Abstract
Acanthamoeba is one of the most common free-living amoebae. It is widespread in the environment and can infect humans causing keratitis. Delayed diagnosis or misdiagnosis leads to extensive corneal inflammation and profound visual loss. Therefore, accurate and rapid diagnosis of Acanthamoeba keratitis is essential for successful treatment and good prognosis. This study was designed to use different staining techniques to facilitate the identification of Acanthamoeba cysts. Acanthamoeba cysts were isolated by cultivation of either corneal scraping specimens or tap water samples onto non-nutrient agar plates seeded with Escherichia coli. Subcultures were done from positive cultures until unique cysts were isolated. Acanthamoeba cysts were stained temporarily using iodine, eosin, methylene blue, and calcofluor white (CFW) stains and as permanent slides after processing for mounting using modified trichrome, Gimenez and Giemsa staining. These stains were compared on the basis of staining quality including clarity of morphological details, differentiation between cytoplasm and nuclei, color and contrast, and also other characteristics of the staining techniques, including ease of handling, time taken for the procedure, and cost effectiveness. The cysts of Acanthamoeba were recognized in the form of double‐walled cysts: the outer wall (ectocyst) that was being differentiated from the variably stained surrounding background and the inner wall (endocyst) that was sometimes stellated, polygonal, round, or oval and visualized as separate from the spherical, sometimes irregular, outline of the ectocyst. Regarding the temporary stains, it was found that they were efficient for visualizing the morphological details of Acanthamoeba cysts. In CFW staining, Acanthamoeba cysts appeared as bluish-white or turquoise oval halos although the internal detail was not evident. On the other hand, the results of permanent-stained slides showed the most consistent stain for identification of Acanthamoeba cysts was modified trichrome followed by Gimenez stain and lastly Giemsa stain that gave poor visibility of Acanthamoeba cysts due to the intense staining background and monochrome staining of parasite. In the present study, multi-attribute ranking of the used staining techniques showed the highest rank for iodine stain (92 %) followed by eosin stain (84 %), Gimenez stain (76 %), methylene blue (72 %), CFW (64 %), modified trichrome (56 %), and the least was Giemsa stain (44 %). In conclusion, the staining techniques enhance the overall visibility of Acanthamoeba cysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthamoebae are free-living amoebae, occurring worldwide in soil and water. They have been isolated from ponds, lakes, brackish water, sea water, filters on heating and ventilating air-conditioning units, medical equipments such as gastric wash tubing, contact lenses, and contact lens solutions, as well as vegetables, cell cultures, and even human and animal tissues (Shoff et al. 2008). Acanthamoebae have two stages in their life cycle: a vegetative or trophozoite stage that reproduces by binary fission and feeds voraciously on bacteria and detritus present in the environment, and a non-dividing cyst stage with a double cyst wall, providing it with a high resistance to unfavoured and adverse environmental conditions, desiccation and disinfecting compounds, e.g., chlorine (Scheid and Schwarzenberger 2012).
Acanthamoeba causes some painful and life-threatening diseases (Marciano-Cabral and Cabral 2003) on immunocompromised individuals (granulomatous encephalitis) or immunocompetent individuals (Acanthamoeba keratitis) (El-Sayed et al. 2012; Ertabaklar et al. 2007; Scheid et al. 2008). Acanthamoeba keratitis is a severe, potentially sight-threatening ocular infection characterized by progressive corneal inflammation and ulceration. Delayed diagnosis or misdiagnosis leads to extensive corneal inflammation; the corneal epithelium becomes ulcerated with stromal infiltration, perforation, and finally loss of vision (Marciano-Cabral and Cabral 2003; Visvesvara et al. 2007). Acanthamoeba keratitis can result from corneal trauma or improper use of contact lenses. It is widely believed that manipulation of contact lenses may result in epithelial breaks that transmit infectious Acanthamoeba trophozoites to the eye (Ibrahim et al. 2009).
Definitive diagnosis of Acanthamoeba keratitis is based on morphological detection of the both trophozoite and cyst stages, either directly or by in vitro cultivation of infective samples. The unstained characteristics of the Acanthamoeba stages are difficult to detect and sometimes are not identified because these stages are transparent and are surrounded by bacteria or other organisms (e.g., fungi) growing together on the agar surface. Therefore, staining is needed to provide a detailed structure of the cellular organelles (Ithoi et al. 2011).
Several stains were currently used in the identification of Acanthamoeba including Gram’s stain, Gimenez stain, Giemsa stain, chlorazol black E stain, hematoxylin-eosin, periodic acid-schiff, Masson’s trichrome, iron hematoxylin-eosin, lactophenol cotton blue, Field’s stain, Gomori methenamine silver, calcofluor white, and acridine orange (Shen et al. 2005; Thomas et al. 2011; Sharma et al. 2001; Pirehma et al. 1999). Some of these staining techniques are tedious and time consuming. For routine diagnostic work, most laboratories select one of the shorter staining procedures. So, the present study was designed to use different staining techniques to facilitate the identification of Acanthamoeba cysts and compare them on the basis of staining quality and other characteristics of these techniques.
Materials and methods
Acanthamoeba cysts isolation
After taking informed consents, corneal scraping specimens were collected from keratitis patients attending outpatient clinic at the Research Institute of Ophthalmology (RIO), Giza, Egypt. Also, tap water samples from the same patients’ localities were collected. For each sample, approximately 1000 ml water was filtered through a 0.45-μm pore-size filter. Both corneal specimens and water filters were inoculated onto non-nutrient agar plates seeded with Escherichia coli. Plates were incubated at 37 °C and observed daily for the presence of amoebae as previously described (Init et al. 2010). Examination of the agar plate surface for the presence of amoebic growth was carried out daily for up to 7 days with light and inverted microscopes using a ×400 objective. Acanthamoeba were identified at the genus level, based on morphological characteristics of trophozoites and cyst. Subcultures were done after 2 weeks from positive cultures confirmed with amoebic growth until unique cysts were isolated. Agar culture plate containing Acanthamoeba cysts was irrigated with 5 ml of Page’s amoeba saline and pipetted several times to detach the amoebae from the agar surface. The suspension was centrifuged at 2500g for 10 min. The supernatant was aspirated, leaving 0.5 ml above the pellet. The leftover supernatant was gently pipetted to resuspend the mixture (Ithoi et al. 2011).
Staining techniques
-
1.
Temporary staining
-
(a)
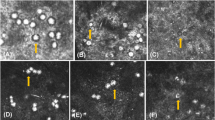
Eosin, iodine, and methylene blue stains were used in wet mount staining. Eosin stain was prepared by adding 1 g in 100 ml distilled water, and 0.5 ml acetic acid was added to sharpen the stain. Lugol’s iodine solution was prepared by adding 20 g potassium iodide to the 100 ml distilled water; when dissolved, 10 g iodine crystals were added. Methylene blue stain was prepared by dissolving 1 g of methylene blue powder in 100 ml physiological saline. The wet mount staining was prepared by spreading out two drops (25 μl) of Acanthamoeba suspension on a glass slide; a drop of the stain was then added, and a coverslip was finally applied to enclose the material. Staining slides were examined under a compound microscope (Zeiss, Axio Scope.A1, Jena, Germany) at a magnification of ×400. Photographs were obtained using a Zeiss AxioCam MRc5 digital camera. The cysts of Acanthamoeba were recognized in the form of double‐walled cysts: the outer wall (ectocyst) that was being differentiated from the variably stained surrounding background and the inner wall (endocyst) that was sometimes stellated, polygonal, round, or oval and visualized as separate from the spherical, sometimes irregular, an outline of the ectocyst. Cysts are uninucleate, and the nucleus has a centrally placed dense nucleolus (Schuster 2002).
-
(b)
Calcofluor white stain (CFW) (comprising of 1 g/l CFW M2R and 0.5 g/l Evans blue). Staining procedure was done according to the manufacturer (Sigma-Aldrich, St Louis, MO, USA). One drop of Acanthamoeba cysts suspension was put onto a clean glass slide. Then, one drop of 10 % potassium hydroxide and one drop of CFW were added, before a coverslip was applied and the slide was allowed to stand for 1–2 min. Lastly, the slide was viewed under a UV microscope (MOTIC BA400 fluorescence compound trinocular microscope with a D350/50× exciter filter at a wavelength of between 395 and 415 nm). Acanthamoeba cysts appeared as bluish-white or turquoise oval halos.
-
(a)
-
2.
Permanent staining
For preparation of permanent-stained smear, Acanthamoeba cysts suspension (50 μl) was dropped on a glass slide and spread to make a smear. It was dried in a moist chamber for 30 min at 37 °C and then fixed in absolute methanol for 3 min (Ithoi et al. 2011). The fixed smears were subsequently stained with the following staining techniques.
-
(a)
Modified trichrome blue staining method (Ryan-Blue) (Ryan et al. 1993). Modified trichrome staining using Ryan modification, by substitution of fast/light green by aniline blue and changing the incubation time and temperature, was adapted for this study. The staining solution was prepared by dissolving 6.0 g of chromotrope 2R (Sigma; C-3143) with 0.5 g of aniline blue (Fisher, Fairlawn, NJ; A-969) and 0.7 g of phosphotungstic acid in 3 ml of glacial acetic acid. This solution stood at room temperature for 30 min, after which 100 ml of distilled water was added and 1.0 M HCl was added to generate a pH 2.5 solution. Methanol-fixed smears were stained in this chromotrope 2R solution for 30 min at 37 °C and then rinsed for 10 s with acid alcohol (4.5 ml of acetic acid in 995.5 ml of 90 % ethyl alcohol). The smears were then dehydrated with a 10-s rinse in 95 % ethyl alcohol, two 5-min incubations in 95 % ethyl alcohol, a 10-min incubation in 100 % ethyl alcohol, and a 5-min incubation in xylene (or xylene substitute). Acanthamoeba cysts appeared bright pink with blue background.
-
(b)
Gimenez staining (Gimenez 1964). The fixed smears were flooded with freshly filtered carbol fuchsin solution prepared from 2 ml of stock solution of basic fuchsin in 5 ml of phosphate buffer for 10 s. The smear was then rinsed in tap water and incubated in malachite green (counterstain) for 9 s. The smear was then rinsed again in tap water and was air-dried. Basic fuchsin stain in aqueous solution with phenol and ethanol stained Acanthamoeba cysts red/magenta against a greenish-blue background.
-
(c)
Giemsa stain (Ithoi et al. 2011). Fix smears were stained with 20 % Giemsa solution (Merck, Darmstadt, Germany) in pH 6.8 of phosphate buffer for 20 min. Acanthamoeba cysts appeared blue and surrounded by a clear halo against a violet background.
-
(a)
All permanently stained slides were examined under a compound microscope (Zeiss Axio Scope.A1, Jena, Germany) at a magnification of ×400. Photographs were obtained using a Zeiss AxioCam MRc5 digital camera.
Assessment of different stains
Different staining techniques were assessed according to staining quality, i.e., clarity of morphological details, differentiation between cytoplasm and nuclei, color and contrast, and other characteristics of these techniques, including ease of handling, time taken for the procedure, and cost effectiveness. The cost calculations for each procedure included material and reagent costs and the cost of the technologist’s time. Comparing between the staining techniques was conducted utilizing the multi-attribute evaluation method (MacPherson and McQueen 1993). This method identifies, characterizes, and then combines different attributes (variables) to assess the true ranking of these techniques. The attributes were given a rank order from one to five, with one being less desirable and five being most desirable.
Results
Results are shown in the Figs. 1, 2, 3, 4, 5, 6, 7, and 8 and Tables 1 and 2.
Discussion
Laboratory diagnosis of Acanthamoeba keratitis relies on the demonstration of trophozoite or cyst in corneal scrapings under microscopic observation directly or isolated from the culture (Whitcher et al. 2003). Wet mount preparation has the advantage of demonstrating the trophozoite motility. However, the internal structures are often poorly visible making definitive diagnosis of cysts or trophozoites difficult leading to misdiagnosis of Acanthamoeba in 60–70 % of Acanthamoeba keratitis cases (Sharma et al. 2004; Yera et al. 2007). So, several staining techniques were used in this study to make Acanthamoeba cysts easy to recognize and identify. It was found that stained preparations gave variable results and the staining quality of Acanthamoeba cysts was affected by the stain type, the concentration of the staining solution, the temperature, and duration of staining. Additionally, the differences in the ability of stains to reveal Acanthamoeba cysts were likely to be due to the thickness of the cyst wall and the extent to which the stain is taken up by the Acanthamoeba depending on specimen processing and fixation.
In this study, the used staining techniques were classified according to their stability into temporary and permanent stains. Several staining solutions have been used for preparation of temporary stained wet mounts including Lugol’s iodine, eosin, and methylene blue. These solutions were able to stain internal structures of Acanthamoeba cysts, thereby in the present study, the wet mount staining was considered as a simple, rapid method for visualization of Acanthamoeba cysts. Comparing these temporary stained wet mounts with the other permanent stains for the detection of Acanthamoeba, they are quick single-step procedures (the entire process taking about 1 min), use inexpensive stains easily available in any clinical parasitology laboratory, and do not require a fluorescent microscope or special filter for evaluation (Grossniklaus et al. 2003). Disadvantage of wet mount staining is that the slide preparations are temporary; therefore, they are not available for future reference and the diagnosis relies on the experience of the technician only.
The other temporary staining method used in this study was calcofluor white which is a non-specific fluorochrome. This stain is able to bind cellulose in the cell wall of Acanthamoeba cysts. The results of the current study proved that CFW is a simple and fast method for detecting Acanthamoeba cysts as reported previously by Sharma et al. (2000, 2001). CFW stain was mixed with a potassium hydroxide mixture to clear up the specimen to facilitate visualization of Acanthamoeba. Also, Evans blue was used to act as a counter stain to diminish background fluorescence of tissues and cells when using blue light excitation (Harrington and Hageage 2003). Calcofluor staining procedure did not produce permanently stained slides for archiving however, if stored in the dark; the calcofluor-stained specimens may be viewed several months later with little loss of fluorescence (Didier et al. 1995). The disadvantages of this technique included its complexity, which required frequent quality control monitoring and technological expertise, and the fact that it is a non-specific stain. It is also important to note that in mixed fungi, Acanthamoeba infections, both pathogens will be stained, since both amoebic cysts and fungi cell walls are possible targets for CFW (Harrington and Hageage 2003). An added disadvantage is the need for a fluorescence microscope, an additional expense for the laboratories not equipped with one. The use of fluorescent dyes may lead to false-positive staining patterns of cell debris (Grossniklaus et al. 2003; Bharathi et al. 2006). Due to the cost involved with evaluating fluorescence staining of calcofluor white to diagnose Acanthamoeba, Waring et al. (2003) did not recommend its use.
Although an experienced microscopist can occasionally identify Acanthamoeba in a wet preparation both unstained and stained, most identification should be considered tentative until confirmed by the permanent stained slide. Permanent stained smears are reported to have many color ranges that either aided or hindered identification of organisms within the samples (Pietrzak-Johnston et al. 2000). The permanent stained smears not only provide the microscopic technician with a permanent record of protozoal organisms identified but also may be used for consultations with specialists when unusual morphologic characteristics are found (Garcia 2007) in which the morphology of Acanthamoeba cysts could be altered by culture age, pH, dryness, and lack of food (Ithoi et al. 2011; Walochnik et al. 2000).
Permanent staining by Gimenez stain was found to be effective for visualizing the morphological details of the parasite. Acanthamoeba cysts appeared as magenta/red-colored pleomorphic cysts against the greenish-blue background. The staining method was originally developed by Gimenez in 1964 (Gimenez 1964) to identify the bacteria. The primary stain for this technique is carbol fuchsin, and the secondary stain is malachite green. It is known that the basic dye carbol fuchsin is retained by the acid-fast organism which has a complex envelope composed of glycolipids and glycopeptidolipids (Brennan et al. 1990). The Gimenez staining technique had fewer steps than the other permanent staining methods and could easily be integrated into the workflow of a parasitology laboratory. It was moderately inexpensive, specimens could be used in batches, and the stained slide could be kept as a permanent record that can be archived. The result of this study was in agreement with Shen et al. (2005) who concluded that Gimenez staining is a rapid method for initial identification of Acanthamoeba cysts.
Alternatively, the slides stained with Giemsa’s stain gave poor visibility of Acanthamoeba cysts due to the intense staining background and monochrome staining of parasite. In spite of this stain does not require time consuming or special equipments for its evaluation (Grossniklaus et al. 2003); the poor color contrast leads to underestimation of Acanthamoeba cysts in positive cases.
In this study, modified trichrome stain was superior to other used stains for the identification of Acanthamoeba cysts; however, it is costly and time consuming (60 min) and it involved many steps. Acanthamoeba cysts stained with modified trichrome appeared bright pink with well-differentiated internal structures. In this method, aniline blue was used as a counterstain (Ryan et al. 1993) that provided good contrast to the pink-staining Acanthamoeba.
The process of accepting one staining procedure over another involves a complex decision analysis of several criteria related to those stains. In the present study, when comparing time efficiencies for performing the used staining methods, wet mount staining required the least time of approximately 1 min, followed by the calcofluor stain (5 min), Gimenez stain (10 min), Giemsa (20 min), and lastly, the modified trichrome blue stain, which required approximately 60 min. In addition, wet mount staining specimens were the easiest to read, requiring approximately 2 to 3 min per slide to view 200 fields while permanent staining specimens required approximately 5 to 10 min to carefully view 200 fields for detecting Acanthamoeba. Considering the total procedure cost, CFW was very expensive; that is why this stain was not suited for the identification of Acanthamoeba cysts in most laboratories (Waring et al. 2003). In addition, ease of handling and the ability to process large numbers of specimens were subjectively evaluated on the basis of normal procedures in a diagnostic laboratory performing parasitological examinations only and the ability to test batches of up to 25 specimens in the routine staining procedure. Adopting the method of multi-attribute evaluation (MacPherson and McQueen 1993), multi-attribute ranking of the used stained techniques showed the highest rank for iodine stain (92 %) followed by eosin stain (84 %), Gimenez stain (76 %), methylene blue (72 %), CFW (64 %), and modified trichrome (56 %) and the least was Giemsa stain (44 %).
In conclusion, the staining techniques enhance the overall visibility of Acanthamoeba cysts and laboratorians should be cautioned not to rely solely on the direct wet mount for detection or identification of this protozoan.
References
Bharathi MJ, Ramakrishnan R, Meenakshi R, Mittal S, Shivakumar C, Srinivasan M (2006) Microbiological diagnosis of infective keratitis: comparative evaluation of direct microscopy and culture results. Br J Ophthalmol 90(10):1271–1276. doi:10.1136/bjo.2006.096230#_blank
Brennan PJ, Hunter SW, McNeil M, Chatterjee D, Daffe M (1990) Reappraisal of the chemistry of mycobacterial cell walls, with a view to understanding the roles of individual entities in disease processes. In: Ayoub EM, Cassell GH, Branche WC Jr, Henry TJ (eds) Microbial determinants of virulence and host response. American Society for Microbiology, Washington, D.C, pp 55–75
Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA (1995) Comparison of three staining methods for detecting Microsporidia in fluids. J Clin Microbiol 33(12):3138–3145
El-Sayed NM, Ismail KA, Ahmed SAG, Hetta MH (2012) In vitro amoebicidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and Pancratium maritimum L. on Acanthamoeba castellanii cysts. Parasitol Res 110(5):1985–1992
Ertabaklar H, Türk M, Dayanir V, Ertuğ S, Walochnik J (2007) Acanthamoeba keratitis due to Acanthamoeba genotype T4 in a non-contact-lens wearer in Turkey. Parasitol Res 100(2):241–246
Garcia LS (2007) Diagnostic medical parasitology I\SM press, Washington DC; part II, pp. 771–812
Gimenez DF (1964) Staining Rickettsiae in yolksack cultures. Stain Technol 39:135–140
Grossniklaus HE, Waring GO IV, Akor C, Castellano-Sanchez AA, Bennett K (2003) Evaluation of hematoxylin and eosin and special stains for the detection of Acanthamoeba keratitis in penetrating keratoplasties. Am J Ophthalmol 136(3):520–526
Harrington H (2003) Calcofluor white: a review of its uses and applications in clinical mycology and parasitology. Lab Med 34(5):361–376
Ibrahim YW, Boase DL, Cree IA (2009) How could contact lens wearers be at risk of Acanthamoeba infection? A review. J Optom 2(2):60–66
Init I, Lau YL, Arin-Fadzlun A, Foead AI, Neilson RS, Nissapatorn V (2010) Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop Biomed 27(3):566–577
Ithoi I, Ahmad AF, Mak JW, Nissapatorn V, Lau YL, Mahmud R (2011) Morphological characteristics of developmental stages of Acanthamoeba and Naegleria species before and after staining by various techniques. Southeast Asian J Trop Med Public Health 42(6):1327–1338
MacPherson DW, McQueen R (1993) Cryptosporidiosis: multi-attribute evaluation of six diagnostic methods. J Clin Microbiol 3 1(2):198–202
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp as agents of disease in humans. Clin Microbiol Rev 16(2):273–307
Pietrzak-Johnston SM, Bishop H, Wahlquist S, Moura H, Da Silva ND, Da Silva SP, Nguyen-Dinh P (2000) Evaluation of commercially available preservatives for laboratory detection of helminths and protozoa in human faecal specimens. J Clin Microbiol 38(5):1959–1964
Pirehma M, Suresh K, Sivanandam S, Anuar KA, Ramakrishnan K, Kumar GS (1999) Field’s stain—a rapid staining method for Acanthamoeba spp. Parasitol Res 85:791–793
Ryan NJ, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird RW, Pedersen J, Dwyer B (1993) A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol 31:3264–3269
Scheid P, Schwarzenberger R (2012) Acanthamoeba spp. as vehicle and reservoir of adenoviruses. Parasitol Res 111(1):479–485
Scheid P, Zöller L, Pressmar S, Richard G, Michel R (2008) An extraordinary endocytobiont in Acanthamoeba sp. isolated from a patient with keratitis. Parasitol Res 102(5):945–950
Schuster FL (2002) Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev 15(3):342–354
Sharma S, Grag P, Roa GN (2000) Patient characteristics, diagnosis and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol 84(10):1103–1108. doi:10.1136/bjo.2006.096230#_blank
Sharma S, Athmanathan S, Ata-Ur-Rasheed M, Garg P, Rao GN (2001) Evaluation of immunoperoxidase staining technique in the diagnosis of Acanthamoeba keratitis. Indian J Ophthalmol 49(3):181–186
Sharma S, Pasricha G, Das D, Aggarwal RK (2004) Acanthamoeba keratitis in non-contact lens wearers in India: DNA typing-based validation and a simple detection assay. Arch Ophthalmol 122(10):1430–1434
Shen J, Jiang QW, Li QX, Chen HY, Li ZH (2005) Gimenez staining: a rapid method for initial identification of legionella pneumophila in Amoeba trophozoite. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 23(4):240–242
Shoff ME, Rogerson A, Kessler K, Schatz S, Seal DV (2008) Prevalence of Acanthamoeba and other naked amoebae in South Florida domestic water. J Water Health 6(1):99–104
Thomas PA, Jesudasan CAN, Kaliamurthy J (2011) Rapid detection of Acanthamoeba cysts in corneal scrapings by chlorazol black E staining. Can J Ophthalmol 46(5):443–444
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living Amoeba: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50(1):1–26
Walochnik J, Obwaller A, Aspock H (2000) Correlations between morphological, molecular biological and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl Environ Microbiol 66(10):4408–4413
Waring GO IV, Akor C, Castellano-Sanchez A, Grossniklaus H E (2003) Hematoxylin and eosin is superior to calcofluor white and acridine orange in detecting Acanthamoeba Keratitis. Investig Ophthalmol Vis Sci 44
Whitcher JP, Srinivasan M, Upadhyay MP (2003) Microbial keratitis. In: Johnson GJ, Minassian DC, Weale RA, West SK (eds) The epidemiology of eye diseases, 2nd edn. Arnold, London, pp 190–195
Yera H, Zamfir O, Bourcier T, Ancelle T, Batellier L, Dupouy- Camet J, Chaumeil C (2007) Comparison of PCR, microscopic examination and culture for the early diagnosis and characterization of Acanthamoeba isolates from ocular infections. Eur J Clin Microbiol Infect Dis 26:221–224
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Sayed, N.M., Hikal, W.M. Several staining techniques to enhance the visibility of Acanthamoeba cysts. Parasitol Res 114, 823–830 (2015). https://doi.org/10.1007/s00436-014-4190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4190-4