet Med Sci 70:501–503
Nagata N (2006) Reevaluation of the prevalence of blood parasites in Japanese Passerines by using PCR based molecular diagnostics. Ornithol Sci 5:105–112
Perkins SL, Schall JJ (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol 88:972–978
Ramsey JM, Bown DN, Aron JL, Beaudoin RL, Mendez JF (1986) Field trial in Chiapas, Mexico, of a rapid detection method for malaria in anopheline vectors with low infection rates. Am J Trop Med Hyg 35:234–238
Sato Y, Tamada A, Mochizuki Y, Nakamura S, Okano E, Yoshida C, Ejiri H, Omori S, Yukawa M, Murata K (2009) Molecular detection of Leucocytozoon lovati from probable vectors, black flies (Simuliudae) collected in the alpine regions of Japan. Parasitol Res 104:251–255
Shirotani A, Shibata A, Ejiri H, Sato Y, Tsuda Y, Hatakeyama Y, Iwano H, Murata K, Yukawa M (2009) Detection of avian malaria DNA from mosquitoes at Kanagawa in Japan. J Jpn Vet Assoc 62:73–79 (in Japanese with English summary)
Stokstad E (2004) Infectious diseases. Hawaii girds itself for arrival of West Nile virus. Science 306:603
Tanaka K (1979) A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contrib Am Ent Inst 16:1–981
Toma T, Miyagi I (1986) The mosquito fauna of the Ryukyu Archipelago with identification keys, pupal descriptions and notes on biology, medical importance and distribution. Mosq Syst 18:1–109
Tsuda Y, Higa Y, Kurahashi T, Hayashi T, Hoshino K, Komegata O, Isawa H, Kasai S, Sasaki K, Tomita T, Sawabe K, Nihei N, Kobayashi M (2006) Dry-ice trap collection of mosquitoes at urban areas surrounding Tokyo, Japan in 2003 and 2004. Med Entomol Zool 57:75–82 (in Japanese with English summary)
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
White BJ, Andrew DR, Mans NZ, Ohajuruka OA, Garvin MC (2006) West Nile virus in mosquitoes of Northern Ohio, 2003. Am J Trop Med Hyg 75:346–349
Acknowledgements
This study was partially supported by the Academic Frontier Project “Surveillance and control for zoonoses” and “High-Tech Research Center” Project for Private Universities: matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology of Japan, Global Environment Research Fund of the Ministry of the Environment of Japan (F-062), and Nihon University Research Grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ejiri, H., Sato, Y., Sawai, R. et al. Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol Res 105, 629–633 (2009). https://doi.org/10.1007/s00436-009-1434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1434-9