Abstract
Purpose
The purpose of this study was to evaluate the impact of [18F]fluorodeoxy-d-glucose positron emission tomography (FDG-PET) on the primary staging of patients with small-cell lung cancer (SCLC).
Methods
FDG-PET was performed in 120 consecutive patients with SCLC during primary staging. In addition, brain examinations with both FDG-PET and cranial magnetic resonance imaging (MRI) or computed tomography (CT) were performed in 91 patients. Results of FDG-PET were compared with those of conventional staging procedures. FDG-PET detected markedly increased FDG uptake in the primary tumours of all 120 patients (sensitivity 100%).
Results
Complete agreement between FDG-PET results and other staging procedures was observed in 75 patients. Differences occurred in 45 patients at 65 sites. In 47 sites the FDG-PET results were proven to be correct, and in ten, incorrect. In the remaining eight sites, the discrepancies could not be clarified. In 14/120 patients, FDG-PET caused a stage migration, correctly upstaging ten patients to extensive disease and downstaging three patients by not confirming metastases of the adrenal glands suspected on the basis of CT. Only 1/120 patients was incorrectly staged by FDG-PET, owing to failure to detect brain metastases. In all cases the stage migration led to a significant change in the treatment protocol. Sensitivity of FDG-PET was significantly superior to that of CT in the detection of extrathoracic lymph node involvement (100% vs 70%, specificity 98% vs 94%) and distant metastases except to the brain (98% vs 83%, specificity 92% vs 79%). However, FDG-PET was significantly less sensitive than cranial MRI/CT in the detection of brain metastases (46% vs 100%, specificity 97% vs 100%).
Conclusion
The introduction of FDG-PET in the diagnostic evaluation of SCLC will improve the staging results and affect patient management, and may reduce the number of tests and invasive procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer mortality in Western society and its prevalence is increasing globally. Approximately 170,000 new cases of lung cancer are diagnosed each year in the USA [1]. About 20% of these tumours are small-cell lung carcinomas (SCLC), which are distinguished from non-small-cell lung carcinomas (NSCLC) by more aggressive biological behaviour. For example, the volume-doubling time of SCLC is about 33 days, which is three times faster than that of most NSCLC [2]. Exact staging of SCLC is important for treatment decisions, since it has been shown that time of diagnosis, staging and performance status have prognostic value [3, 4]. Surgery plays a minor role in the treatment of SCLC [4, 5]. However, most SCLC are chemosensitive, with about 80% of patients showing a major response to therapy. Unfortunately, relapses develop early. When the extent of the disease is limited, radiation is added to chemotherapy. With respect to the therapeutic strategy, TNM classification is usually collapsed into a simple binary classification—limited disease (LD) and extensive disease (ED). LD is restricted to a hemithorax that can be encompassed in a tolerable radiation field, whereas ED indicates distant metastases [6].
Computed tomography (CT) of the thorax and upper abdomen plays an important role in the initial staging of SCLC. Histological confirmation is usually obtained during bronchoscopy or mediastinoscopy. Staging is completed by performing bone scan, abdominal ultrasound, abdominal CT or magnetic resonance imaging (MRI), cranial CT or MRI, and bone marrow biopsy [4, 7, 8].
In the past decade, positron emission tomography (PET) with [18F]fluorodeoxy-d-glucose (FDG) has emerged as an important staging tool for NSCLC. Metabolic imaging with the glucose analogue FDG reflects intracellular glucose metabolism, which is markedly increased in a variety of tumour cells [9]. Numerous studies and meta-analyses have impressively demonstrated that FDG-PET is not only highly accurate but also superior to CT for assessing lymph node involvement and distant metastases in NSCLC [10–14].
Unlike in the case of NSCLC, little is known about the impact of FDG-PET on the diagnostic evaluation of SCLC. Preliminary studies with small patient numbers presumed a potential use of FDG-PET as a staging tool in SCLC [15–21]. Furthermore, it has been shown that FDG-PET improves management of SCLC, influencing both staging and therapy [20, 21]. However, larger studies are needed before FDG-PET can be recommended for routine use in the staging of SCLC [4]. Therefore, the aim of this prospective study with 120 patients with newly detected SCLC was to confirm the clinical impact of FDG-PET on primary staging. The following questions were addressed: (1) Does FDG-PET reveal additional diagnostic information that influences staging and patient management? (2) Does the use of FDG-PET allow simplification of the staging procedure by reducing the number of tests required?
Materials and methods
Patient characteristics
This prospective study was started following approval by the local Ethics Commission. Between 1999 and 2003, 120 consecutive patients (90 males, 30 females, age 60.8±8.9 years) with histologically confirmed SCLC were examined with FDG-PET during primary staging. All patients had granted informed consent.
The tumour stage was classified using a simple system introduced by the Veterans Administration Lung Cancer Study Group (VALSG). In the VALSG system, LD is defined as disease limited to one hemithorax, including mediastinal, contralateral hilar and ipsilateral supraclavicular lymph nodes, while ED represents tumour spread beyond these manifestations [6].
PET protocol
In all patients, whole-body PET imaging was performed after CT imaging, with a mean time interval of 12 days (range of 1–26 days).
The isotope and the radiopharmaceutical ([18F]FDG) were produced and synthesised as previously reported [22]. After the patients had fasted for 12 h, 5 MBq of [18F]FDG per kilogram body weight was injected into a peripheral vein. In eight cases, an elevated fasting plasma glucose level (>6.0 mmol/l) was normalised using fast-acting insulin (1 unit per 0.5 mmol/l increase) before FDG injection. Patients rested during the FDG uptake period.
Static 2D whole-body PET was performed with an ECAT-EXACT 922 tomograph (CTI Siemens, Knoxville, TN). This device simultaneously records 47 planes, which encompass a 16.2-cm field of view. The spatial resolution is about 7.0 mm full-width at half-maximum. The scanner is calibrated for absolute activity concentration (kBq/ml). Static 2D brain scans (performed in 91/120 patients) were started 60 min p.i. (six frames for 5 min each). Beginning 90 min after tracer injection, an emission image and a transmission image (for subsequent photon attenuation correction using an external germanium source) were recorded at each bed position for 8 min and 2 min, respectively. Data were corrected for the dead time of the scanner, decay and photon attenuation. Coronal, sagittal and transaxial images were reconstructed on the basis of an iterative reconstruction algorithm [23, 24] using ordered-subset expectation-maximisation and segmented attenuation correction (final voxel size 4.2×4.2×4.2 mm3).
The images were viewed on hard copy and on a computer workstation (SunSPARK 20; SUN Microsystems, Palo Alto, CA). The latter enabled the use of multiple operator-defined planes. The PET images were read independently by two experienced investigators who were blinded to other data. The physicians interpreted any hot spots as either benign or malignant. Lesions were classified as malignant (a) if there was focally increased tracer uptake that exceeded the normal limits of regional FDG accumulation in the area and (b) if the lesion was located at a typical metastatic site. Images were subsequently compared, and consensus was reached by discussion.
Verification
Conventional staging consisted of patient history, physical findings, bronchoscopy and thoracic and abdominal contrast-enhanced CT scans in all patients. In CT images, lymph nodes up to a diameter of 1 cm were rated as tumour-free and lymph nodes with a diameter >1 cm were rated as malignant. In 91 patients who also underwent PET brain scans, cranial MRI (n=55) or cranial CT (n=36) was carried out. Iliac crest bone marrow biopsy was performed in 84/120 patients; the remaining 36 patients refused the biopsy.
Whole-body planar bone scintigraphy was performed in 76/120 patients 3 h after injection of 700 MBq 99mTc-dicarboxypropane methylene diphosphonate (Schering, Berlin, Germany) using a dual-head camera equipped with high-resolution collimators. When necessary, additional static planar views or single-photon emission computed tomography (SPECT) images were acquired.
If discrepancies between conventional staging tools and PET examination appeared, selective additional examinations were performed (e.g. a negative bone scan but PET suggestive for bone or bone marrow metastasis led to targeted MRI of the affected area or histological proof) or pre-existing image files were re-evaluated. In some cases the clinical course resolved inconsistent findings (e.g. PET-detected tumour viability became evident).
Since not all of the lesions could be histologically proven and the different imaging procedures frequently showed discrepancies, the results were evaluated as follows. First, the results of morphological imaging were assessed by an experienced radiologist, and the results of the clinical diagnostics were analysed by two experienced physicians specialising in either surgery or internal medicine. The radionuclide investigations were assessed by two experienced nuclear medicine specialists, without knowledge of the clinical and morphological imaging data. For further evaluation, a committee consisting of two clinicians and two nuclear medicine specialists achieved a consensual diagnosis for each patient in respect of the extent of disease and the involved tumour sites. This consensus, which served as the reference standard against which the results of the individual procedures were measured, was based on histology in about 20% of all lesions. In general, when discordant lymph node results between the staging examinations did not influence the disease stage, no validation was performed and patients were treated without histological determination of nodal status. When histological results were not available, the consensus was based on the sum of all the available data, including results of follow-up examinations. Moreover, in six patients discrepant findings at eight tumour sites could not be clarified because the patients did not attend follow-up investigations. None of these findings influenced the disease stage. Non-validated results were excluded from statistical analysis. FDG-PET results showing discrepancies vis-à-vis morphological imaging were not considered sufficient grounds for the consensual verdict until they had been confirmed by other examinations. This consensus procedure resulted in a set of data for each patient with respect to primary tumour, lymph node involvement and distant metastases. Sensitivity and specificity were calculated from these data.
Statistical analysis
The degree of inter-observer agreement for FDG-PET studies was quantified with the κ statistic. The McNemar test was performed to compare the sensitivity and specificity of FDG-PET versus CT or MRI [25]. Statistical analysis was performed with SPSS for Windows version 10.0.7 (SPSS, Chicago, IL).
Results
Patient characteristics and stage migration after PET
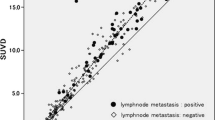
LD was detected in 44/120 patients (37%) and ED in 76/120 patients (63%). Complete agreement between PET results and other staging procedures was observed in 75 patients (Fig. 1). Differences between PET and the other staging procedures occurred in 45 patients at the following 65 sites: lymph nodes (n=17), bone (n=12), lung (n=5), liver (n=8), adrenal gland (n=13), brain (n=9) and spleen (n=1). In 47/65 sites the PET results were proven to be correct, and in 10/65, incorrect. No validation could be obtained in 8/65 discrepant findings that did not influence the disease stage. In 14/120 patients, PET caused a stage migration, with correct upstaging of 10/120 patients to ED (Table 1). As a result, these patients were treated with chemotherapy alone instead of combined radio-chemotherapy. Furthermore, PET correctly downstaged 3/120 patients due to exclusion of CT-diagnosed metastases of the adrenal glands. As they had limited stage disease, these three patients received radio-chemotherapy. Failure of PET to detect brain involvement led to incorrect classification as LD instead of ED in one patient without further distant metastases, which also affected the treatment protocol.
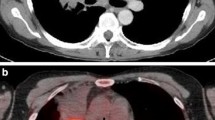
Concordant FDG-PET and CT findings in a 52-year-old woman with centrally located SCLC and a pretracheal lymph node metastasis. a FDG-PET demonstrates high FDG uptake in a large lesion in the right lung and right pulmonary hilus. Furthermore, FDG-PET shows a pretracheal hot spot on the right side. Physiological FDG uptake in the urinary tract is also apparent. b A representative axial CT scan confirms the pretracheal lymph node metastasis and c shows that the tumour mass is infiltrating the mediastinum and surrounding the trachea and oesophagus
Inter-observer agreement
With respect to the detection of pathological FDG uptake on FDG-PET images, the degree of inter-observer agreement (κ) was 0.94 (95% confidence interval 0.87–1.00).
Detection of primary thoracic tumours and lung metastases
PET showed markedly increased glucose metabolism in the primary tumours of all 120 patients (sensitivity 100%). Furthermore, additional pulmonary lesions could be observed in 28 patients. CT demonstrated additional lung metastases in only 24 patients. Comparing the two methods regarding lung metastases, differences that had no influence on the staging were observed in five patients (4× only PET true positive, 1× only CT true positive). In one of these discrepant cases, PET incorrectly located a hypermetabolic lesion to the lung which could later be proven to be a lymph node metastasis in the hilus.
Detection of lymph node metastases
Thoracic CT, abdominal CT and FDG-PET were performed in all 120 patients. CT showed enlarged lymph nodes that fulfilled the criteria of LD in 94/120 patients. On PET scans, lymph node metastases staged as LD could be detected in only 48/120 patients. In 52 PET studies, extensive tumour uptake precluded differentiation between the primary tumour and nearby lymph node metastases, i.e. in the mediastinum. In general, when discordant lymph node results between the staging examinations did not influence the disease stage, no validation was performed and patients were treated without histological determination of nodal status.
Extrathoracic lymph node metastases fulfilling the criteria of ED were detected in 53/120 patients. FDG-PET demonstrated malignant nodes in all 53 patients. CT identified only 37 affected patients and was false positive in four cases. The single false positive PET finding was caused by a reactive node. In two cases with discordant results between FDG-PET and CT, no validation could be obtained. Table 2 shows the statistical analysis of the remaining 118 patients. Evaluation by McNemar test showed PET to be significantly more sensitive than CT in the detection of lymph node metastases fulfilling the criteria of ED (p<0.01). The difference in specificity was not significant.
Detection of distant metastases
In a site-based analysis, FDG-PET was revealed to be significantly more sensitive and specific than the sum of the conventional staging procedures in the detection of distant metastases, excluding brain metastases (Table 2). The statistics included 70 patients with validated results, who were all investigated by FDG-PET, thoracic and abdominal CT, bone scintigraphy, and iliac crest biopsy.
Forty-three of the 120 patients had bone or bone marrow metastases at the time of primary staging, and these metastases could be demonstrated in all 43 patients by FDG-PET. However, PET was false positive in one patient with active myositis ossificans.
Bone marrow biopsy was performed in 84 patients, of whom 23 were found to have bone or bone marrow metastases. Biopsy identified bone marrow involvement in 14 of these 23 patients. There were no false positive biopsy results (sensitivity of bone marrow biopsy 61%, specificity 100%).
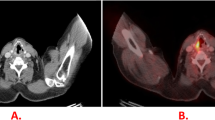
Skeletal scintigraphy was performed in 76 patients, of whom 23 suffered from bone metastases. Skeletal scintigraphy demonstrated bone metastases in 14/23 (61%) patients and missed 9/23 (Fig. 2). In two cases that were later proven by MRI to be degenerative disc disease, skeletal scintigraphy yielded false positive results (sensitivity of skeletal scintigraphy 61%, specificity 96%).
A 69-year-old male with SCLC and multiple metastases. a Coronal FDG-PET images show the primary tumour in the right lung and mediastinum, and metastases in the liver and right adrenal gland, the thoracic and lumbar spine, ribs, upper limbs, femora and pelvis. b Corresponding whole-body planar scintigraphy is unsuspicious except for slight inhomogeneous accumulation in the medial thoracic spine. The images confirm that FDG-PET is clearly superior to bone scintigraphy in the detection of bone marrow involvement
Brain examinations with both FDG-PET and cranial MRI/CT (55 MRI, 36 CT) were performed in 91 patients. Brain metastases were detected in 13/91 patients by MRI or CT (Table 2). PET detected brain metastases in only 6/13 patients. In one patient, the false negative PET scan caused incorrect staging as LD owing to the absence of other distant metastases. Furthermore, two suspicious spots on PET scans classified as brain metastases could not be proven by CT or MRI and were adjudged false positive. Neither of these false positive PET brain scans influenced the disease stage. Cranial MRI/CT were significantly more sensitive than FDG-PET in the detection of brain metastases (p<0.001, Table 2). The difference in specificity was not significant. Liver metastases were found in 26 patients; PET identified 25 of these cases, and CT only 23. One false positive FDG-PET scan and two false positive CT scans were obtained with respect to liver metastases. The latter findings were caused by haemangioma.
Twenty-four patients suffered from adrenal gland metastases and all of these cases could be detected by FDG-PET. CT missed nine non-enlarged but malignant adrenals and yielded four false positive adrenal findings. In three of these cases, exclusion of adrenal gland metastases by FDG-PET was decisive for staging. Spleen metastases occurred in 4/120 patients; FDG-PET demonstrated all four spleen metastases, but CT detected only three.
Discussion
This prospective study investigated the performance of FDG-PET in the primary staging of 120 consecutive patients with newly detected SCLC. The results demonstrated a very high level of agreement between FDG-PET alone and a battery of staging procedures. Even more impressively, FDG-PET improved diagnosis by detecting more lymph node and distant metastases, which resulted in correct stage migration in 11% of the patients when compared with the sum of the established staging procedures. Only 1/120 patients was incorrectly staged by PET, owing to failure to detect brain metastases in a patient without further cancer spread. The stage migration led to significant changes in the treatment protocol of all affected patients. These encouraging results confirm observations from pilot studies on SCLC with small patient numbers [15–21] and are comparable to reports from numerous studies demonstrating the value of FDG-PET in NSCLC [10–14].
A basic limitation of this study was that only about 20% of the lesions could be verified histologically, which would have been the real reference standard. This weakness was unavoidable because the institutional review board found it unjustified to obtain a biopsy for each individual lesion in each patient. However, histological proof was obtained in all stage-relevant cases with discordant results. Other discrepancies between PET results and established staging procedures were clarified by additional or follow-up examinations. Thus, while bias in our study data was not completely eliminated, it was markedly reduced.
In addition to the improvement in staging results, FDG-PET may permit a reduction in the number of staging procedures applied for stage definition of SCLC and thereby save time in patient management. But how can FDG-PET be implemented in a diagnostic strategy in SCLC?
The central role of thoracic CT in the diagnostic assessment of lung cancer appears unquestionable. Most lung carcinomas are discovered as solitary pulmonary nodules by CT or chest X-ray. CT not only detects pulmonary lesions with a higher spatial resolution than PET but also provides important anatomical information about the local tumour extent and tumoural invasion of the chest wall, vertebra or mediastinal structures. In addition, CT scans are helpful for guiding biopsy when histological clarification is needed. However, the present study also indicates that FDG-PET is more accurate than CT in the staging of extrathoracic lymph node metastases, which affects the disease stage and leads to changes in the therapeutic protocol. Comparable observations have been reported in the staging of NSCLC, where it has been demonstrated that FDG-PET is superior to CT for the detection of lymph node metastases of stage N2 but not stage N1 [12–14, 26]. With the exception of nodes located directly adjacent to the primary tumour, the strength of PET in depicting the metabolic tumour activity is obviously advantageous compared with the size criterion used in CT and may improve the staging results not only in lymph node but also in distant metastases. Comparable to the high impact of FDG-PET on NSCLC, the present study revealed FDG-PET to be superior to CT in detecting adrenal gland and liver metastases [27–29].
Also, not unexpectedly, FDG-PET proved superior to conventional bone scintigraphy and bone marrow biopsy in identifying sites of bone or bone marrow metastasis. It might be assumed that the initial bone marrow involvement caused by haematogenous spread of tumour cells cannot be detected by bone scintigraphy. An inadequately representative site for aspiration is a common source of false negative results in bone marrow biopsy. The high sensitivity and specificity of FDG-PET in the detection of skeletal metastases are well known from studies in NSCLC and breast cancer [30, 31].
The performance of FDG-PET in the diagnosis of brain metastases was disappointing. PET detected brain metastases in less than 50% of the affected patients. Furthermore, false positive brain metastases were described in two patients. Problems of FDG-PET in the detection of brain metastases have previously been reported in NSCLC [32] and, in a small patient population, also in SCLC [21]. Reasons for false positive PET scans might be the high normal cortical FDG uptake and limited spatial resolution of the scanner. Therefore, cranial MRI cannot be replaced by FDG-PET. However, it has been shown that amino acids are superior to FDG in the detection of brain tumours [33]. To our knowledge, data on PET imaging with amino acids in SCLC are still lacking.
The current results suggest that with the introduction of FDG-PET in the diagnostic evaluation of SCLC, the routine use of abdominal CT, bone scintigraphy and bone marrow biopsy might be abandoned in the future. A strategy of choice could be a combination of thoracic CT, cranial MRI and whole-body FDG-PET.
Conclusion
FDG-PET improves the accuracy of SCLC staging, particularly regarding the involvement of extrathoracic lymph nodes, bone marrow and adrenal glands, and affects treatment decisions. It may reduce the number of tests and invasive procedures and save time in the management of SCLC. Future studies will investigate the influence of FDG-PET staging on the clinical outcome of SCLC patients.
References
Jemal A, Taylor Murray AS, Ghafoor A, Ward E, Thun MJ. Cancer statistics. CA Cancer J Clin 2003;53:5–26.
Payne D, Naruke T. Lung cancer. In: Pollock RE, ed. Manual of clinical oncology, 7th edn. New York: Wiley; 1999:385–405.
Pasemans M, Sculier JP, Lecombe J, et al. Prognostic factors for patients with small cell lung carcinoma. Cancer 2000;89:523–33.
Simon GR, Wagner H. Small cell lung cancer. Chest 2003;123(Suppl):259S–271S.
Szczesny TJ, Szczesny A, Shepherd FA, et al. Surgical treatment of small cell lung cancer. Semin Oncol 2003;30:47–56.
Mountain CF. Revision in the international system for staging lung cancer. Chest 1997;111:1710–7.
Richardson GE, Daniel CI. Staging of small cell lung cancer. In: Carney DN, ed. Lung cancer. London: Arnold; 1995:114–21.
Darling GE. Staging of the patient with small cell lung cancer. Chest Surg Clin North Am 1997;7:81–94.
Warburg O, Posener K, Negelein E VIII. The metabolism of cancer cells. Biochem Zool 1924;152:129–69.
Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. J Am Med Assoc 2001;285:914–24.
Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s—meta-analytic comparison of PET and CT. Radiology 1999;213:530–6.
Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology 1999;212:803–9.
Pieterman RM, Van Putten JWG, Meuzelaar JJ, et al. Preoperative staging of non-small cell lung cancer with positron emission tomography. N Engl J Med 2000;393:254–61.
Ahuja V, Coleman RE, Herndon J, et al. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer 1998;83:918–24.
Schumacher T, Brink I, Mix M, et al. FDG-PET imaging for the staging and follow-up of small cell lung cancer. Eur J Nucl Med 2001;28:483–8.
Pandit N, Gonen M, Krug L, et al. Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 2003;30:78–84.
Zhao DS, Valdivia AY, Y Li, Blaufox MD. 18F-fluorodeoxyglucose positron emission tomography in small cell lung cancer. Semin Nucl Med 2002;32:272–5.
Hauber HP, Bohuslavizki KH, Lund CH, et al. Positron emission tomography in the staging of small cell lung cancer—a preliminary study. Chest 2001;119:950–4.
Chin R, McCain TW, Miller AA, et al. Whole body FDG-PET for the evaluation and staging of small cell lung cancer: a preliminary study. Lung Cancer 2002;37:1–6.
Shen YY, Shiau YC, Wang JJ, et al. Whole-body 18F-2-deoxyglucose positron emission tomography in primary staging small cell lung cancer. Anticancer Res 2002;22:1257–64.
Kamel EM, Zwahlen D, Wyss MT, Stumpe K, Schulthess GK, Steinert HC. Whole-body 18F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med 2003;44:1911–7.
Hamacher K, Coenen HH, Stöcklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-d-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 1986;27:235–8.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1982;13:601–9.
Mix M, Nitzsche EU. PISAC. A post-injection method for segmented attenuation correction in whole body PET. J Nucl Med 1999;40:297P [abstract].
Weiss C. Basiswissen Medizinische Statistik, 2nd edn. Berlin Heidelberg New York: Springer; 2001:249–51.
Imdahl A, Jenkner S, Brink I, et al. Validation of FDG positron emission tomography for differentiation of unknown pulmonary lesions. Eur J Cardiothorac Surg 2001;20:324–9.
Boland GW, Goldberg MA, Lee MJ, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-d-glucose. Radiology 1995;194:131–4.
Erasmus JJ, Patz EF Jr, McAdams HP, et al. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. Am J Roentgenol 1997;168:1357–60.
Schumacher T, Brink I, Moser E, et al. Imaging of an adrenal cortex carcinoma and its metastasis with FDG-PET. Nuklearmedizin 1999;38:124–6.
Bury T, Barreto A, Daenen F, et al. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med 1998;25:1244–7.
Cook GJ, Fogelman I. The role of positron emission tomography in the management of bone metastases. Cancer 2000;88:2927–33.
Coleman RE. PET in lung cancer staging. Q J Nucl Med 2001;45:231–4.
Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–45.
Acknowledgements
The authors thank the “German Cancer Foundation” for their financial support of this study (#70-2594-Schu I). Furthermore, we are indebted to Claudia Santini-Böttcher and Harald Dietsche for their technical assistance and to Ursula Sahm, PhD, Kenneth Stålmo and Bernd Morasch for producing the radioisotope and radiopharmaceutical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brink, I., Schumacher, T., Mix, M. et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging 31, 1614–1620 (2004). https://doi.org/10.1007/s00259-004-1606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1606-x