Abstract
In the present article, the detection and the development of a parasitic endocytobiont within host amoebae (Acanthamoeba sp.) recently isolated from the contact lens and the inflamed eye of a patient with keratitis is presented. An otherwise healthy 55-year-old female patient presented with keratitis in her inflamed left eye. She was a contact lens wearer and had no history of a corneal trauma. Acanthamoebae as well as other smaller free-living amoebae could be detected from the fluid of the contact lens storage cases by culture methods. A successful therapy could be provided consequently. Two of these Acanthamoeba strains showed intracellular aggregating organisms. Within 2 to 3 days, the host amoebae ruptured, and numerous microorganisms were released. We succeeded in detecting the mechanism of infection and intrusion of this organisms by using light and electron microscopy. Infection with this endocytobiont is a suitable model for studying the host–parasite relations while the parasites use their hosts as so-called Trojan horses (see Barker, Lambert, Brown, Infect Immun 61:3503–3510, 1992).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free-living amoebae (FLA) occur ubiquitously in many aquatic habitats and humid soils. In addition to their role as pathogens, FLA are known to serve as natural hosts and vehicles of various intracellular organisms (bacteria, viruses, and eucaryonts)
FLA belonging to the genus Acanthamoeba are able to cause a painful sight-threatening disease of the eyes designated as Acanthamoeba keratitis (AK; Marciano-Cabral and Cabral 2003) in addition to their role as causative agents of the well-known Acanthamoeba granulomatous encephalitis (GAE). Amoebae responsible for those diseases are commonly found in nearly all freshwater habitats and moist soils. They can be found in artificial habitats such as swimming pools, drinking water systems (Rohr et al. 1998; Seal et al. 1999), mineral water, eye wash stations, in water conduit systems of dental units (Michel and Just 1984; Just and Michel 1984; Michel and Borneff 1989), and in contact lens storage cases (Müller et al. 1999; Marciano-Cabral and Cabral 2003), the latter of which has the greatest significance for a potential eye infection resulting in keratitis. Moreover, growth of populations of acanthamoebae can occur on the nasal mucosa and throat of healthy and immunocompromised persons (Rivera et al. 1984; Michel et al. 1982; De Jonckheere and Michel 1988; Matias et al. 1991). The life cycle of Acanthamoeba sp. comprises an actively feeding and dividing trophozoite and a dormant cyst with a double cyst wall providing it with a high resistance (tenacity) to desiccation and disinfecting compounds, e.g., chlorine. The trophozoites phagocytically feed on bacteria, algae, and yeast they find in the environment. The cysts remain viable for at least several months.
The use of lens storage cases combined with poor lens hygiene are considered major preconditions for an eye infection by these Acanthamoebae (Marciano-Cabral and Cabral 2003). Especially soft contact lenses are involved. Consequently, acanthamoebae have been isolated frequently from lens storage cases and lens cleaning solutions. Acanthamoebae isolated from keratitis patients were identified as A. castellanii, A. griffini, A. polyphaga, A. hatchetti, A. culbertsoni, A. rhysodes, A. quina, and A. lugdunensis (Marciano-Cabral and Cabral 2003). Finally, the role of acanthamoebae as hosts of intracellularly replicating bacteria, e.g., Legionellae (Rowbotham 1980) or chlamydia-like bacteria belonging to Parachlamydiaceae (Everett et al. 1999) among others should be mentioned because this feature gained great relevance in the case presented here. Host amoebae can, thus, highly protect the endoparasitic bacteria against the adverse action of various disinfectants, especially when forming cysts.
Case report
A 55-year-old woman, wearer of soft contact lenses, presented to the Clinic and Policlinic of Ophthalmology at the University Clinic Hamburg-Eppendorf with “severe keratitis” in the left eye, which had already persisted for 14 days without showing any improvement after local therapy with Ofloxacin eye drops. In her self-reported medical history, the patient stated that she suffered from occasional recurrences of labial herpes. A concomitant reduction in visual acuity was diagnosed (vision 0.6). An initial examination yielded the following results:
Findings in the left anterior section: hyperaemic conjunctiva, epithelial unrest such as dendritic lesions typical of herpes. Based on these findings, herpes therapy was initiated with five daily administrations of Aciclovir 800 mg p.o. and four daily administrations of L Aciclovir eye ointment AS. Administration of Ofloxacin eye drops was continued.
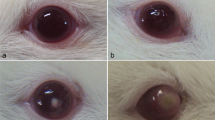
An ophthalmological follow-up examination performed 4 days later revealed a deterioration of the patient’s condition (Fig. 1): visual acuity of the affected eye continued to decrease (vision 0.16). Epithelial alteration had further progressed; moreover, endothelial precipitates caused by anterior chamber irritation as well as a reduction in sensitivity were observed. The woman was admitted to hospital as an inpatient. The contact lenses, along with the fluid container, were sent for examination for Acanthamoeba to the Laboratory of Medical Parasitology at the Central Institute of the Bundeswehr Medical Service at Koblenz.
During the inpatient period, a further ophthalmological examination was performed which revealed a deep, brownish circular infiltrate, epithelial defects, superficial clouding, as well as fibrin reaction in the anterior chamber. At this time already, the working diagnosis of “Acanthamoeba keratitis” was established, and Brolene eye drops, Lava Sept eye drops (Biguanid), and Polyspektran eye drops were alternately administered at intervals of 15 min for a period of 72 h (without breaks for sleep) in conjunction with Atropin 1% eye drops (three administrations per day). Aciclovir therapy was continued in parallel. The patient’s condition improved noticeably under this therapy. Within 3 days, vision increased to 0.4. Therapy was reduced to half-hourly administrations, and night breaks were included. The patient was discharged from hospital with a vision of 0.5 and a hardly identifiable subephitelial circular clouding with an otherwise smooth and clear cornea; the anterior chamber was free of irritation. Medication was continued using Brolene eye drops, Biguanid eye drops (half-hourly), Prednisolon 2.5% eye drops (three administrations per day), as well as Corneregel Fluid (half-hourly) and Aciclovir 800 mg (five administrations per day p.o.).
Seventeen days after admission, the patient presented with a marked eyelid edema with skin ulcerations and large central corneal epithelial erosion, which again led to hospitalization. Thanks to local care with, amongst others, therapeutic contact lenses and cortisone ointment for eyelids, the allergic contact dermatitis of the lid skin healed fast. Ever since, no further irritations have been diagnosed. An examination performed 6 weeks after the patient’s initial presentation (and after successful therapy) revealed a vision of 0.6 and a paracentral scar of the cornea stroma (Fig. 2).
Materials and methods
Organisms and cultures
Pieces of contact lenses and fluid from their storage case were transferred to non-nutrient agar plates (NNA), according to (Page 1988) seeded with Enterobacter cloacae and incubated at 29°C and 35°C. The plates were inspected daily by light microscope. The developing amoebal populations were subcultured and maintained on NNA as well. Isolation of pure cultures was achieved by separating organisms with different cyst morphology after a couple of days. The Acanthamoeba strains harboring endocytobionts were maintained on NNA until the organisms could be successfully transferred to axenic SCGYE medium (De Jonckheere 1977). The Acanthamoeba strains were submitted to incubation temperature tolerance tests at 37°, 40°, and 42°C (De Jonckheere 1980). To decide whether the organisms grow on usual microbiological culture media, Columbia agar (5% SB; sheep blood), Mac Conkey II agar, Müller–Hinton Choco agar, Sabouraud (G11+C) agar, and Chrom agar were used in co-cultivation assays.
Light microscopy
The light-microscope photos were taken with the Leica DM 5000B microscope with the JVC 3CCD 3-Chip Camera. The software used for documentation purposes was DISKUS 4.30 from Hilgers, Königswinter.
Electron microscopy
For electron microscopical investigations, infected amoebae were harvested from 3 to 5 days old NNA plates and from 5 to 7 days old SCGYE (fluid-) cultures and pelleted by centrifugation (500×g) for 10 min. The resulting pellets were fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 1 h, washed twice in the same buffer, postfixed for 1 h in 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.2), and embedded in Spurr resin. Finally, the sections were stained with uranyl acetate and Reynold’s lead citrate and examined using a Leo EM 910 transmission electron microscope.
Results
There were three major findings during the diagnostic procedure:
-
1.
Within a period of 3 days of incubation, trophozoites of acanthamoebae could be observed by light microscopy at 100× magnification (Fig. 3) After encystation, beginning with day 4, they could be identified as Acanthamoeba sp. (group II; Fig. 4). Temperature tolerance tests showed that the acanthamoebae grew well at 37°C, but their growth stopped at 40°C. Depending on the different cyst morphology of the amoebal host, two strains could be separated: A. triangularis and a second strain, not yet determined, with polygonal cysts.
-
2.
Very small trophozoites forming tiny smooth round cysts could be distinguished and were isolated as a pure culture (Figs. 5 and 6) beneath the Acanthamoebae.
-
3.
Some of the Acanthamoeba trophozoites appeared to harbor small bacteria-like organisms (KLaHel) of round-oval shape (Fig. 7) which replicated intracellularly leading to the rupture of the host amoebae, leaving plaques of those organisms within the border of the shape of the disintegrated amoeba. Both Acanthamoeba strains were permissive to infection with KLaHel.
One of the preconditions for further investigations (e.g., a sequence-based identification) of the endocytobionts was to get rid of the food bacteria of the host amoeba. This could finally be achieved by establishing an axenic culture. To get an idea of the ultrastructure of these organisms acting as endocytobionts (endoparasites), cultures from plates and from axenic SCGYE medium as well were fixed and investigated by transmission electron microscopy. As a first result, the organisms appeared enveloped by a massive electron-dense outer wall containing only homogeneous granular material—no morphologic characteristics of bacteria, such as ribosomes or nucleoid-like structures, could be distinguished. Depending on the section plane, a prominent apical pore is visible (Fig. 8). A more detailed investigation of these peculiar organisms is in progress.
It could be shown that the organisms at the cell membrane of strain KLaHel were taken up into the host amoebae by phagocytosis after adhesion of the organisms and food cup formation (infective phase). The organisms were transported into the cytoplasma of the acanthamoebae in food vacuoles. Phase contrast microscopy revealed early stages of the endocytobionts as well as stages of proliferation within the cytoplasma (proliferative phase). With the rupture of the host cell membrane, the cycle started again from the beginning, the released infectious stages being ingested by other host amoebae.
As the infected amoebal population was destined to break down, cultures of endoparasite-free acanthamoebae, derived from the original plates, had to be used as new host organisms. If necessary, a subculture of them could be re-infected with the endocytobionts, thus, enabling a continuous maintenance of the host–parasite model. Additionally, it could be shown that there was no growth of these extraordinary organisms on five on the usual bacterial culture media plates.
Discussion
In cases of AK, trophozoites can be observed in cultures on NNA within a period of 2–3 days. It is recommended not to fall short of an incubation period of 10 days, as sometimes, amoebal populations may develop very slowly if very few amoebae are present in the corneal sample. The detection of other FLA and other accompanying organisms can be accomplished more reliably by culture (Kirkness et al. 1993; Kennedy et al. 1995). Additionally, the choice of different incubation temperatures may enable the identification of unusual amoebae, e.g., the small unidentified species which multiplied much faster at 20°C than at 35°C.
As amoebae are able to survive as cysts within the corneal tissue, treatment has to be continued for at least several months or even longer. During the treatment period, patients have to be monitored for recurrence of disease. Because cysts are resistant to many drugs, treatment failures are frequently reported and may be attributed to poor penetration of the drug, too short duration of treatment, or to resistance.
At the late stage of infection especially, if the amoebae have already permeated the cornea, keratoplastic or cryotherapy may be unavoidable for patients with poor response to anti-amoebal treatment. An overview on the current drugs for Acanthamoeba keratitis therapy is given by Marciano-Cabral and Cabral (2003). In the present case, the detection of Acanthamoeba sp. led to a successful therapy (see case report).
Ineffective disinfectants (Beattie et al. 2003), self-made storage fluids (Reul 2004), the use of tap water, and the contamination of the lens store cases (Houang et al. 2001) have been described as important risk factors concerning AK. Soft contact lenses are especially suited for contamination with bacteria, etc.
As they consist of a sponge-like plastic soaked with tear fluid, they favor the growth of microorganisms which can serve as food for free-living amoebae, e.g., acanthamoebae.
In contrast to GAE infections, AK can also occur in immunocompetent patients. The infection does not leave protective immunity (Marciano-Cabral and Cabral 2003). Potential synergy between amoebae and bacterial contaminants may have an effect on the genesis of corneal infection (Bottone et al. 1992), yet the significance of endoparasitic organisms for the course of infection (pathogenesis) remains still unclear. At least a considerable number of AK cases have been reported to be due to strains of acanthamoebae harboring cocoid or rod-shaped endocytic bacteria (Fritsche et al. 1993). One of the cocoid endocytobionts turned out later as a member of the family of Parachlamydiaceae. Two of the rod-shaped endocytic bacteria could be affiliated to the Rickettsiales as a potential new genus (Fritsche et al. 1999). In a recent study, a microsporidian-like endocytobiont was analyzed, which was also isolated from a female patient with keratitis, with Vannella sp. as host amoebae (Michel et al. 2000; Scheid 2007).
As shown by morphology, the present isolate was absolutely different from any of those endocytobionts reported as yet. It was shown that the organisms are taken up by phagocytosis after adhesion of the organisms to the cell membrane, followed by a food-cup formation (infective phase). Active penetration processes were not observed. The enodocytobionts were transported into the cytoplasma of the acanthamoebae within food vacuoles. The examinations with the help of electron microscopy, in particular, revealed both the endocytobionts’ morphology and their development inside the host amoeba.
The meaning of these endoparasitic organisms with respect to the pathogenesis of the keratitis remains unresolved. Staining methods, molecular analysis, and mass spectrometry will be used for taxonomic classification of this extraordinary organism applied to obtain further information on the taxonomic position of the endocytobiont.
Infection of Acanthamoeba sp. with the parasitic organism KlaHel is another suitable model for studies of the host–parasite relations of endocytobionts using their hosts as so-called Trojan horses.
References
Barker J, Lambert PA, Brown MRW (1992) Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun 61:3503–3510
Beattie TK, Seal DV et al (2003) Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J Clin Microbiol 41(7):2992–3000
Bottone EJ, Madayag RM, Qureshi MN (1992) Acanthamoeba keratitis: synergy between amebic and bacterial cocontaminants in contact lens care systems as a prelude to infection. J Clin Microbiol 30(9):2447–2450
De Jonckheere JF (1977) Use of an axenic medium for differentiation between pathogenic an non-pathogenic Naegleria fowleri isolates. Appl Environ Microbiol 33:751–757
De Jonckheere JF (1980) Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol 39:681–685
De Jonckheere JF, Michel R (1988) Species identification and virulence of Acanthamoeba strains from human nasal mucosa. Parasitol News 74:314–316
Everett KDE, Bush RM, Anderssen AA (1999) Embedded description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five species, and standards for the identification of organisms. Int J Syst Bacteriol 49:415–440
Fritsche TR, Gautom RK, Seyedirashti S, Bergeron DL, Lindquist TD (1993) Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol 31:1122–1126
Fritsche TR, Horn M, Seyedirashti S, Gautom RK, Schleifer KH, Wagner M (1999) In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl Environ Microbiol 65:206–212
Houang E, Lam D, Fan D, Seal D (2001) Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg 95:361–367
Just H-M, Michel R (1984) Infektionsgefährdung durch Bakterien, Pilze und Amöben in Kühl- und Spülwasser zahnärztlicher Einheiten. Dtsch Zahnärtzl Z 39:60–64
Kennedy SM, Devine P et al (1995) Corneal infection associated with Hartmannella vermiformis in contact-lens wearer. Lancet 346:637–638
Kirkness CM, Aiken D et al (1993) Vahlkampfiids keratitis simulating Acanthamoeba infection associated with disposable contact lens wear (an overlooked diagnosis). Invest Ophthalmol Vis Sci 334:853
Matias R, Schottelius J, Raddatz CHF, Michel R (1991) Species identification and characterisation of an Acanthamoeba strain from human cornea. Parasitol Res 77:469–474
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307
Michel R, Just H-M (1984) Acanthamoeben, Naeglerien und andere freilebende Amöben in Kühl- und Spülwasser von Zahnbehandlungseinheiten. Zbl Hyg 179:56–72
Michel R, Borneff M (1989) Über die Bedeutung von Amöben und anderen Protozoen in wasserführenden Systemen von Dentaleinheiten. Zbl Bakt Hyg 187:312–323
Michel R, Röhl R, Schneider H (1982) Isolierung von freilebenden Amöben durch Gewinnung von Nasenschleimhautabstrichen bei gesunden Probanden. Zbl Bakt Hyg 176:155–159
Michel R, Schmid EN, Böker T, Hager DG, Müller K-D, Hoffmann R, Seitz HM (2000) Vannella sp. harboring Microsporidia-like organisms isolated from the contact lens and inflamed eye of a female keratitis patient. Parasitol Res 86:514–520
Müller K-D, Schmid EN, Michel R (1999) Intracellular bacteria of Acanthamoebae resembling Legionella spp. turned out to be Cytophaga sp. Zbl Bakteriol 189:389–397
Page FC (1988) A new key to freshwater and soil Gymnamoebae with instructions for culture. Freshwater Biological Association, Ambleside, UK
Reul M (2004) Entnahme- und Versandtechniken von mikrobiologischem Probenmaterial: Infektionen der Augen und Ohren; Krankenhaushygiene + Infektionsverhütung 26 Heft 2:72–76
Rivera F, Medina F et al (1984) Pathogenic and free-living protozoa cultured from nasopharyngeal and oral regions of dental patients. Environ Res 33:428–440
Rohr U, Weber S, Michel R, Selenka F, Wilhelm M (1998) Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl Environ Microbiol 64:1822–1824
Rowbotham TJ (1980) Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol 33:1179–1183
Scheid P (2007) Mechanism of intrusion of a microsporidian-like organism into the nucleus of host amoebae (Vannella sp.) isolated from a keratitis patient. Parasitol Res 101:1097–1102
Seal DV, Kirkness CM, Bennet HGB, Peterson M, Keratitis Study Group (1999) Acanthamoeba keratitis in Scotland: risk factors for contact lens wearers. Contact Lens Anterior Eye 22:58–68
Acknowledgments
We like to thank Dr. B. Hauröder for her help and assistance to obtain the impressive electron microscopical records.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheid, P., Zöller, L., Pressmar, S. et al. An extraordinary endocytobiont in Acanthamoeba sp. isolated from a patient with keratitis. Parasitol Res 102, 945–950 (2008). https://doi.org/10.1007/s00436-007-0858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0858-3