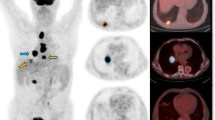
Abstract
Purpose
Metabolic parameters measured by [18F]-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT) are important prognostic factors in several types of cancers. We evaluated the predictive value of tumor metabolic parameters measured by 18F-FDG PET/CT in limited-disease small-cell lung cancer (LD-SCLC).
Methods
This retrospective study included 30 LD-SCLC patients who underwent standard chemotherapy after radiotherapy with 18F-FDG PET/CT. The maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and blood glucose-corrected values were used to evaluate metabolic parameters in primary tumors.
Results
For the median follow-up of 41.1 months, median overall survival (OS) was 75.0 months [95% confidence interval (CI) 20.9–129.1 months], and median progression-free survival (PFS) was 9.5 months (95% CI 6.8–12.1 months). Two-year OS was 78.6%, and PFS was 32.7%. OS analysis indicated that MTV and TLG were significant predictors of OS following standard treatment. High glucose-corrected SUVmax (glu-SUVmax) was related to shorter median PFS. On multivariate analysis, MTV was an independent factor of OS, and glu-SUVmax was significantly related to PFS.
Conclusions
MTV and glu-SUVmax measured on pretreatment 18F-FDG PET/CT were independent prognostic factors for LD-SCLC patients after chemoradiotherapy with curative intent. These metabolic markers need validation in larger prospective studies but may be useful in the clinical care of LD-SCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small-cell lung cancer (SCLC) consists of 15–20% of primary lung cancers (Parkin et al. 2005). The standard treatment for patients with good performance status and limited disease SCLC (LD-SCLC) is concurrent chemoradiotherapy (Pignon et al. 1992). Compared with non-SCLC, SCLC has a shorter doubling time, an elevated growth fraction, and earlier development of extensive metastases, all of which lead to frequent relapse and reduced survival, despite initially favorable responses to treatment (Simon et al. 2004). Presently, tumor stage is the most important prognostic factor of SCLC over other clinical factors [e.g., performance status, weight loss, serum lactate dehydrogenase (LDH)], which is classified LD and extensive disease (ED) (Yip and Harper 2000; Zhu et al. 2011). However, the stage system is insufficient for predicting survival in certain patients (Micke et al. 2002). In addition, the clinical response to treatment for LD-SCLC shows substantial interindividual variation (Jiang et al. 2016). Thus, more discriminative prognostic markers are needed, allowing for better stratification for appropriate therapy and more accurate predictions of treatment outcome and survival.
For SCLC, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT) is the main imaging tool for staging and influences patient management and early assessment of tumor response. 18F-FDG PET/CT results with maximum standardized uptake value (SUVmax) have been suggested as a valuable prognostic gauge (Brink et al. 2004; Azad et al. 2010; Yamamoto et al. 2009; Kamel et al. 2003; Pandit et al. 2003; Onitilo et al. 2008; Lee et al. 2009; Zhu et al. 2011; Park et al. 2014). While volumetric metabolic factors such as metabolic tumor volume (MTV) or total lesion glycolysis (TLG) are investigated for independent prognostic parameters in other kinds of cancer (Lee et al. 2010, 2014; Dibble et al. 2012; Seol et al. 2010), evidence for their clinical value in SCLC patients is limited (Zhu et al. 2011; Oh et al. 2012; Park et al. 2014) Previous studies investigated patients with ED as well as LD and had limitation due to heterogeneous groups with different clinical stages. Furthermore, blood glucose level should be considered for metabolic factor analysis with PET/CT considering that blood glucose decreased FDG uptake by tumor cells through competitive inhibition (Lee et al. 2011). Glucose-corrected SUVmax has been reported to have better accuracy in predicting outcome than SUVmax in other cancers (Lee et al. 2011). However, the significance of glucose-corrected metabolic parameters has not yet been evaluated in SCLC patients.
The objective of this study was to assess the prognostic role of 18F-FDG PET/CT pretreatment metabolic parameters and glucose-corrected values in LD-SCLC patients who underwent concurrent chemoradiotherapy.
Methods
Patients
This was a retrospective study of 83 consecutive SCLC patients who underwent pretreatment 18F-FDG PET/CT at Seoul National University Bundang Hospital (Seongnam-si, Korea) from May 2009 to October 2012. Of these patients, we retrospectively enrolled 30 with limited-stage cancer who met the following inclusion criteria: (1) histologically or cytologically confirmed diagnosis of primary SCLC; (2) sufficient clinical information in the medical chart; and (3) treatment with at least two cycles of chemotherapy. Excluded from analysis were 53 patients with inadequate PET imaging or medical records. The remaining 30 patients were analyzed to assess clinicopathological characteristics, tumor responses, and survival outcomes using a predesigned data-collection format. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1402-238-107).
Treatment and response evaluation
Patients were treated with two cycles of induction chemotherapy, followed by definitive three-dimensional, conformal, involved field radiotherapy and concurrent chemotherapy. The chemotherapy regimen was etoposide plus cisplatin (EP) or irinotecan plus cisplatin (IP). The EP regimen was etoposide 100 mg/m2 (days 1–3) and cisplatin 75 mg/m2 (day 1). The IP regimen was irinotecan 60 mg/m2 (days 1 and 8) and cisplatin 60 mg/m2 (day 1). Cycles of combination chemotherapy were administered at 3-week intervals. Chest irradiation was administered after two cycles of induction chemotherapy. Patients received 1.8 Gy once daily in 30 fractions. Patients with LD-SCLC who showed complete or partial response after chemoradiotherapy were treated with prophylactic cranial irradiation of 25 Gy in 2.5-Gy fractions. Response evaluation was performed with a CT scan every two cycles, according to the guidelines of the Response Evaluation Criteria in Solid Tumor Committee version 1.0 (Therasse et al. 2000).
PET/CT imaging
Patients fasted for at least 6 h before 18F-FDG PEC/CT. Median blood glucose was 98.5 mg/dL (range 85.0–142.0 mg/dL). Approximately 5.18 MBq/kg 18F-FDG was intravenously injected 50 min before imaging. Using a dedicated PET/CT scanner (DVCT, GE Healthcare, Milwaukee, WI, USA) after an initial low-dose CT (120 kVp, tube current modulation), a PET scan was obtained from the skull base to proximal thighs, with an acquisition time of 2.5 min per bed position in three-dimensional mode. PET images were reconstructed with ordered-subset expectation maximization with attenuation correction using vendor-provided software (VUE Point High Definition, GE Healthcare, Milwaukee, WI, USA).
Data analysis and statistical methods
18F-FDG PET-CT images were evaluated by two nuclear medicine physicians using an Advantage Workstation 4.5 (GE Healthcare, USA). Maximum standard uptake value (SUVmax), mean SUV and MTV of PET images were evaluated using volume viewer software. Tumors were measured with a spherical volume of interest (VOI) that included the entire lesion in the axial, sagittal, and coronal planes. Using CT images, 18F-FDG uptake of normal organs such as stomach, intestine, and liver was not counted in the VOI. SUVmax of the VOI was assessed as (decay-corrected activity/tissue volume)/(injected dose/lean body weight). MTV was defined as total tumor volume with SUV 2.5 or greater, and MTV and mean SUV of the VOI were mechanically analyzed. TLG was computed as (mean SUV) × (MTV). SUVmax corrected for blood glucose level (glu-SUVmax) was estimated as (SUVmax) × (blood glucose level)/100. Glucose-corrected TLG (glu-TLG) was also estimated using glucose-corrected SUVmax. Values corresponding to the 75th percentile of SUVmax, MTV, TLG, glu-SUVmax, and glu-TLG were used as arbitrary cutoffs. The 75th percentile values better discriminated patients according to the main clinical outcome endpoints compared to other cutoffs.
Overall survival (OS) was defined as the time interval between the date of 18F-FDG PET/CT and the date of death from any cause, date of last contact, or last date known to be alive. Progression-free survival (PFS) was evaluated from the date of 18F-FDG PET/CT until the date of first recurrence or death. Survival time was calculated using the Kaplan–Meier method and survival differences between groups assessed by log-rank test. Multivariate Cox regression analyses assessed prognostic significance of 18F-FDG PET/CT variables and clinical factors that were significant in univariate analyses. We used both forward stepwise methods and a block entry method (all variables entered together in a single block). The forward stepwise method was chosen because many individual 18F-FDG PET/CT parameters are highly correlated. Stepwise regression solves the problem of multicollinearity because two highly correlated characteristics generally are not both entered in the model (Braun et al. 2011). SPSS 20.0 for Windows software was used for statistical analysis.
Results
Patient characteristics
The clinical characteristics of the 30 patients are described in Table 1. The median age was 65 years (range 37–76 years). Initial chemotherapy regimens were mainly EP (80%). Follow-up data were available through June 2016, and the median follow-up time was 41.1 months [95% confidence interval (CI) 31.7–50.4 months]. Median OS was 75.0 months (95% CI 20.9–129.1 months), and median PFS was 9.5 months (95% CI 6.8–12.1 months). Two-year OS and PFS were 78.6% and 32.7%, respectively.
Tumor response
Tumor response for concurrent chemoradiotherapy was evaluable in 29 patients after treatment, while one patient showed disease progression after induction chemotherapy. The objective tumor response rate for CCRT was 96.5%, with six complete responses and 22 partial responses. Stable disease was observed in one case (3.5%). No significant difference was seen in response to CCRT according to metabolic parameters.
Metabolic parameters for survival
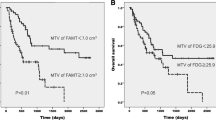
High MTV and TLG were associated with significantly shorter OS. Median OS for MTV < 166.6 mL vs. MTV ≥ 166.6 mL was 75.0 months (95% CI not calculated) vs. 22.2 months (95% CI 7.9–36.7) (P < 0.001). Median OS for TLG < 780.3 g vs. TLG ≥ 780.3 was 75.0 months (95% CI not calculated) vs. 22.3 months (95% CI 5.7–38.8) (P < 0.001) (Fig. 1a, b; Table 2). Median OS for high SUVmax was shorter than for low SUVmax, but the difference was not significant. SUVmax < 10.1 vs. SUVmax ≥10.1 was 75.0 months (95% CI 20.9–129.0) vs. 22.3 months (95% CI 2.6–41.9) (P = 0.066). Median OS for glu-SUVmax and glu-TLG was associated with higher risk of death. Median OS for glu-SUVmax < 10.1 vs. glu-SUVmax ≥10.1 was 75.0 months (95% CI 21.6–128.9) vs. 22.3 months (95% CI 2.5–41.9) (P = 0.047). Median OS for glu-TLG < 856.0 g vs. glu-TLG ≥ 856.0 g was 75.0 months (95% CI not calculated) vs. 22.3 months (95% CI 1.6–42.9) (P < 0.001). No other clinical factors of age, sex, performance status, or tumor response were predictors of OS in univariate analysis. In multivariate analysis for OS, MTV was confirmed as an independent predictor. High MTV patients had higher risk of death than low MTV patients, with an adjusted hazard ratio of 16.7 (95% CI 3.26–85.1, P = 0.001).
For PFS analysis, glu-SUVmax was associated with significantly shorter PFS. Median PFS for glu-SUVmax < 10.1 vs. glu-SUVmax ≥ 10.1 was 19.6 months (95% CI 6.6–36.7) vs. 8.37 months (95% CI 0.83–15.9) (P = 0.011) (Fig. 2). Median PFS for high SUVmax was shorter than for low SUVmax, though the difference was not significant. SUVmax < 10.1 vs. SUVmax ≥ 10.1 was 13.2 months (95% CI 0–28.5) vs. 8.37 months (95% CI 0.84–15.8) (P = 0.06). No other factors of age, sex, performance status, tumor response, MTV, TLG, or glu-TLG were identified as predictors in univariate analysis of PFS. In multivariate analysis of PFS, glu-SUVmax was confirmed as an independent predictor. High glu-SUVmax patients had a higher risk of recurrence/progression than patients with low glu-SUVmax, with an adjusted hazard ratio of 3.38 (95% CI 1.24–9.18, P = 0.017). No significance difference was observed in OS or PFS according to sex, age, performance status, serum albumin, or hemoglobin.
Discussion
This study explored the prognostic value of specific metabolic parameters from pretreatment 18F-FDG PET/CT of patients with LD-SCLC treated with chemoradiotherapy as the primary treatment. Our results showed that MTV of SCLC lesions was a significant prognostic factor of OS, and glu-SUVmax was a significant prognostic factor of PFS. To our knowledge, this is the first study to report the clinical worth of glu-SUVmax for predicting survival of LD-SCLC patients.
Conservatively, tumor volume measured by CT is used to characterize tumors (Zhu et al. 2011). However, CT-based tumor volume does not perfectly represent tumor size or burden, because tumors do not always have a uniform shape and can have necrotic portions with nonviable tissues. Functional imaging, which can obtain metabolic data on malignant tissues, can more precisely reflect tumor burden. SUVmax is used to evaluate outcomes in patients with SCLC (Lee et al. 2009; Pandit et al. 2003; Azad et al. 2010). Although convenient to measure and commonly used, SUVmax has limitations. It gives a single-pixel value representing the most intense 18F-FDG uptake by the tumor and may not be a sufficient surrogate marker of tumor biology (Dibble et al. 2012). It may not reveal the heterogeneous nature of the tumor and is affected by statistical noise and pixel size (Soret et al. 2007). MTV and TLG represent the extent of FDG uptake for the entire tumor and have been proposed as better prognostic parameters of clinical results than SUVmax (Zhu et al. 2011; Dibble et al. 2012). Our study showed that MTV, TLG, glu-SUVmax, and glu-TLG were significantly associated with OS. Only MTV was a significant independent prognostic factor of OS on multivariate analysis. However, analysis of PFS showed that glu-SUVmax was significantly associated with PFS in both univariate and multivariate analyses. This result could be explained by the inhibitory effect of glucose on tumor FDG uptake, which is seen for other cancer types. Langen et al. (1993) reported that FDG uptake in lung cancer decreases in response to elevated plasma glucose. In head and neck cancer patients, decreased FDG uptake is seen after glucose loading (Lindholm et al. 1993). Adding glucose level to metabolic parameters seems to offset the underestimation of FDG uptake, revealing the true tumor FDG uptake (Lee et al. 2006).
An essential question in assessing tumor biology is whether total MTV or the maximally active portion of the tumor is more important for predicting outcome. Our results showed that MTV was strongly associated with OS, probably because this volume-based parameter reflects overall tumor burden. SUVmax was more highly related to PFS, presumably because SUVmax may represent current disease activity. Relationships among pretreatment metabolic parameters, disease progression, and survival may be quite different depending on the intrinsic biological characteristics of tumor cells, treatment modality, and chemotherapeutic agents.
New treatment strategies by MTV and SUVmax are required for subgroups of patients with LD-SCLC, who are at higher risk for death or progression. LD-SCLC patients with high MTV and/or SUVmax relapsed more frequently with distant metastasis and needed more enhanced systemic chemotherapy than patients with low MTV and/or SUVmax. Combinations of chemoradiotherapy and induction chemotherapy using non-cross resistant drugs or chemoradiotherapy and consolidation chemotherapy with novel agents may help these high-risk patients with the LD-SCLC (Han et al. 2005; Ready et al. 2015).
This study had several limitations such as its retrospective nature. In addition, some medical data such as LDH were missing for some patients, although LDH is rarely elevated in LD-SCLC (Osterlind 2000). SUVmax is usually measured with high reproducibility; however, it is vulnerable to image noise (Nahmias and Wahl 2008; Lodge et al. 2012). In addition, the partial-volume effect strongly depends on tumor size (Soret et al. 2007). In patients with tumors smaller than 2.0 cm, partial-volume effects can affect 18F-FDG uptake by tumors, resulting in undervaluing of MTV and TLG. Despite these limitations, this study is noteworthy because the patient population was homogenous, including only patients with LD-SCLC who received chemoradiotherapy as an initial treatment.
In conclusion, MTV and glu-SUVmax measured on pretreatment 18F-FDG PET/CT were independent and significant prognostic factors in LD-SCLC patients after chemoradiotherapy with curative intent. Patients with low MTV or glu-SUVmax had significantly better OS and PFS than patients with high MTV or glu-SUVmax, respectively. These biomarkers need to be validated in larger prospective studies but may be valuable for treatment stratification of LD-SCLC patients.
References
Azad A, Chionh F, Scott AM, Lee ST, Berlangieri SU, White S, Mitchell PL (2010) High impact of 18F-FDG-PET on management and prognostic stratification of newly diagnosed small cell lung cancer. Mol Imaging Biol 12(4):443–451
Braun DP, Gupta D, Staren ED (2011) Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer 11:353
Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, Henke M, Ghanem N, Moser E, Nitzsche EU (2004) Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging 31(12):1614–1620
Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM (2012) 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med 53(5):709–715
Han JY, Cho KH, Lee DH, Kim HY, Kim EA, Lee SY, Lee JS (2005) Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol 23(15):3488–3494
Jiang W, Bi N, Zhang WJ et al (2016) MicroRNA-related polymorphisms in apoptosis pathway genes are predictive of clinical outcome in patients with limited disease small cell lung cancer. Oncotarget 7(16):22632–22638
Kamel EM, Zwahlen D, Wyss MT, Stumpe KD, von Schulthess GK, Steinert HC (2003) Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med 44(12):1911–1917
Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, Feinendegen LE (1993) The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med 34(3):355–359
Lee WW, Chung JH, Jang SJ, Eo JS, Park SY, Sung SW, So Y, Chung JK, Lee MC, Kim SE (2006) Consideration of serum glucose levels during malignant mediastinal lymph node detection in non-small-cell lung cancer by FDG-PET. J Surg Oncol 94(7):607–613
Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, Moon JW, Park IK, Choi HJ, Kim JH (2009) High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res 15(7):2426–2432
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, Ahn MJ, Kim TS, Yi CA, Chung MJ (2010) Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 17(10):2787–2794
Lee SM, Kim TS, Lee JW, Kim SK, Park SJ, Han SS (2011) Improved prognostic value of standardized uptake value corrected for blood glucose level in pancreatic cancer using F-18 FDG PET. Clin Nucl Med 36(5):331–336
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, Lee JD (2014) Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative (1)(8)F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 55(6):898–904
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H (1993) Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 34(1):1–6
Lodge MA, Chaudhry MA, Wahl RL (2012) Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med 53(7):1041–1047
Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, Buhl R (2002) Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 37(3):271–276
Nahmias C, Wahl LM (2008) Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med 49(11):1804–1808
Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, Bom HS (2012) Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 39(6):925–935
Onitilo AA, Engel JM, Demos JM, Mukesh B (2008) Prognostic significance of 18 F-fluorodeoxyglucose—positron emission tomography after treatment in patients with limited stage small cell lung cancer. Clin Med Res 6(2):72–77
Osterlind K (2000) LDH or NSE or LDH and NSE as pretreatment prognostic factors in small cell lung cancer? A commentary. Lung Cancer 30(1):51–53
Pandit N, Gonen M, Krug L, Larson SM (2003) Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 30(1):78–84
Park SB, Choi JY, Moon SH, Yoo J, Kim H, Ahn YC, Ahn MJ, Park K, Kim BT (2014) Prognostic value of volumetric metabolic parameters measured by [18F]fluorodeoxyglucose-positron emission tomography/computed tomography in patients with small cell lung cancer. Cancer Imaging 14:2
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Pignon JP, Arriagada R, Ihde DC et al (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327(23):1618–1624
Ready NE, Pang HH, Gu L et al (2015) Chemotherapy with or without maintenance Sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase II study-CALGB 30504 (Alliance). J Clin Oncol 33(15):1660–1665
Seol YM, Kwon BR, Song MK et al (2010) Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol 49(2):201–208
Simon M, Argiris A, Murren JR (2004) Progress in the therapy of small cell lung cancer. Crit Rev Oncol Hematol 49(2):119–133
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48(6):932–945
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Yamamoto Y, Kameyama R, Murota M, Bandoh S, Ishii T, Nishiyama Y (2009) Early assessment of therapeutic response using FDG PET in small cell lung cancer. Mol Imaging Biol 11(6):467–472
Yip D, Harper PG (2000) Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer 28(3):173–185
Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, Yu J (2011) Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 73(3):332–337
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Author H.C. declares that he/she has no conflict of interest. Author S.J.L. declares that he/she has no conflict of interest. Author J.L. declares that he/she has no conflict of interest. Author J.S.L declares that he/she has no conflict of interest. Author Y.J.K declares that he/she has no conflict of interest. Author W.W.L declares that he/she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The requirement for informed consent was waived in view of the retrospective nature of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, H., Lee, S.J., Lim, J. et al. Prognostic significance of metabolic parameters measured by 18F-FDG PET/CT in limited-stage small-cell lung carcinoma. J Cancer Res Clin Oncol 145, 1361–1367 (2019). https://doi.org/10.1007/s00432-019-02848-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02848-9