Abstract
Macroscopic cysts of Sarcocystis in ducks were recorded in Europe, but they were not investigated in more detail. Results of light and electron microscopy as well as 18S rDNA, 28S rDNA and ITS-1 region sequences of Sarcocystis macrocysts isolated from naturally infected mallard duck (Anas platyrhynchos) from Lithuania are presented in this paper. According to ultrastructure results, macrocysts examined corresponds to S. rileyi. Phylogenetic investigation showed S. rileyi to be the most closely related to two unnamed Sarcocystis species from anseriforms and to the S. mucosa. This is the first well-documented case of S. rileyi in Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic protists of genus Sarcocystis are considered to be common in many species of mammals, birds and reptiles (Dubey et al. 1989). Birds can serve as intermediate or definitive hosts for these parasites. Representatives of the order Anseriformes usually serve as intermediate hosts, i.e. asexual reproduction of Sarcocystis occurs in their muscles. Sarcocystis cysts have been investigated most exhaustively in ducks, and it has been established that ducks are intermediate hosts for several species of this genus (Drouin and Mahrt 1980; Kutkienė and Sruoga 2004). S. rileyi was found by C.V. Riley in 1869 and named by Stiles (1893) and is best known and most thoroughly investigated Sarcocystis species in ducks. This was one of the first described and named Sarcocystis species, and its intermediate host is the shoveler (Anas clypeata). Later the striped skunk (Mephitis mephitis) was found to be a definitive host for this species (Cawthorn et al. 1981; Wicht 1981). S. rileyi cysts are macroscopic, resembling a grain of rice mainly localised in the breast muscles. Macrocysts found in ducks by most authors are characterised as S. rileyi, however, it is not clear whether they all belong to this species. To establish the final species dependence, ultrastructural investigations into macrocysts are necessary. S. rileyi has a type-23 tissue cyst wall, which has not been identified for any other species (Dubey et al. 1989; Dubey and Odening 2001). Thus far in Europe, S. rileyi in wild ducks has been investigated only grossly or by light microscopy (Kutkienė and Sruoga 2004). Identification of the species is made more difficult by the fact that its definitive host in the North America is the skunk, and in Europe, it lives in captivity only. Hence, the question whether S. rileyi species is found in Europe arises.
In many cases morphological and DNA analysis are needed to identify Sarcocystis species (Kutkienė et al. 2009). However, S. rileyi identification is relatively easy and is based on cyst morphology, i.e. macroscopic cysts, which resemble a grain of rice and a unique type of the cyst wall. Nevertheless the DNA investigation of S. rileyi is useful for comparing it with other Sarcocystis species at present, and also in the future, and possibly for evaluating intraspecific genetic diversity. 18S rRNA and 28S rRNA genes, as well as the first internal transcribed spacer (ITS-1), are mainly used in genetic investigations into different Sarcocystis species (Dahlgren and Gjerde 2008a; Mugridge et al. 2000; Olias et al. 2010). Therefore, these three genetic markers are applied in this study.
The ultrastructure of the macrocysts from the mallard duck (Anas platyrhynchos) and the results of DNA analysis are presented in this article.
Material and methods
Ten mallard ducks (Anas platyrhynchos) were hunted in Ukmergė district (Lithuania) in the winter of 2010. All ducks were examined for Sarcocystis macrocysts.
Light microscopy
For Sarcocystis microcysts, neck, leg and breast muscles of all birds were examined. For this purpose, 28 oath-size pieces of muscles were cut off, stained with water (1:500) methylene blue solution, lightened with 1.5% water acetic acid solution, pressed into glass compressor and examined by light microscope. The morphometric analysis of the micro–macrocysts walls and cystozoites was carried out in fresh preparations after the cysts had been isolated from the muscle fibres with two preparation needles. The cyst wall thickness, the size and shape of protrusions and the morphology of cystozoites were evaluated by a computerised image analysis system.
Transmission electron microscopy (TEM)
For TEM, mature Sarcocystis macrocysts isolated from pectoral muscles of one mallard were fixed in Karnovsky‘s fixative, postfixed in 1% osmium tetroxide and dehydrated and embedded in Epon. Ultrathin sections were stained with 2% uranyl acetate and lead citrate and examined under the JEOL JEM-100B TEM.
DNA analysis
For a DNA analysis, some cysts were isolated from muscle fibres and placed in 1.5-ml Eppendorf tubes containing 75% ethanol. Genetic characteristics of S. rileyi were evaluated using ITS-1 region, 18S rRNA gene and partial 28S rRNA gene sequences. The investigated 28S rRNA gene fragment contained the most variable D2 and D3 domains of this gene. The genetic markers analysed could be distinguished according to a different evolutionary rate—from the most rapidly evolving ITS-1 to the most conservative 18S rRNA gene. Genomic DNA was extracted from one macrocyst using the Qiagen DNeasy tissue kit. The 18S rRNA gene was amplified using four primer pairs SarAF\SarAR, SarBF\SarBR, SarCF\SarCR and SarDF\SarDR; the 28S rRNA gene fragment was amplified using two primer pairs KL-P1F\KL-P1R and KL-P2F\KL-P2R; the ITS-1 fragment was amplified using P-ITSF\P-ITSR primer pair (Kutkienė et al. 2010). Polymerase chain reactions (PCRs) were carried out in the final 25-μl volume consisting of 2.5 μl 10× PCR buffer, 2.5 μl dNTP (2 mM), 0.2 μM each primer, 1 μl Taq polymerase, 2.5 μl MgCl2 and 0.2 μg template DNA. PCR was performed using “hot start” of 95°C for 7 min, followed by 35 cycles of 94°C for 45 s, 50–59°C depending on the primer pair for 1 min, 72°C for 1.30 min and the final extension at 72°C for 10 min. Annealing temperatures and extension times varied seeking to achieve the highest amount of the amplified product and trying to avoid amplification of non-specific fractions. Amplification products were analysed using 1.7% agarose gel electrophoresis and were purified using the Cyclo-Pure gel extraction kit (Amresco, USA). PCR products were sequenced directly with the ABI Prism 377 automatic DNA sequencer using the same primers as for the PCR reactions. Sequences identity values were determined on the European Molecular Biology Open Software Suite (http://www.ebi.ac.uk/emboss/align/) using the default options. Sequences were aligned using ClustalW algorithm. The beginning and the end of some sequences were truncated to have all the sequences beginning with and ending in the same nucleotide positions. The alignment was then checked manually in order to correct the ambiguously placed nucleotides. Phylogenetic position of S. rileyi was determined using two separate phylogenetic analyses of 18S rRNA and 28S rRNA gene sequences. Species that were involved in one phylogenetic group together with S. rileyi in the primary phylogenetic analysis of the Sarcocystidae family served as an ingroup in further analyses. A total of 18 representatives belonging to the Sarcocystis and Frenkelia genera, which in their turn belong to the Sarcocystinae subfamily, were included in the ingroup. Besnoitia besnoiti, which belongs to the subfamily Toxoplasmatinae and is noted for having the shortest evolutionary distance to the subfamily Sarcocystinae was chosen as the outgroup. Phylogenetic trees were constructed using the Bayesian method and the MrBayes programme, version 3.1.2 (Ronquist and Huelsenbeck 2003). Phylogenetic relationships were assessed using the most complex available model, GTR + I + G evolutionary model, which allows all six possible substitutions to vary with a proportion of invariable sites and a gamma-shaped distribution of rates across the sites. The trees were drawn using TreeView version 1.6.6 (Page 1996).
Results
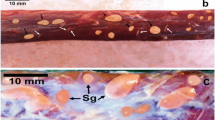
Having examined ten mallard ducks grossly, Sarcocystis macrocysts were found in breast muscles of one duck (Fig. 1). They were yellowish white, in the size of 5.0–7.0 × 2.0 mm and resembled a grain of rice. Using a computerised image analysis system, the cyst wall structure seems to be complicated and reaches up to 5.6 μm. Cystozoites were straight and 15.3–16.8 μm in length (Fig. 2a).
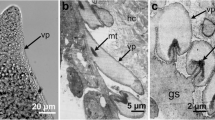
a–e Structure of Sarcocystis rileyi cysts from the breast muscles of the mallard duck (Anas platyrhynchos). a Light micrograph of cystozoites. Fresh preparation. b, c Electron micrographs of the cyst wall. Arrows pointed at the protrusions that differ in size and shape. d High magnification of protrusion. Note microgranules (small arrows) and microfilamentous structures (big arrows). e High magnification of protrusion. The electron dense layer under parasitophorous vacuolar membrane at invaginations in some places is interrupted (arrows). Ground substance (g)
Ultrastructurally, the primary cyst wall consisted of parasitophorous vacuolar membrane, and the electron dense layer under it was highly wavy and formed branched protrusions of a very complicated shape, which differed greatly in size (Fig. 2b–c). The protrusions were up to 4.2 μm high. The electron dense layer was up to 0.08 μm thick and, in some invaginations, interrupted (Fig. 2e). The majority of the protrusions contained filamentous structures and fine granules (Fig. 2d). The ground substance layer (up to 0.7 μm) continued into interior of the cyst as septa and divided it into large compartments filled with cystozoites. Based on data obtained by visual identification and ultrastructural examination of cysts wall, we suggested that examined macrocysts belong to S. rileyi species.
By light microscopy, microcysts were detected in the neck muscles of one duck and these microcysts were identified as belonging to Sarcocystis sp. (cyst type II) whose morphology was presented in earlier publications (Kutkienė and Sruoga 2004; Kutkienė et al. 2008).
ITS-1 (942-bp long), 18S rDNA (1,792-bp long) and 28S rDNA (1,510-bp long) sequences of S. rileyi derived from the macrocyst which was examined ultrastructurally and prepared from the muscle tissue of the same mallard individual were deposited in GenBank (HM185744, HM185742 and HM185743). The comparison of 18S rRNA and 28S rRNA gene sequences showed that S. rileyi had the highest sequence identity values with homological genes of Sarcocystis sp. cyst type III from Anser albifrons, 99.5% and 97.1%, respectively. According to 18S rRNA and 28S rRNA gene sequences, Sarcocystis sp. cyst type II from Anas platyrhynchos was the second Sarcocystis species that genetically was most identical to S. rileyi. The similarity between sequences of S. rileyi and Sarcocystis sp. cyst type II from Anas platyrhynchos accounted for 99.5% and 96.3% within 18S rRNA and 28S rRNA genes, respectively. According to the highly variable ITS-1 region, S. rileyi sequences differed sharply from other Sarcocystis species. The enormous number of gaps in the alignment of Sarcocystis species sequences of ITS-1 region hinders an accurate evaluation of sequences identity. The highest sequences identity within ITS-1 of S. rileyi was detected for S. neurona, S. falcatula and S. dasypi and approximately accounted for 57%. When comparing S. rileyi with other Sarcocystis spp., greater identity in ITS-1 region could be expected if Sarcocystis sp. cyst type III from Anser albifrons and Sarcocystis sp. cyst type II from Anas platyrhynchos sequences were available in the GenBank. The initial phylogenetic analysis of the family Sarcocystidae showed that S. rileyi belonged to a well-supported phylogenetic group, which encompassed Sarcocystis and Frenkelia species whose intermediate or definitive hosts are birds and other Sarcocystis species: S. gallotiae, S. lacerate, S. mucosa, S. muris, S. neurona, S. rodentifelis, S. zamani and Sarcocystis sp. from the shrew. This phylogenetic group is distinct from other phylogenetic groups of the subfamily Sarcocystinae: representatives of one of these groups use snake-rodent life cycle and representatives of the other group has even-toed ungulate as their intermediate host and a canine, feline or unknown predator as their definitive host (Dahlgren and Gjerde 2008b). According to 18S rRNA gene phylogenetic tree, S. rileyi is the most closely related to Sarcocystis sp. cyst type III from Anser albifrons, Sarcocystis sp. cyst type II from Anas platyrhynchos and to S. mucosa (Fig. 3a). In the phylogenetic tree of 28S rRNA gene, S. rileyi is grouped with Sarcocystis sp. cyst type III from Anser albifrons and Sarcocystis sp. cyst type II from Anas platyrhynchos (Fig. 3b).
a–b Bayesian phylogenetic trees based on a the 18S rDNA and b the 28S rDNA. Trees were rooted on Besnoitia besnoiti and scaled according to the branch length. GenBank accession numbers of analysed Sarcocystidae species are in brackets. The numbers in the figure show posterior probability support values
Discussion
Mallard ducks have been investigated most extensively for macroscopically visible sarcocysts in North America. Erickson (1940) indicated that having examined 279 ducks belonging to 18 species, they detected macrocysts in eight individuals, six of which were mallard ducks. According to the data presented by Chabreck (1965), in Louisiana, they were recorded in 27.2% of 250 adult mallard ducks, whereas no sarcocysts were found in 154 immature individuals. No macrocysts were detected in 169 juveniles of mallards from North Dakota either, whereas having examined 307 adult individuals, they were found in 7.82% of cases (Hoppe 1976). According to Fedynich and Pence (1992) in the southern high plains of Texas, 6% prevalence in adults was recorded; while juveniles were not infected. Macrocysts were found only in 3% of adult mallards from western Canada (Drouin and Mahrt 1979). Thus, the data presented by different authors show that prevalence of Sarcocystis macrocysts in mallards depends on the age of the birds and geographic regions. The juveniles did not have macrocysts because it takes approximately 3 months for easily visible sarcocysts to develop.
Infection of mallard ducks with Sarcocystis species, which form macrocysts, has not been thoroughly investigated in Europe. In the period of 1997 and 2008, Sarcocystis spp. were found in 48 (19.7%) individuals out of 244 mallards examined. Having most often examined the neck muscles, one type of macrocysts was detected. Macrocysts were found in five (2.1%) cases and, grossly and by light microscopy, were identified as S. rileyi (Kutkienė and Sruoga 2004). In north-western Poland, Kalisińska et al. (2003) recorded only one case of macrocysts out of 148 (0.7%) mallards investigated. Sarcocysts in mallards and in its domesticated forms were also detected in Bulgaria and Germany (Kalyakin and Zasukhin 1975). In our opinion, to answer to the question about prevalence of macrocysts in mallards in Europe much more detailed investigations are needed, which would be carried out in different regions of the continent examining breast muscles of the birds. Furthermore, the birds should be differentiated according to their age, which, unfortunately, has not been determined in the above-mentioned publications.
Phylogenetic trees constructed from the complete 18S rRNA gene and the partial 28S rRNA gene sequences showed congruous topologies. Discrepancies between these trees occurred due to the fact that 18S rRNA and 28S rRNA gene sequences have been established for a different number of Sarcocystis species. The phylogenetic tree constructed from 28S rRNA gene sequences was more reliable, and the arrangement of branches was based on higher probability values as compared with the phylogenetic tree formed from 18S rRNA gene sequences. The reason for this most likely could be the fact that 28S rRNA gene is more variable than 18S rRNA gene, and therefore it carries a greater amount of valuable phylogenetic information (Butkauskas et al. 2007). Phylogenetic results indicate that S. rileyi is most closely related to two Sarcocystis spp. whose intermediate hosts are from the order Anseriformes and to S. mucosa which exists as macroscopic sarcocysts in the gastrointestinal tract of several marsupial species (Jakes 1998; O’Donoghue et al. 1987). This close affinity with S. mucosa could be related to the fact that S. mucosa, like S. rileyi, forms macroscopic sarcocysts. In the analysed 18S rRNA gene fragment, S. rileyi only insignificantly differed from other related Sarcocystis species. When comparing the analysed 28S rRNA gene and ITS-1 region sequences, S. rileyi differed from the most related Sarcocystis species by 3% and 43%, respectively. The DNA analysis certainly reveals that S. rileyi significantly differs from all other genetically characterised Sarcocystis species.
The ultrastructural investigations into macrocysts from the mallard duck carried out in this work showed that the cyst wall structure corresponds to the type-23 tissue cyst wall, which has been established for macrocysts of the mallard duck from the USA (Dubey et al. 2010) and which resembled S. rileyi from type host shoveler (Dubey et al. 2003). Thus, S. rileyi is found in Europe; however, its definitive host has not been determined yet. What could replace the skunk, which lives in zoos in Europe and hardly takes part in the distribution of these species on this continent? The red fox (Vulpes vulpes) is the main predatory species of mallard ducks because birds account for 35% of its food in spring and autumn (Logminas 1990). Birds (including ducks) constitute main food of racoon dogs (Nyctereutes procyonoides), so participation of latter as definitive host species in the life cycle of S. rileyi is also possible. Other mammalian species (the family of Mustelidae) and birds of prey (of Accipitridae, Falconidae and Laridae families) found in the breeding and wintering grounds of ducks from Europe can also be potential definitive hosts of this species.
References
Butkauskas D, Sruoga A, Kutkienė L, Prakas P (2007) Investigation of the phylogenetic relationships of Sarcocystis spp. from greylag (Anser anser) and white-fronted (Anser albifrons) geese to other cyst forming coccidia using 18S and 28S rRNA gene sequences. Acta Zool Lituanica 17:124–128
Cawthorn RJ, Rainnie D, Wobeser G (1981) Experimental transmission of Sarcocystis sp. (Protozoa: Sarcocystidae) between the shoveler (Anas clypeata) duck and striped skunk (Mephitis mephitis). J Wildl Dis 17:389–394
Chabreck RH (1965) Sarcosporidiosis in ducks in Louisiana. Trans N Am Wildl Conf 30:174–184
Dahlgren SS, Gjerde B (2008a) Sarcocystis in moose (Alces alces): molecular identification and phylogeny of six Sarcocystis species in moose, and a morphological description of three new species. Parasitol Res 103:93–110
Dahlgren SS, Gjerde B (2008b) Sarcocystis in Norwegian roe deer (Capreolus capreolus) molecular and morphological identification of Sarcocystis oviformis n. sp. and Sarcocystis gracilis and their phylogenetic relationships with other Sarcocystis species. Parasitol Res 104:993–1003
Drouin TE, Mahrt JL (1979) The prevalence of Sarcocystis Lankester, 1882, in some bird species in western Canada, with notes on its life cycle. Can J Zool 57:1915–1921
Drouin TE, Mahrt JL (1980) The morphology of cysts of Sarcocystis infecting birds in western Canada. Can J Zool 58:1477–1482
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. CRC, Boca Raton
Dubey JP, Odening K (2001) Toxoplasmosis and related infections. In: Samuel WM, Pybus MJ, Kocan AA (eds) Parasitic diseases of wild mammals. Iowa State University Press, Ames, pp 478–519
Dubey JP, Cawthorn RJ, Speer CA, Wobeser GA (2003) Redescription of the sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae). J Eukaryot Microbiol 50:476–482
Dubey JP, Rosenthal BM, Felix TA (2010) Morphologic and molecular characterization of the sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) from the mallard duck (Anas platyrhynchos). J Parasitol 96:765–770
Erickson AB (1940) Sarcocystis in birds. Auk 57:514–519
Fedynich AM, Pence DB (1992) Sarcocystis in mallards on the southern high plains of Texas. Avian Dis 36:1067–1069
Hoppe DM (1976) Prevalence of macroscopically detectable Sarcocystis in North Dakota ducks. J Wildl Dis 12:27–29
Jakes KA (1998) Sarcocystis mucosa in Bennetts wallabies and pademelons from Tasmania. J Wildl Dis 34:594–599
Kalisińska E, Betlejewska KM, Schmidt M, Goździcka-Józefiak A, Tomczyk G (2003) Protozoal macrocysts in the skeletal muscles of a mallard duck in Poland: the first recorded case. Acta Parasitol 48:1–5
Kalyakin VN, Zasukhin DN (1975) Distribution of Sarcocystis (Protozoa: Sporozoa) in vertebrates. Folia Parasitol 22:289–307
Kutkienė L, Sruoga A (2004) Sarcocystis spp. in birds of the order Anseriformes. Parasitol Res 92:171–172
Kutkienė L, Sruoga A, Butkauskas D (2008) Sarcocystis sp. from the goldeneye (Bucephala clangula) and the mallard (Anas platyrhynchos): cyst morphology and ribosomal DNA analysis. Parasitol Res 102:691–696
Kutkienė L, Prakas P, Sruoga A, Butkauskas D (2009) Sarcocystis in the birds family Corvidae with description of Sarcocystis cornixi sp. nov. from the hooded crow (Corvus cornix). Parasitol Res 104:329–336
Kutkienė L, Prakas P, Sruoga A, Butkauskas D (2010) The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol Res 107:879–888
Logminas V (comp.) (1990) Fauna of Lithuania. Aves 1. Mokslas, Vilnius
Mugridge NB, Morrison DA, Jäkel T, Heckeroth AR, Tenter AM, Johnson AM (2000) Effects of sequence alignment and structural domains of ribosomal DNA on phylogeny reconstruction for the protozoan family Sarcocystidae. Mol Biol Evol 17:1842–1853
O’Donoghue PJ, Obendorf DL, O'Callaghan MG, Moore E, Dixon BR (1987) Sarcocystis mucosa (Blanchard 1885) Labbe 1889 in unadorned rock wallabies (Petrogale assimilis) and Bennett’s wallabies (Macropus rufogriseus). Parasitol Res 73:113–120
Olias P, Olias L, Lierz M, Mehlhorn H, Gruber AD (2010) Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet Parasitol 171:7–14
Page RDM (1996) Treeview: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Wicht RJ (1981) Transmission of Sarcocystis rileyi to the striped skunk (Mephitis mephitis). J Wildl Dis 17:387–388
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kutkienė, L., Prakas, P., Sruoga, A. et al. Identification of Sarcocystis rileyi from the mallard duck (Anas platyrhynchos) in Europe: cyst morphology and results of DNA analysis. Parasitol Res 108, 709–714 (2011). https://doi.org/10.1007/s00436-010-2117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2117-2