Abstract
An otherwise healthy 22-year-old man presented with Acanthamoeba keratitis (AK) in the right eye. He was not a contact lens wearer and had no history of corneal trauma. The Acanthamoeba strain isolated from a corneal scraping was identified as morphological group II and genotype T4. Three more Acanthamoeba strains isolated from sites of possible human contact with acanthamoebae in the same geographical region, including a lens storage case, tap water and soil, were subjected to morphological and molecular biological identification. Whereas the strain from tap water also exhibited genotype T4, the two other isolates were identified as morphological group I and genotype T9. To the best of our knowledge, this is the first study identifying an AK-causing Acanthamoeba strain in Turkey and the first isolation of genotype T9 in this country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthamoebae are potentially pathogenic free-living amoebae (FLA) that are widely distributed in freshwater, seawater, soil and the air and that can frequently be isolated also from man-made habitats such as tap water, bottled mineral water, laboratory distilled water wash bottles, chlorinated swimming pools, sewage and contact lens containers (De Jonckheere 1991; Kilvington and White 1994; Khan and Paget 2002). They can cause two different disease entities, the so-called Acanthamoeba keratitis (AK) occurring mainly in contact lens wearers on the one hand, and several disseminating infections in the immunocompromised host on the other hand, including skin lesions, pneumonitis and the almost always fatal granulomatous amebic encephalitis (GAE) (Marciano-Cabral and Cabral 2003; Schuster and Visvesvara 2004). Besides their active pathogenicity, acanthamoebae are also of clinical relevance by acting as vehicles for fungi, viruses and bacteria, including quite a variety of bacterial pathogens, such as Legionella pneumophila, Pseudomonas aeruginosa and Mycobacterium avium (Barker and Brown 1994, Horn and Wagner 2004).
At least 24 Acanthamoeba species have been described to date, and these have been assigned to one of the three morphological groups established by Pussard and Pons (1977) according to the size and the shape of their cysts. However, the described species are not supported by molecular analyses, and thus, the genus has been reclassified into 15 different genotypes (i.e. T1–T15) based on 18S rDNA sequence analyses (Gast 2001; Gast et al. 1996; Hewett et al. 2003; Horn et al. 1999; Stothard et al. 1998).
In the current study, we present a case of AK in a non-contact-lens wearer without a history of corneal trauma. The causative agent was isolated from a corneal scraping and was identified as Acanthamoeba morphological group II and genotype T4. To the best of our knowledge, this is the first study identifying an AK-causing Acanthamoeba strain in Turkey. Three more Acanthamoeba strains isolated from sites of possible human contact with acanthamoebae in the same geographical region, including a lens storage case, tap water and soil, were subjected to morphological and molecular biological identification. Two of these strains were identified as genotype T9, representing the first isolation of Acanthamoeba genotype T9 in Turkey.
Case report
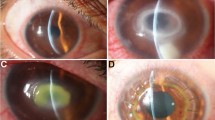
An otherwise healthy 22-year-old man presented with severe pain, redness and photophobia in the right eye. According to his statement, the symptoms had already been present for 15 days and had been treated with 1% cyclopentholate (Sikloplejin, Abdi Ibrahim, Turkey) and dexamethasone sodium phosphate (Cebedex, Abdi Ibrahim, Turkey) for 2 weeks without any sign of recovery. The patient had no history of contact lens wear or corneal trauma. Biomicroscopic examination revealed multiple scattered subepithelial infiltrates, enlarged corneal nerves and limbitis in the right eye. Result of biomicroscopic examination of the left eye was normal. Visual acuity (Snellen) in the right and left eye was 0.8 and 1.0, respectively. Intraocular pressure measured with a non-contact tonometer was 14 mmHg in the right and 16 mmHg in left eye. Result of fundus examination was normal in both eyes. Corneal epithelial scrapings were taken under topical anesthesia and were subjected to Acanthamoeba diagnostics. The scrapings were culture positive for Acanthamoeba, and the patient was treated with propamidine isethionate 0.1% (Brolene), neomycin (Neosporin), polyhexamethylene biguanide (Klorhex) and fluconazole (Klorhex) eye drops for altogether 1 year. The patient was reexamined after 1, 2, 3, 4, 5, 6, 8 and 12 months. After 1 year, the patient was completely free of symptoms. In the last follow-up, the visual acuity in the right eye was 1.0 and the result of biomicroscopic examination was within normal limits.
Materials and methods
Samples
Sample 1 was the corneal scraping from the AK patient. Sample 2 was a contact lens storage case of a 40-year-old female asymptomatic contact lens wearer who had been using her lenses for 10 years and had been washing them with tap water. Sample 3 was a 2.5-l sample of domestic tap water from the city of Izmir, and sample 4 was a 1-g soil sample from a garden in the centre of Izmir.
Amoeba culture
All samples were cultured on non-nutrient agar plates (1.5%) precoated with Escherichia coli. The corneal scraping and the contact lens fluid were directly inoculated onto the middle of the respective plate. The tap water sample (2.5 l) was filtered through a sterile 5.0-μm pore size cellulose acetate membrane (47 mm in diameter), and then the membrane was inoculated onto a plate upside down. The soil sample (1 g) was suspended in a few drops of amoeba saline (Page 1991) and was then inoculated onto the centre of a plate. The inoculated plates were sealed with parafilm, incubated at 35°C and examined daily under an inverted microscope. Two to 5 days after inoculation, Acanthamoeba spp. trophozoites and, sporadically also, cysts were clearly visible. All isolates were cloned using a micromanipulator in order to obtain genetically uniform cultures. Subsequently, all isolates were ascribed to one of the morphologic groups according to the identification key of Page (1991), investigated for their physiologic capabilities and subjected to 18S rDNA sequencing.
Temperature tolerance test
Subcultures of all samples were incubated at 37, 40 and 42°C, respectively. After 48 h of incubation, samples were investigated for amoebal growth by phase contrast microscopy.
Isolation of DNA
For molecular biological investigations, actively growing amoebae (∼106 cells) were harvested from culture plates with a sterile cotton-tipped applicator and washed three times in sterile 0.9% NaCl by centrifugation at 500×g for 7 min. Whole-cell DNA was isolated by a modified UNSET procedure (Hugo et al. 1992). Briefly, the pellet was resuspended in 500 μl of UNSET lysis buffer, overlaid with 500 μl phenol-chloroform-isoamylalcohol (PCI) and shaken gently for 5 h. DNA was extracted by multiple PCI extraction, precipitated in alcohol, air dried and resuspended in 30 μl of sterile double-distilled water.
Polymerase chain reaction and sequence analysis
The 18S rRNA gene was amplified using the SSU1 and SSU2 primers (Gast et al. 1996). These are universal eukaryotic primers, complementary to the strongly conserved ends of the eukaryotic 18S rRNA genes. A standard amplification programme with 30 cycles of 1 min 95°C, 2 min 52°C and 3 min 72°C was used for polymerase chain reaction (PCR). The amplification of the 18S rRNA gene was visualized by ethidium bromide in an agarose gel electrophoresis, and the amplified gene was sequenced stepwise by direct sequencing from the PCR product using the Thermo Sequenase TM II sequencing kit (Amersham Pharmacia Biotech GmbH, Wien, Austria) and the P1–3 forward and reverse internal primers (Walochnik et al. 2004). Sequencing was carried out in a 310 ABI PRISM automated sequencer (PE Applied Biosystems, Langen, Germany), and sequences were obtained from both strands. The sequences were compared to published sequences from other Acanthamoeba strains, and the genotypes were assessed with the model assumption of a <5% sequence dissimilarity within one genotype.
Sequence data were deposited at GenBank and are available under the following accession numbers: DQ264391 (sample 1, strain Fa03), DQ185607 (sample 2, strain PSH), DQ185606 (sample 3, strain PS) and DQ185605 (sample 4, strain PJ).
Results
All samples investigated revealed acanthamoebae that were able to grow at 35°C, the approximate temperature of the human eye. No representative of any of the other three genera of FLA of clinical relevance, Balamuthia, Naegleria and Sappinia, was present in any of the samples.
The Acanthamoeba strain isolated from the AK patient and the strain isolated from tap water were identified as morphological group II and genotype T4, and the strains isolated from the lens storage case and from soil were identified as morphological group I and genotype T9 (Table 1). All isolates were able to grow at 37°C, and except the strain isolated from the soil, strain PJ genotype T9, all strains were also able to grow at 40°C. Strain Fa03 of genotype T4 isolated from the corneal scraping of the AK patient was the only strain that showed growth also at 42°C.
Strain Fa03 genotype T4 has an average number of six or less truncate rays, and the ectocyst is not pronounced and wrinkled as it is described in the closely related A. castellanii; thus, this isolate would morphologically be identified as A. mauritaniensis (Fig. 1a). However, A. mauritaniensis has recently been shown to be a synonym of A. rhysodes, as it does not show more than 0.2% sequence dissimilarity to the Singh strain of A. rhysodes (Liu et al. 2005). The Singh strain again and also our Fa03 strain belong to genotype T4, and Stothard et al. (1998) have suggested that all T4 strains should be reclassified as A. castellanii, as the type strain of A. castellanii, strain Castellani, shows T4. We support this aim to reconcile the species designations with genetic relatedness and thus reclassify our isolate as A. castellanii.
Strain PS isolated from tap water exhibits a typical ‘A. hatchetti morphology’ with four rays (Fig. 1c), which also correspond to its ability to grow at 40°C. However, this strain also is a representative of genotype T4—as are many, but not all, of the A. hatchetti isolates known to date—and is thus also reclassified as A. castellanii.
The strain isolated from the contact lens container, strain PSH, has a mean number of 12 rays (Fig. 1b), and although strain PJ has an average number of only 9 rays (Fig. 1d), in both cases, the rays are not all in the same plane; thus, both isolates have to be classified as A. comandoni. This close relationship between strain PSH and strain PJ despite their differing morphology and their differing temperature tolerance is also reflected by the fact that both are representatives of genotype T9, showing as much as 99.8% sequence identity to one another.
Discussion
Approximately 80% of the more than 3,000 documented AK cases were associated with contact lens wear, and of these patients, again at least 80% wore soft hydrogel lenses (Schuster and Visvesvara 2004; Seal 2003). However, most documented cases originated from the USA or the UK, where contact lens wear is highly common and other risks of infection are low. Recent studies from India demonstrate that most AK patients there are non-contact-lens wearers, with most cases occurring in the rural population after corneal trauma or a dirty water splash into the eye (Sharma et al. 2000, 2004; Srinivasan et al. 2003). In the current study, we present a case of AK that was neither linked to contact lens wear nor to a history of corneal trauma. Interestingly, however, the last AK case in Turkey, a 5-year-old boy, was also not associated with contact lens wear or corneal trauma (Demirci et al. 2006). Thus, obviously, also in non-tropical regions, risk factors other than contact lens wear, such as water splashes into the eyes, must be taken into account for AK.
The AK-causing strain of the presented case was identified as Acanthamoeba genotype T4, as was also the strain isolated from tap water, strain PS. The relatively high abundance of Acanthamoeba isolates with genotype T4 in the environment has been proposed as one explanation for the preponderance of genotype T4 in AK infections; in particular, tap water has been assumed to be the most important source of infection (Stothard et al. 1998; Tsvetkova et al. 2004). Unfortunately, in neither of the previous AK cases in Turkey—the first case was reported in 1996 (Akyol et al. 1996), and after this, only two other cases have been published, one in 1999 (Akisü et al.1999) and one in 2006 (Demirci et al. 2006)—the Acanthamoeba subpopulations have been characterized. Worldwide, most AK-causing strains are associated with genotype T4, but AK-causing strains belonging to T2, T3, T5, T6 and T11 have also been isolated (Gast et al. 1996; Khan et al. 2002; Maghsood et al. 2005; Spanakos et al. 2006; Stothard et al. 1998; Walochnik et al. 2000). A recent comparative study from the UK and Iran has demonstrated that whereas in the UK, 81.8% of the investigated AK isolates belonged to T4, in Iran, it was only 61.5%—the rest belonged to T3 and T2 (Maghsood et al. 2005).
The strains isolated from the lens storage case and from the soil were both identified as A. comandoni and genotype T9. A. comandoni and genotype T9, respectively, are widely seen in nature and are considered to be non-pathogenic (Schroeder et al. 2001; Booton et al. 2002; Kilvington et al. 2004). This corresponds very well to the fact that the contact lens wearer from whose contact lens case our strain had been isolated was completely asymptomatic. Several studies have shown that pathogenic but also non-pathogenic acanthamoebae can frequently be isolated from contact lens cases (Gray et al. 1995; Hiti et al. 2000; Walochnik et al. 2000). Nevertheless, non-pathogenic acanthamoebae can also be of clinical relevance, as they can act as vehicles for pathogenic bacteria as for example the common eye pathogen Pseudomonas aeruginosa or the causative agent of Legionnaires’ disease, Legionella pneumophila (Barker and Brown 1994). Interestingly, a recent study has shown that legionellae are widely distributed in hot water samples from hotels in Izmir province, the same geographical area where our strains had been isolated (Uzel et al. 2005). Acanthamoebae have repeatedly been isolated from soil and also from other environmental samples including fresh water, a thermal spring and sewage in Turkey (Saygı 1979; Saygı et al. 2000; Saygı and Polat 2003); however, in these studies, the amoebae have not been identified below the genus level. In a recent study (Kilic et al. 2004), Acanthamoeba isolates belonging to T2, T3, T4 and T7 genotypes—but not T9—have been found in environmental samples from Ankara, Turkey.
Altogether, our study corroborates that also in non-tropical regions, risk factors other than contact lens wear, such as water splashes into the eyes, must be taken into account for AK. Temperature-tolerant acanthamoebae seem to be common in the environment in Turkey; furthermore, in Turkey, Acanthamoeba genotype T4 is a typical causative agent of AK. To the best of our knowledge, this is the first study identifying an AK-causing Acanthamoeba strain below the genus level in Turkey. Moreover, it is the first time that Acanthamoeba genotype T9 was isolated in this country.
References
Akisü Ç, Baka M, Durak I, Orhan V (1999) A case of Acanthamoeba keratitis: light and electron microscope findings. Acta Parasitol Turc 23:340–342
Akyol N, Aşçı Z, Kükner S (1996) Acanthamoeba keratitis: the first reported case from Turkey. Ophtal Practice Asia Ed 2:46–48
Barker J, Brown MR (1994) Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253–1259
Booton GC, Kelly DJ, Chu Y-W, Seal DV, Houang E, Lam DSM, Byers TJ, Fuerst PA (2002) 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J Clin Microbiol 40:1621–1625
De Jonckheere JF (1991) Ecology of Acanthamoeba. Rev Infect Dis 13:385–387
Demirci G, Ay GM, Karabas LV, Altintas O, Tamer GS, Caglar Y (2006) Acanthamoeba keratitis in a 5-year-old boy without a history of contact lens usage. Cornea 25:356–358
Gast RJ (2001) Development of an Acanthamoeba-specific reverse dot-blot and the discovery of a new ribotype. J Eukaryot Microbiol 48:609–615
Gast RJ, Ledee DR, Fuerst PA, Byers TJ (1996) Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J Eukaryot Microbiol 43:498–504
Gray TB, Cursons RT, Sherwan JF, Rose PR (1995) Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol 79:601–605
Hewett MK, Robinson BS, Monis PT, Saint CP (2003) Identification of a new Acanthamoeba 18S rRNA gene sequence type, corresponding to the species Acanthamoeba jacobsi Sawyer, Nerad and Visvesvara, 1992 (Lobosea: Acanthamoebidae). Acta Protozool 42:325–329
Hiti K, Faschinger C, Haller-Schober EM, Hiti H, Walochnik J, Aspöck H (2000) Acanthamoeba in asymptomatic contact lens wearers? Examination of storage-boxes. Spektrum Augenheilkd 14:163–166
Horn M, Wagner M (2004) Bacterial endosymbionts of free-living amoebae. J Eukaryot Microbiol 51:509–514
Horn M, Fritsche TR, Gautom RK, Schleifer K, Wagner M (1999) Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryphilus. Environ Microbiol 1:357–367
Hugo ER, Stewart VJ, Gast RJ, Byers TJ (1992) Purification of amoeba mtDNA using the UNSET procedure. In: Soldo AT, Lee JJ (eds) Protocols in Protozoology. Allen Press, Lawrence, Kansas, PD-7.1
Khan NA, Paget TA (2002) Molecular tools for speciation and epidemiological studies of Acanthamoeba. Curr Microbiol 44:444–449
Khan NA, Jarroll EL, Paget TA (2002) Molecular and physiological differentiation between pathogenic and non-pathogenic Acanthamoeba. Curr Microbiol 45:197–202
Kilic A, Tanyuksel M, Sissons J, Jayasekera S, Khan N (2004) Isolation of Acanthamoeba isolates belonging to T2, T3, T4, and T7 genotypes from environmental samples in Ankara, Turkey. Acta Parasitol 49:246–252
Kilvington S, White DG (1994) Acanthamoeba: biology, ecology and human disease. Rev Med Microbiol 5:12–20
Kilvington S, Gray T, Dart J, Morlet N, Beeching JR, Frazer DG, Matheson M (2004) Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci 45:165–169
Liu H, Moon EK, Yu HS, Jeong HJ, Hong YC, Kong HH, Chung DI (2005) Evaluation of taxonomic validity of four species of Acanthamoeba: A. divionensis, A. paradivionensis, A. mauritaniensis, and A. rhysodes, inferred from molecular analyses. Korean J Parasitol 43:7–13
Maghsood AH, Sissons J, Rezaian M, Nolder D, Warhurst D, Khan NA (2005) Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J Med Microbiol 54:755–759
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307
Page FC (1991) Nackte Rhizopoda. In: D. Matthes (eds) Protozoenfauna, Band 2. G. Fischer, Stuttgart-New York, 297 pp
Pussard M and Pons R (1977) Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebidae). Protistologica 8:557–598
Saygı G (1979) Erzurum’da topraktan Acanthamoeba türünün soyutlanması. T Parazitol Derg 2:109–114
Saygı G, Polat Z (2003) Özgür yaþayan amipler ve neden oldukları parazitozlar. Primer amibik meningoensefalit-Granülomatöz Amibik Ensefalit-C.Ü. Tıp Fak Derg 25:140–149
Saygı G, Akın Z, Tecer H (2000) Isolation of Acanthamoeba and Naegleria spp. from soil and thermal water specimens in Sivas. Acta Parasitol Turc 24:237–242
Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol 39:1903–1911
Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34:1001–1027
Seal D (2003) Treatment of Acanthamoeba keratitis. Expert Rev Anti Infect Ther 1:205–208
Sharma S, Garg P, Rao GN (2000) Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol 84:1103–1108
Sharma S, Pasricha G, Das D, Aggarwal RK (2004) Acanthamoeba keratitis in non-contact lens wearers in India: DNA typing-based validation and a simple detection assay. Arch Ophthalmol 122:1430–1434
Spanakos G, Tzanetou K, Miltsakakis D, Patsoula E, Malamou-Lada E, Vakalis NC (2006) Genotyping of pathogenic Acanthamoebae isolated from clinical samples in Greece—report of a clinical isolate presenting T5 genotype. Parasitol Int 55:147–149
Srinivasan M, Burman S, George C, Nirmalan PK (2003) Non-contact lens related Acanthamoeba keratitis at a tertiary eye care center in south India: Implications for eye care programs in the region. Med Sci Monit 9:CR125–129
Stothard DR, Schroeder-D JM, Awwad MH, Gast RJ, Ledee DR, Rodriguez-Zaragoza S, Dean CL, Fuerst PA, Byers TJ (1998) The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rNA gene sequence types. J Eukaryot Microbiol 45:45–54
Tsvetkova N, Schild M, Panaiotov S, Kurdova-Mintcheva R, Gottstein B, Walochnik J, Aspöck H, Lucas MS, Muller N (2004) The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res 92:405–413
Uzel A, Ucar F, Hames-Kocabas EE (2005) Prevalence of Legionella pneumophila serogroup 1 in water distribution systems in Izmir province of Turkey. APMIS 113:649–664
Walochnik J, Haller-Schober E, Kolli H, Picher O, Obwaller A, Aspöck H (2000) Discrimination between clinically relevant and non-relevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J Clin Microbiol 38:3932–3936
Walochnik J, Michel R, Aspöck H (2004) A molecular biological approach to the phylogenetic position of the genus Hyperamoeba. J Eukaryot Microbiol 51:433–444
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare that all experiments performed comply with the current laws of Turkey and Austria.
Rights and permissions
About this article
Cite this article
Ertabaklar, H., Türk, M., Dayanir, V. et al. Acanthamoeba keratitis due to Acanthamoeba genotype T4 in a non-contact-lens wearer in Turkey. Parasitol Res 100, 241–246 (2007). https://doi.org/10.1007/s00436-006-0274-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0274-0