Abstract
Sarcocystis species are among the most common and widespread protozoan parasites of mammals and birds. The current study provides the first record of infection with Sarcocystis species in the common moorhens from Brolos Lake, KafrElsheikh province, Egypt. Morphology of the parasite cysts was described using light and transmission electron microscopy. Out of 25 examined birds, sarcocysts were found in neck, thigh, and legs muscles of two birds. The cysts were microscopic and measured 150–650 μm in length × 45–185 μm in width. Histologically, the sarcocyst wall appeared striated and characterized by the presence of radial spines. Ultrastructurally, it measured 2–4.5 μm in thickness and had irregularly shaped crowded finger-like villar protrusions that measured 1.5–4 μm in length and up to 0.4–2 μm in width with the presence of dense electron ground substance of 200–750 nm thick. Several septa derived from the ground substance were present and divided the cyst into compartments containing both bradyzoites and metrocytes. The bradyzoites were banana-shaped and measured 6–12 × 1–2 μm with centrally or posteriorly located nuclei. The ultrastructural features of the cyst wall belonged to type 10 cyst wall according the classification of Dubey et al. (1989) and Dubey and Odening (2001).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcocystis species are obligate heteroxenous parasites which require two hosts to complete life cycle between prey–predator hosts (Mehlhorn and Heydorn 1978), and they possess life cycle that alternate between herbivores or omnivores as intermediate host and carnivores as definitive host (Olias et al. 2010a; Xiang et al. 2010). So far, more than 150 species of Sarcocystis were known as parasitic of domestic and wild animals (Morsy et al. 2011). Most of previous literatures on such parasites focused on infections in domestic mammals in which some species might be pathogenic to their intermediate hosts causing weight loss and reduced milk production (Mehlhorn and Heydorn 1978; Dubey and Odening 2001). Members of the genus Sarcocystis were found to have a wide range of phylogenic distribution when studying non-mammalian wildlife hosts (Xiang et al. 2010).
Infections with Sarcocystis spp. have been reported in domestic animals such as horse, dogs, cats, swine, cattle, water buffalo, sheep, goats, camels and wild animals (Dubey et al. 2001a, b; Moré et al. 2008), monkeys (Prathap 1973; Mehlhorn et al. 1977;Yabsley et al. 2006), and humans (Kannan and Dissanaike 1975; Mehrotra et al. 1996). Different species of wild animals have been investigated and were found to be infected with sarcocysts such as raccoons, black bear, roe deer, rein deer, moose, mink, armadillos, sea otter, Pacific harbor seal, and reptiles (Drouin and Mahrt 1979; Zhu et al. 2009; Xiang et al. 2010). Odening (1998) reported that there were 20 identified Sarcocystis species in birds with description of cysts in the muscles of the intermediate hosts. Thereafter, eight more Sarcocystis species parasitizing birds were named, Sarcocystis otus from long-eared owl (Asio otus) (Krone et al. 2000), Sarcocystis lindsayi from experimentally infected budgerigars (Melapsittacus undulatus; Dubey et al. 2001c), Sarcocystis ramphastosi and Sarcocystis sulfuratusi from the keel-billed toucan (Ramphastos sulfuratus) (Dubey et al. 2004). Kutkiene et al. (2006) reported a new Sarcocystis sp. from the white fronted goose (Anser albifrons); meanwhile, Kutkiene et al. (2008) reported a Sarcocystis sp. from the golden eye (Bucephala clangula) and another novel one from the mallard duck (Anas platyrhynchos). Sarcocystis cornixi from the hooded crow (Corvus cornix; Kutkiene et al. 2009), Sarcocystis calchasi from the domestic pigeon (Columba livia f. domestica; Olias et al. 2010a), Sarcocystis columbae from the wood pigeon (Columba palumbus; Olias et al. 2010b) and Sarcocystis wobeseri from the barnacle goose (Branta leucopsis; Kutkiene et al. 2010).
Sarcocysts were found in leg muscles of wood pigeon (C. palumbus) hunted in Lithuania and were identified as Sarcocystsis columae (Prakas et al. 2011a). Microcysts were observed in herring gulls (Larus argentatus) and were morphologically and genetically similar to S. wobeseri and named S. wobeseri-like (Prakas et al. 2011b). Prakas and Butkauskas (2012) stated that sarcocysts were detected in the muscles of the white-fronted goose, bean goose (Anser fabalis), lesser white-fronted goose (Anser erythropus), mallard, gadwall (Anas strepera), garganey (Anas querquedula), common teal (Anas crecca), common scoter (Melanitta nigra), velvet scoter (Melanitta fusca), shelduck, tufted duck (Aythya fuligula), long-tailed duck (Clangula hyemalis), common goldeneye (B. clangula), common merganser (Mergus merganser), and common eider (Somateria mollissima). Kutkienė et al. (2012) detected a novel Sarcocystis species from the common black bird (Turdus merula) and named it Sarcocystis turdusi. Prakas et al (2013) recently identified a new Sarcocystis sp. from the jackdaw (Corvus monedula) and named it Sarcocystis corvusi.
To our knowledge, this is first study investigated Sarcocystis spp. from common moorhens (Gallinula chloropus) (Aves: Gruiformes: Rallidae) in Egypt, with morphological identification and description of the revealed sarcocysts using light and transmission electron microscopy (TEM).
Materials and methods
Birds and area of study
Twenty-five moorhens (G. chloropus) were hunted by shooting from Brolos lake located at the northern region of Kafr El-Sheikh province (coordinates: 31°06′42″N 30°56′45″E), Egypt during the period from November 2012 to March 2013, and investigated for Sarcocystis species infection. The birds were examined as fast as possible after death. Skeletal muscles (neck, breast, wing, thigh, leg, abdomen, and heart) were grossly examined for the presence of macrocysts and histologically for microcysts.
Histopathology
From each bird, pieces of muscle tissues from the previously mentioned muscles of about 1 cm2 were fixed in 10 % neutral-buffered formalin and routinely processed for histopathology, sectioned at 5 μm, stained by hematoxylin and eosin and examined by light microscopy at magnification powers 40×, 100×, 400×, and 1,000×. Other small pieces were fixed in 2.5 % glutaraldehyde for TEM examination.
Transmission electron microscopy
When the formalin-fixed samples were found positive for Sarcocystis species infection, using light microscopy, the corresponding glutaraldehyde fixed samples were further processed for TEM. After fixation of muscle samples in 2.5 % glutaraldehyde, they were postfixed in 1 % osmium tetraoxide, dehyderated in ethanol, and finally embedded in epoxy resin. Semithin sections were prepared using glass knives on Leica EMUC6 Ultramicrotome at a thickness of 1 μm stained with toluidine blue 1 % and examined by light microscopy at magnifications of 40×, 100×, 400×, and 1,000×. Ultrathin sections were obtained using diamond knife at a thickness of 50–100 nm, collected on copper grids, stained with uranyl acetate and lead citrate, and examined by TEM JEOL-JEM-1400 operated at 80 kV.
Results
Light microscopy
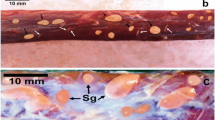
Sarcocysts of the recovered species were present in the neck, thigh, and leg muscles of two moorhens while other remaining birds were negative. All the detected sarcocysts from both birds were morphologically similar. These cysts were microscopic and measured from 150 to 650 μm in length × 45 to 185 μm in width (n = 25). The sarcocyst wall appeared striated and characterized by presence of striations or radial spines. The cyst wall measured approximately 2–4.5 μm in thickness (n = 15). The sarcocysts were packed with bradyzoites (Z; Fig. 1a).
a Histological section of the revealed Sarcocystis sp. in leg muscles of a common moorhen showing cyst wall with radial spines or striations (two opposing arrows) and crescent-shaped bradyzoites (Z). Scale bar 10 μm. b Semithin section of the identified Sarcocystis sp. stained with tolouidine blue 1 % showing cyst wall having crowded finger-like villar protrusions VP (two opposite white arrows) and banana-shaped bradyzoites (Z). Scale bar 10 μm. c TEM photograph showing the sarcocyst wall. Note the outer layer of the cyst wall (parasitophorus vacuolar membrane) PVM that is underlined by an electron dense layer. The PVM formed irregularly shaped densely packed finger-like villar protrusions VP containing multiple electron dense microgranules (Ed) and vesicle-like structures (V). Notice the dense electron ground substance (GS) that has no evident structures, from which septa (S) originated dividing the cyst into several compartments containing peripherally located metrocytes (Met) and bradyzoites (BR). Scale bar 1 μm. d TEM micrograph showing a centrally located bradyzoite within the sarcocyst with posteriorly located nucleus (N). Notice the double membranous pellicle (PE) surrounding the bradyzoite, the presence of conoid (C) at the anterior end of the bradyzoite. Micronemes (Mn) are distributed in the anterior third. Rhoptries (Rh) and amylopectin granules (Ag) are scattered. Notice the mitochondrion (Mit) located anterior to the nucleus with the presence of many other crossly sectioned mitochondria and their characteristic cristae, the septa (S) dividing the cyst into different compartments, villar protrusions (VP) and ground substance (GS). Scale bar 1 μm
Examination of semithin sections
The thickness of cyst wall amounted from 2 to 4.5 μm and characterized by presence of crowded finger-like villar protrusions (VP). The cavity of the cyst was filled with crescent or banana-shaped bradyzoites (Fig. 1b).
Ultrastructural examination
The identified sarcocysts were characterized by the presence of a cyst wall ranging from 2 to 4.5 μm and had crowded, densely packed and irregularly shaped finger-like VP of variable dimensions resting on a thin electron-dense ground substance (GS), from which septa (S) originated and divided the cyst into several compartments containing few peripherally located faintly stained metrocytes and many bradyzoites. The cores of the VP contained several electron-dense microgranules and vesicle-like structures (V) with the absence of microfilaments. The diameter of VP ranged from 1.5 to 4 μm in length and 0.4 to 2 μm in width (n = 20). There were narrow spaces between the VP ranging from 50 to 150 nm in diameter. The outer layer of the sarcocyst wall (parasitophorus vacuolar membrane; PVM) was underlined with dense electron layer that ranged from 50 to 100 nm in thickness. The GS was electron dense with no evident structures and measured 200–750 nm in thickness (Fig. 1c).
The bradyzoites were crescent or banana-shaped cells that measured 6–12 × 1–2 μm (n = 25) and had centrally or posteriorly located nuclei (N) with clear nuclear envelope and ranged from 1 to 2 μm in diameter and contained osmiophilic particles alternating with paler ones. Bradyzoites were also characterized by the presence of the apical complex, the basic unique structure, which was present in the anterior end of infective stages of all members of phylum Apicomplexa and formed of several organelles. The organelles were conoid (C), a cone-like structure situated at the most anterior end of the bradyzoite and measured 300 nm in length and nearly 350 nm at its base, micronemes that were distributed in the cytoplasm of the anterior third of the bradyzoite, rhoptries that were distributed anteriorly and posteriorly to the nucleus, pellicle, and amylopectin granules which were distributed inside the cytoplasm of the bradyzoites. Mitochondia that measured 1–3 μm in length with their characteristic cristae were obviously observed in many bradyzoites (Fig. 1d).
Metrocytes were mainly peripherally located, immediately under the cyst wall, globular to ovoid in shape, sometimes irregularly shaped faintly stained cells, and measured from 3.5 to 6.5 × 8.5 to 9.5 μm in diameter with centrally located nuclei (n = 15).
Discussion
Complete life cycles of parasites in the genus Sarcocystis are known for only a few species of animals, mostly those in livestock (Dubey et al. 1989). Parasitic protists of the genus Sarcocystis have the ability to infect a large scale of hosts such as mammals, birds, and reptiles (Odening 1998; Dubey et al. 1989). Most Sarcocystis species have been named based on their intermediate host occurrence and their structure (Dubey et al. 1989, 2008; Odening 1998; Dubey and Morales 2006). The ultrastructure of the cyst wall is the basic criterion used for its identification, Dubey et al. (1989) and Dubey and Odening (2001) recognized 37 distinct species of Sarcocystis. Each species possess a sarcocyst wall that often has unique ultrastructural characteristics which can be used to distinguish it from other species within the same intermediate host (Abdel Ghaffar et al. 1978; Dubey et al. 1989; 2006, 2008; Dubey and Morales 2006; Hilali et al. 2011), however, a similarity between species might be found (Odening 1998).
Two out of 25 moorhens (G. chloropus) were found positive for microscopic sarcocysts of the identified species, while no macroscopic cysts were detected. . All the detected cysts from both birds were morphologically identical. The cyst wall ultrastructure of Sarcocystis species revealed in the present investigation belonged to type 10 based on the classification of Dubey et al. (1989) and Dubey and Odening (2001).The VP of the newly revealed species were crowded and densely packed finger-like with broader bases and to some degree narrower tips. They contained electron dense microgranules and vesicle-like structures while the microfilaments were absent. The PVM was underlined with an electron dense layer. There were no characteristic structures within the ground substance and its thickness ranged from 200 to 750 nm in thickness depending on variations in the plane of section.
All the examined sarcocysts were mature as they contained greater number of bradyzoites with fewer metrocytes (Dubey et al. 1989). Metrocytes of the present Sarcocystis sp. measured 3.5–6.5 × 8.5–9.5 μm. The structure and measurements of the metrocytes alone are not helpful for species identification because metrocytes are often irregularly shaped and their size is highly variable depending on the stage of division (Dubey et al. 1989). Furthermore, length of bradyzoites ranged from 6 to 12 μm that may allow them to be densely packed in some sarcocysts or sparsely distributed in others, therefore, affecting size and shape of cystozoites. In addition, bradyzoites of the current Sarcocystis sp. and in most of others are banana-shaped, with a great curvature, so it seems difficult to measure them accurately (Dubey et al. 1989).
Ultrastructurally, features of the cyst wall described herein were distinct and characteristic for the Sarcocystis sp. under investigation, while showed few similarities to some of those previously detected in other wild bird species. Spalding et al. (1994) found sarcocysts in the striated muscles of 4.83 % (7/145) adult wading birds examined grossly and 20 % (14/70) examined histologically. Cysts were filamentous, usually extended along the entire length of the muscle fiber, and were grossly visible in 33 % of the positive cases. Using TEM, they found that the detected Sarcocystis sp. resembled type 9 sarcocysts described by Dubey et al. (1989).The VP of such species were finger-like that contained microfilaments and the VP were located at variable distances on the cyst wall and not crowded and the PVM was undulating and uneven. The ground substance ranged from 0.32 to 0.99 μm in thickness.
The sarcocyst wall in Sarcocystis falcatula was thick, striated with evenly spaced finger-like VP that had an uneven thickness in the outer electron dense layer (hobnail appearance). Microtubules were obvious in the center of the projections and did not extend to the ground substance layer (Drouin and Mahrt 1980; Box et al. 1984; Dubey et al. 2000; Luznar et al. 2001).
Sarcocysts of S. lindsayi were described in budgerigars by Dubey et al. (2001c); they were microscopic, up to 600 μm long and up to 50 μm wide. The cyst wall was up to 2 μm thick. Ultrastructurally, the sarcocyst wall consists of numerous slender VP (up to 2.0 μm long and up to 0.3 μm wide), each with a stylet-like structure at its tip.
Two new Sarcocystis spp from the keel-billed toucan were identified by Dubey et al. (2004); S. ramphastosi which was macroscopic and another microscopic one that was named S. sulfuratusi. Under light microscopy, cyst walls of both species were smooth and have no clear protrusions. However, ultrastructurally, the cyst wall of S. ramphastosi had finger-like VP that were up to 6.5 μm long and up to 3 μm wide and contained microtubules that were smooth and confined to the villi. Whereas, S. sulfuratusi VP measured 4.3 μm long and 1.4 μm wide and were characterized by the existence of microtubules those extended deeply into the ground substance and were more electron dense than that present in the VP.
Whereas Kutkiene et al. (2006) identified a new Sarcocystis sp. from the white fronted goose (A. albifrons); the cysts were ribbon-shaped up to 4 mm in length and up to 750 μm in width. By light microscope, the cyst wall (up to 2.4 μm) had teat- or finger-like protrusions with gaps between them. Ultrastructurally, the cyst wall had teat- or finger-like VP (up to 2.3 μm long) different in length and width. Within the protrusions, there were microfilaments that extend from the villi tips into the ground substance of the cyst.
A new Sarcocystis sp. from two Buffoons macaw (Ara ambigua) was reported by Dubey and Morales (2006) from Costa Rica. They revealed that the cyst wall was less than 1 μm thick and smooth using light microscopy. Ultrastructurally, the cyst wall was characterized by sloping VP with a wavy PVM. The VP was finger-like but they were narrow at both the tips and bases and wider at their middle portions with clear electron dense layer immediately under the PVM. The terminal end of one villus appeared to be bifid and the tip of another one was bent over. The microtubules did not extend into the ground substance. The VP were 4 μm long and up to 0.6 μm wide, and were folded over the sarcocyst wall giving it the thin-walled appearance.
Meanwhile, Dubey et al. (2006) reported a Sarcocystis species from a naturally infected African grey parrot, Psittacus erithacus, from Costa Rica. Mature sarcocysts, measuring up to 2 mm in length and up to 750 μm in width. Histologically, sarcocysts were seen with a smooth cyst wall. The VP were up to 5 μm long and up to 1.1 μm wide and they were finger-like and the microtubules inside the VP were smooth, well-defined, and did not extend to the ground substance .The PVM was convoluted due to presence of indentations; however, the VP were folded over the sarcocyst wall giving a thin-walled appearance. Moreover, Kutkiene et al. (2008) detected sarcocysts, using light microscopy, from one mallard duck which were ribbon shaped, very long, and thin. The cyst wall measured up to 1.5 μm and had palisade-like VP that were closely crowded together they named it (cyst type II). Ultrastructurally, using TEM examination, the sarcocysts showed relatively short VP (up to 1.3 μm) different in size and shape that extended from the surface of the cyst wall and were spaced at irregular intervals. The primary cyst wall had dense electron microprojections.
Meanwhile, Kutkiene et al. (2009) investigated 14 hooded crows (C. cornix) out of 67 birds of the family Corvidae. Sarcocysts in hooded crows had radial spines or a smooth outer surface of the cyst wall using light microscopy but ultrastructurally, the cyst wall was characterized by stump like protrusions that were different in size and shape with the existence of electron dense microgranules in the ground substance. Sarcocysts with a striated cyst wall isolated from hooded crows were described as a new species of Sarcocystis, S. cornixi.
Sarcocysts of S. rielyi were identified by Dubey et al. (2010) from the breast muscles of two mallard ducks hunted in Colorado, USA, and they mentioned that this Sarcocystis species was previously detected in the northern shoveler duck (Anas clypeata), with the same wavy and highly branched cyst wall. The VP were up to 4 μm long and belonged to cyst wall type 21 in the classification of Dubey et al. (1989) and Dubey and Odening (2001). In addition, Kutkienė et al. (2011) identified the same species from 10 mallard ducks hunted in Lithuania using ultrastructural and molecular studies, and detected that the recovered macrocysts were correspondent to S. rileyi detected previously by Dubey et al. (2010).
The herring gull (Larus agentatus) was investigated for Sarcocystis species infection by Prakas et al. (2011a, b); they stated that ultrastructural examination of a novel Sarcocystis sp. revealed that it had tissue cyst wall type 1 which was thin (∼1.0 μm), smooth, or slightly wavy cyst wall without clearly visible protrusions. Its cyst wall was similar to those were described for S. calchasi from the domestic pigeon (C. livia f. domestica), S. columbae from the wood pigeon (C. palumbus), and S. wobeseri from the barnacle goose (B. leucopsis).
S. turdusi was recently identified from the common black bird (T. merula) by Kutkienė et al. (2012) and characterized by a cyst wall belonging to type 4 according to the classification of Dubey et al. (1989) and Dubey and Odening (2001). The cyst wall reached up to 3.5 μm and had finger-like protrusions. Under TEM, the cyst wall was 2.5–4.4 μm thick, had club or irregularly shaped and sometimes branched protrusions that differed in their sizes.
Chen et al. (2012) identified cysts of Sarcocystis wenzeli in 17 out of 191 chickens examined at Yunnan province, China. Morphologically, the cysts were thread-like, ranging in size from 334 to 3,169 × 41–117 μm. Histologically, sarcocysts were septate with dense, short finger-like protrusions which appeared radially striated. The cyst wall was 1.4–3.5 μm thick. Ultrastucturally, the primary sarcocyst wall had stubby VP, corresponding to the type 9 cyst wall. The protrusions measured 0.87–1.89 × 0.47–0.91 μm and contained obvious criss-crossing microtubules that extended to the region of the ground substance. The primary cyst wall was supported by a thin osmiophilic material that was occasionally interrupted by small vesicular invaginations that averaged 0.05–0.08 μm in depth. Prakas et al (2013) reported a new Sarcocystis sp. from the jackdaw (C. monedula) that had type-1 cyst wall according to Dubey et al. (1989) which was characterized by many invaginations and knolls in the PVM giving the wall wavy appearance. They named it S. corvusi.
Populations of common moorhens (G. chloropus) are predominantly sedentary or locally dispersive but make partial or full migratory movements in the northern parts of its range, from (Northern Europe and Eurasia) to the south, during the period extending from September to December returning again from March to May (del Hoyo et al. 1996; Taylor and Van Perlo 1998). Moorhen is an omnivorous and opportunistic bird feeding on vegetable matters, algae, aquatic plants, grasses, cereal crops, mollusks, adult and larval insects, worms, leeches, bugs, fish eggs, and frogs. It might suffer from predation by the American mink (Neovison vison) in the UK (Ferreras and MacDonald 1999; BirdLife International 2012). Therefore, the latter animal species is suggested to be definitive hosts for the present Sarcocystis species or humans, as it may be hunted as a source of food and trade in Sumatra and Malawi, for sport in the USA, and for commercial and recreational purposes in Gilan Province, northern Iran (BirdLife International 2012). Moorhen is hunted for local consumption in Egypt by fishermen and people inhabiting the northern coast and the regions of northern lakes bordering the Mediterranean Sea especially Brolos lake in KafrElsheikh province; therefore, man may play the role of the final host for this apicomplexan species. In Egypt, there were no previous investigations into Sarcocystis species infecting wild birds, especially in the common moorhen; as the majority of studies were focusing on Sarcocystis species infecting domestic animals such as cattle (Sayed et al. 2008; Badawy et al. 2012), water buffaloes (Abdel Ghaffar et al. 1978; El-Morsey 2010; Hilali et al. 2011), and sheep (Morsy et al. 2011). To the best knowledge of the authors, this is the first report of infection of the common moorhen (G. chloropus) (Aves: Gruiformes: Rallidae) with a Sarcocystis species in Egypt, a new host record.
References
Abdel Ghaffar F, Hilali M, Scholtyseck E (1978) Ultrastructural studies of Sarcocystis fusiformis (Railliet 1897) infecting the Indian water buffalo (Bubalus bubalis) of Egypt. Tropenmed Parasitol 29:283–294
Badawy AII, Abouzaid NZ, Ahmed HA (2012) Sarcocystis hominis and other Sarcocystis species infecting cattle in Sharkia province, Egypt. J Am Sci 8:271–275
BirdLife International (2012) Gallinula chloropus. In: IUCN 2013. IUCN red list of threatened species. Version 2013.1. www.iucnredlist.org
Box ED, Meier JL, Smith JH (1984) Description of Sarcocystis falcatula Stiles, 1893, a parasite of birds and opossums. J Protozool 31:521–524
Chen X, He Y, Liu Y, Olias P, Rosenthal BM, Cui L, Zuo Y, Yang Z (2012) Infections with Sarcocystis wenzeli are prevalent in the chickens of Yunnan Province, China, but not in the flocks of domesticated pigeons or ducks. Exp Parasitol 1:31–34
del Hoyo J, Elliott A, Sargatal J (1996) Handbook of the birds of the world III. Hoatzin to auks. Lynx Edicions, Barcelona, Spain
Drouin TE, Mahrt JL (1979) The prevalence of Sarcocystis Lankester, 1882, in some bird species in western Canada, with notes on its life cycle. Can J Zool 57:1915–1921
Drouin TE, Mahrt JL (1980) The morphology of cysts of Sarcocystis infecting birds in western Canada. Can J Zool 58:1477–1482
Dubey JP, Morales JA (2006) Morphologic characterization of Sarcocystis sp. sarcocysts from the Buffon's macaw (Ara ambigua). Acta Parasitol 51:231–237
Dubey JP, Odening K (2001) Toxoplasmosis and related infections. In: Samuel WM, Pybus MJ, Kocan AA (eds) Parasitic diseases of wild mammals. Iowa State University Press, Ames, Iowa, pp 478–519
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. CRC Press, Boca Raton, p 215
Dubey JP, Lindsay DS, Rezende PCB, Costa AJ (2000) Characterization of an unidentified Sarcocystis falcatula-like parasite from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol 47:538–544
Dubey JP, Lindsay DS, Fritz D, Speer CA (2001a) Structure of Sarcocystis neurona sarcocysts. J Parasitol 87:1323–1327
Dubey JP, Rosenthal BM, Speer CA (2001b) Sarcocystis lindsayi n. sp. (Protozoa: Sarcocystidae) from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol 48:595–603
Dubey JP, Saville WJ, Stanek JF, Lindsay DS (2001c) Sarcocystis neurona infections in raccoons (Procyon lotor): evidence for natural infection with sarcocysts, transmission of infection to opossums (Didelphis virginiana), and experimental induction of neurologic disease in raccoons. Vet Parasitol 100:117–129
Dubey JP, Lane E, van Wilpe E (2004) Sarcocystis ramphastosi n. sp. and Sarcocystis sulfuratusi n. sp. from the keel-billed toucan (Ramphastos sulfuratus). Acta Parasitol 49:93–101
Dubey JP, Rosenthal BM, Morales JA, Alfaro A (2006) Morphologic and genetic characterization of Sarcocystis sp. from the African grey parrot, Psittacus erithacus, from Costa Rica. Acta Parasitol 51:161–168
Dubey JP, Humphreys G, Fritz D (2008) A new species of Sarcocystis (Apicomplexa: Sarcocystidae) from the black bear (Ursus americanus). J Parasitol 2:496–949
Dubey JP, Rosenthal BM, Felix TA (2010) Morphologic and molecular characterization of the sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) from the mallard duck (Anas platyrhynchos). J Parasitol 96:765–770
El-Morsey A (2010) Studies on Sarcocystis species infecting water buffaloes (Bubalus bubalis) in Egypt. M.V.Sc. thesis. Faculty of Veterinary Medicine, Kafr El-Sheikh University, Egypt
Ferreras P, MacDonald DW (1999) The impact of American mink (Mustela vison) on water birds in the upper Thames. J Appl Ecol 36:701–708
Hilali M, El-seify M, Zayed A, El-Morsey A, Dubey JP (2011) Sarcocystis dubeyi (Huong and Uggla 1999) infection in water buffaloes (Bubalus bubalis) from Egypt. J Parasitol 97:527–528
Kannan KM, Dissanaike AS (1975) A case of human Sarcocystis infection in west Malaysia. Trans R Soc Trop Med Hyg 69:503–504
Krone O, Rudolph M, Jakob W (2000) Protozoa in the breast muscle of raptors in Germany. Acta Protozool 39:35–42
Kutkiene L, Sruoga A, Butkauskas D (2006) Sarcocystis sp. from white-fronted goose (Anser albifrons): cyst morphology and life cycle studies. Parasitol Res 99:562–565
Kutkiene L, Sruoga A, Butkauskas D (2008) Sarcocystis sp. from the goldeneye (Bucephala clangula) and the mallard (Anas platyrhynchos): cyst morphology and ribosomal DNA analysis. Parasitol Res 102:691–696
Kutkiene L, Prakas P, Sruoga A, Butkauskas D (2009) Sarcocystis in the birds family Corvidae with description of Sarcocystis cornixi sp. nov. from the hooded crow (Corvus cornix). Parasitol Res 104:329–336
Kutkiene L, Prakas P, Sruoga A, Butkauskas D (2010) The mallard duck (Anas Platyrhynchos) as intermediate host for Sarcocystis wobeseri sp.nov. from the barnacle goose (Branta leucopsis). Parasitol Res 107:879–888
Kutkienė L, Prakas P, Sruoga A, Butkauskas D (2011) Identification of Sarcocystis rileyi from the mallard duck (Anas platyrhynchos) in Europe: cyst morphology and results of DNA analysis. Parasitol Res 108:709–714
Kutkienė L, Prakas P, Butkauskas D, Sruoga A (2012) Description of Sarcocystis turdusi sp. nov. from the common blackbird (Turdus merula). Parasitology 139:1438–1443
Luznar SL, Avery ML, Dame JB, MacKay RJ, Greiner EC (2001) Development of Sarcocystis falcatula in its intermediate host, the brown-headed cowbird (Molothrus ater). Vet Parasitol 95:327–334
Mehlhorn H, Heydorn AO (1978) Sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure. Adv Parasitol 16:43–91
Mehlhorn H, Heydorn AO, Janitschke K (1977) Light and electron microscopical study on Sarcocystis from muscles of the rhesus monkey (Macaca mulatta), baboon (Papio cynocephalus) and tamarin (Saguinus (=Oedipomidas) oedipus). Parasitol Res 51:165–178
Mehrotra R, Deepti B, Sing PA, Gupta SC, Gupta RK (1996) Diagnosis of human Sarcocystis infection from biopsies of the skeletal muscle. Pathology 28:281–282
Moré G, Pardini L, Basso W, Marín R, Bacigalupe D, Auad G, Venturini L, Venturini MC (2008) Seroprevalence of Neospora caninum, Toxoplasma gondii and Sarcocystis sp. in llamas (Lama glama) from Jujuy, Argentina. Vet Parasitol 155:158–160
Morsy K, Saleh A, Al-Ghamdi A, Abdel-Ghaffar A, Al-Rasheid K, Bashtar A, Al Quraishy S, Mehlhorn H (2011) Prevalence pattern and biology of Sarcocystis capracanis infection in the Egyptian goats: a light and ultrastructural study. Vet Parasitol 181:75–82
Odening K (1998) The present state of species-systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst Parasitol 41(3):209–233
Olias P, Gruber AD, Hafez HM, Heydorn AO, Mehlhorn H, Lierz M (2010a) Sarcocystis calchasi sp. nov. of the domestic pigeon (Columba livia f. domestica) and the Northern goshawk (Accipiter gentilis): light and electron microscopical characteristics. Parasitol Res 106:577–585
Olias P, Olias L, Lierz M, Mehlhorn H, Gruber AD (2010b) Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet Parasitol 171:7–14
Prakas P, Butkauskas D (2012) Protozoan parasites from genus Sarcocystis and their investigations in Lithuania. Ekologija 58:45–58
Prakas P, Butkauskas D, Sruoga A, Svazas S, Kutkiene L (2011a) Identification of Sarcocystis columbae in wood pigeon (Columba palumbus) in Lithuania. Vet Med Zootec 77:33–39
Prakas P, Kutkiene L, Sruoga A, Butkauskas D (2011b) Sarcocystis sp. from the herring gull (Larus agentatus )identity to S. wobeseri based on cyst morphology and DNA results. Parasitol Res 109:1603–1608
Prakas P, Kutkienė L, Butkauskas D, Sruoga A, Žalakevičius M (2013) Molecular and morphological investigations of Sarcocystis corvusi sp. nov. from the jackdaw (Corvus monedula). Parasitol Res 112:1163–1167
Prathap K (1973) Sarcocystis in the Malaysian long-tail monkey, Macaca irus. Trans R Soc Trop Med Hyg 67:651
Sayed FG, Shaheen MSI, Arafa MI, Koraa HM (2008) Sarcocystis infection in cattle at Assiut abattoir: microscopical and serological studies. Ass Univ Bull Environ Res 11:47–56
Spalding MG, Atkinson CT, Carleton RE (1994) Sarcocystis sp. in wading birds (Ciconiiformes) from Florida. J Wildl Dis 30:29–35
Taylor B, van Perlo B (1998) Rails: a guide to the rails, crakes, gallinules and coots of the world. Pica Press, Robertsbridge, UK
Xiang Z, Rosenthal BM, He Y, Wang W, Wang H, Song J, Shen PQ, Li ML, Yang Z (2010) Sarcocystis tupaia, sp. nov., a new parasite species employing tree shrews (Tupaiidae, Tupaia belangeri chinensis) as natural intermediate hosts. Parasitol Int 59:128–132
Yabsley MJ, Jordan CN, Mitchell SM, Norton TM, Lindsay DS (2006) Seroprevalence of Toxoplasma gondii, Sarcocystis neurona, and Encephalitozoon cuniculi in three species of lemurs from St. Catherines Island, GA, USA. Vet Parasitol 144:28–32
Zhu BY, Hartigan A, Reppas G, Higgins DP, Canfield PJ, Slapeta J (2009) Looks can deceive: molecular identity of an intra erythrocytic apicomplexan parasite in Australian gliders. Vet Parasitol 159:105–111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Morsey, A., El-Seify, M., Desouky, AR.Y. et al. Morphologic identification of a new Sarcocystis sp. in the common moorhen (Gallinula chloropus) (Aves: Gruiformes: Rallidae) from Brolos Lake, Egypt. Parasitol Res 113, 391–397 (2014). https://doi.org/10.1007/s00436-013-3667-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3667-x