Abstract
Purpose
We investigated whether the whole-body metabolic tumour volume (WBMTV) measured by 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) can improve the prediction of prognosis in patients with small cell lung cancer (SCLC).
Methods
We reviewed 106 consecutive patients (mean age 67 years, range 42–89 years, limited stage 45 patients, extensive stage 61 patients) with pathologically proven SCLC who underwent pretreatment FDG PET/CT. WBMTV and maximum standardized uptake value (SUVmax) were measured in all malignant lesions. The Cox proportional hazards model was used with age, sex, performance status, lactate dehydrogenase (LDH), treatment, stage, SUVmax and WBMTV to predict overall survival (OS) and progression-free survival (PFS). Subgroup analysis was performed using WBMTV combined with conventional staging and tumour node metastasis (TNM) staging.
Results
The uni- and multivariate analyses showed that both stage and WBMTV were independent prognostic factors for death and progression. Patients with high WBMTV were associated with poor prognosis compared with patients with low WBMTV [hazard ratio = 2.11 (95% confidence interval 1.31–3.39) for death (p = 0.002) and 1.80 (95% confidence interval 1.16–2.80) for progression (p = 0.009)]. Incorporation of conventional staging and WBMTV could classify four subgroups with different prognoses (log-rank test, p < 0.001). Incorporation of TNM staging and WBMTV could classify six subgroups with different prognoses (log-rank test, p < 0.001).
Conclusion
WBMTV is an independent predictor for progression and death in patients with SCLC. Incorporation of WBMTV with TNM staging can provide a more detailed prediction of prognosis than WBMTV with conventional staging as well as tumour staging alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC), accounting for 15–20% of all new cases of lung cancer, is a rapidly disseminating tumour with poor prognosis. Patients with SCLC are staged according to a two-stage system, which was developed by the Veterans Administration Lung Cancer Study Group, as having limited disease (LD) and extensive disease (ED) [1]. LD is defined as disease confined to one hemithorax, which can be encompassed in a tolerable radiation field, whereas ED is defined as disease extending outside the thorax or the existence of malignant pleural effusion [1, 2]. Although suitability for concurrent radiation therapy is a strong prognostic indicator for SCLC, it gives limited prognostic information for some patients. According to the International Association for the Study of Lung Cancer (IASLC) which analysed more than 12,000 patients with SCLC, including 349 cases treated by surgery [2], the tumour node metastasis (TNM) staging system seems to be of relevance for SCLC, and thus it became the standard for all cases of SCLC, which prompted the use of LD and ED staging to be discouraged in the future.
Positron emission tomography/computed tomography (PET/CT) using 2-deoxy-2-[18F]fluoro-D-glucose (FDG) has already been successfully applied in oncological practice. Most FDG PET/CT studies in SCLC have been focused on its usefulness in accurate staging and impact on the management of SCLC [3–7]. Metabolic tumour volume (MTV), defined as the volume of tumour tissues with increased FDG uptake, is a recently investigated index in FDG PET/CT. Because it represents the extent of FDG uptake by tumour tissues beyond the intensity of FDG uptake, it has been suggested as an independent prognostic indicator of clinical outcomes in certain cancers [8–10]. Several very recent studies have validated the use of whole-body MTV (WBMTV), which represents systemic tumour burden encompassing the primary tumour, regional lymph node and all distant metastases, as an independent prognostic marker [11, 12]. Zhu et al. demonstrated that MTV measured in the whole body can identify subgroups of patients at a high risk of progression or death in both LD and ED SCLC [11]. However, no report has validated the prognostic value of WBMTV combined with conventional and TNM staging in patients with SCLC so far.
Therefore, we aimed to investigate whether the WBMTV incorporated with conventional and TNM staging can improve the prediction of prognosis in patients with SCLC.
Materials and methods
Patients
From January 2004 to December 2010, consecutive patients with pathologically proven SCLC who underwent pretreatment FDG PET/CT were enrolled. Routine staging included history and physical examination, complete blood cell counts and chemistry panel, CT of chest and upper abdomen, brain MRI and FDG PET/CT. Patients with disease confined to one hemithorax and with contralateral hilar and ipsilateral supraclavicular lymph node involvements were defined as LD [3]. Patients with contralateral supraclavicular involvement were also defined as LD only if it was encompassed in a tolerable radiation field [13, 14]. TNM classification of each patient for non-small cell lung cancer (NSCLC) based on the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual was also recorded [15]. All staging workups were completed before initiation of therapy. Patients with LD were treated with combined concurrent chemoradiotherapy and patients with ED were treated with chemotherapy only. The chemotherapy regimen consisted of platinum (cisplatin or carboplatin) with either etoposide or irinotecan administered every 3 weeks for six cycles. Chest irradiation was initiated on day 1 of the second cycle of chemotherapy with 1.8–2.0 Gy daily in 30 fractions. Patients showing complete remission after initial therapy received prophylactic cranial irradiation which consisted of 25 Gy in 10 fractions. The standard response evaluation consisted of chest X-ray prior to each cycle and CT scan every two cycles of chemotherapy. Follow-up FDG PET scan was performed 3 weeks after the last cycle of chemotherapy and when disease progression or recurrence was suspected by standard examinations. All patients were followed up for at least 9 months after diagnosis or until death. The local Ethics Committee approved the study and all enrolled patients gave written informed consent for PET/CT study.
Imaging acquisitions
PET/CT studies were performed using combined PET/CT scanners (Discovery ST System, GE Medical Systems, Milwaukee, WI, USA). All patients fasted for at least 6 h prior to the intravenous administration of FDG. Their blood glucose levels were measured before the injection of FDG; if the level was over 8.3 mmol/l, then PET/CT was deferred. No oral or intravenous contrast material was administered. Image acquisitions for torso scanning were started at approximately 1 h after the injection of 7.4 MBq FDG per kilogram of body weight. CT images were acquired from the skull base to the upper thigh using parameters with a peak voltage of 120 kV, a tube current of automated from 10 to 130 mA, a rotation time of 0.7 s, a field of view (FOV) of 50 cm, a scan length of 40–50 s and a slice thickness of 3.75 mm. Immediately following the CT acquisition, the PET data were acquired in the same anatomical locations with 15.7 cm axial FOV acquired in 2-D mode with 150 s/bed position. The CT data were used for attenuation correction and the images were reconstructed using a conventional iterative ordered subsets expectation maximization (OSEM) algorithm.
Image analysis
Image display and analysis was performed using an Advantage Workstation 4.4 (GE Medical Systems, Milwaukee, WI, USA) providing multiplanar reformatted images. Maximum standardized uptake values (SUVmax) based on body weight and WBMTV were determined by the attenuation-corrected PET data using volume viewer software as follows: First, a rectangular parallelepiped-shaped volume of interest (VOI) fully encasing the primary tumour, regional lymph node and all distant metastases in the axial, coronal and sagittal PET/CT images was drawn. Then, the boundaries of voxels presenting SUV intensity exceeding 3.0 were automatically produced. Second, normal organs such as the heart, stomach, liver, intestines, kidney, ureter and bladder were manually subtracted from the product of the previous step. Third, false-positive lesions such as inflammation or other benign FDG-avid lesions based on histopathological reports, other imaging modalities, follow-up PET/CT and response to therapy were subtracted. Finally, SUVmax and MTV of whole-body tumour burden produced by previous steps were recorded in each patient.
Statistical analysis
Statistical analysis was performed using SPSS 18 for Windows (SPSS Inc., Chicago, IL, USA).
For the detailed classification for prognosis, median values of SUVmax or WBMTV in each stage were used [16]. Correlation between WBMTV and clinical/haematological parameters was assessed by the χ2 test. Survival time was derived from the date of PET/CT scan to the date of death/recurrence or last follow-up. Cox regression analysis was used to develop the univariate and multivariate models describing the association of the independent variables with overall survival (OS) and progression-free survival (PFS). Independent variables analysed included sex, age, smoking, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD), Eastern Cooperative Oncology Group (ECOG) performance status, lactate dehydrogenase (LDH), initial chemotherapy regimen, number of lines of chemotherapy, stage, SUVmax and WBMTV. OS and PFS curves were produced using Kaplan-Meier methods and survival difference between groups was assessed by the log-rank test. A value of p < 0.05 was considered statistically significant. The 95% confidence interval (CI) was determined for each parameter.
Results
Patient characteristics
The patient characteristics in this study are outlined in Table 1. A total of 106 eligible patients (89 men and 17 women, mean age 67 years, range 42–89 years) consisting of 45 (42%) patients with LD and 61 (58%) with ED were enrolled in this study. TNM classification was IA in 2 patients, IB in 2, IIA in 4, IIB in 3, IIIA in 10, IIIB in 26 and IV in 59 patients. The ED group comprised 59 patients with stage IV and 2 patients with contralateral supraclavicular involvement (stage IIIB) beyond the radiation field. The median WBMTV was 127 cm3 (range 5–1,355 cm3) and the median SUVmax was 10.4 (range 5.5–52.6). The patients with higher WBMTV (≥ 127 cm3) were more likely to have extensive disease (p = 0.002), higher TNM staging (p < 0.001) and higher SUVmax (≥ 10.4, p = 0.020) compared with the patients that had low WBMTV (Table 1).
Survival analysis
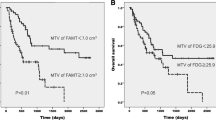
At the time of analysis, 80 (75%) patients had died and 26 (25%) patients were still alive. The median follow-up for the surviving patients was 22.1 months, with a range of 11.5–67.1 months. The median OS and PFS were 12.5 and 6.6 months, respectively (range 0.5–67.1 months, both). The median survival of patients with low WBMTV (< 127 cm3) was significantly longer than that of patients with high WBMTV (≥ 127 cm3) [20.9 vs 8.7 months for death (p < 0.001) and 9.1 vs 5.4 months for progression (p < 0.001)] (Fig. 1a, b), whereas the median survival between the patients with low SUVmax (< 10.4) and high SUVmax (≥ 10.4) was not significantly different [13.8 vs 13.3 months for death (p = 0.315) and 7.3 vs 6.5 months for progression (p = 0.791)] (Fig. 1c, d). The patients with LD showed significantly longer survival compared with the patients with ED [22.2 vs 8.7 months for death (p < 0.001) and 11.6 vs 5.4 months for progression (p < 0.001)] (Fig. 2a, b). The patients with early TNM staging also showed significantly longer survival compared with the patients with advanced TNM staging (Fig. 2c, d). TNM staging could classify the entire cohort into three subgroups with different prognoses.
Following univariate analysis, high LDH (≥ 472 U/l), ED and high WBMTV (≥ 127 cm3) were significant predictors for poor OS, and two or more lines of chemotherapy, ED and high WBMTV (≥ 127 cm3) were significant predictors for poor PFS (Table 2). On multivariate analysis, only ED [hazard ratio (HR) 3.43 for death (p < 0.001) and 3.75 for progression (p < 0.001)] and high WBMTV (≥ 127 cm3) [HR 2.11 for death (p = 0.002) and 1.80 (p = 0.009) for progression] were significant predictors for poor outcome. High SUVmax (≥ 10.4) was not a significant factor for poor prognosis [HR 1.19 for death (p = 0.462) and 0.96 for progression (p = 0.848)] (Table 2).
Conventional staging with WBMTV
Incorporation of conventional staging and WBMTV could classify the entire cohort into four groups with different prognoses (p < 0.001, Fig. 3). The median WBMTV was 72 cm3 in patients with LD and 230 cm3 in patients with ED. The number of deaths was 8/22 (36%), 16/23 (70%), 26/30 (87%) and 30/31 (97%) with OS of 38.2, 20.9, 14.8 and 8.0 months in LD with low WBMTV (< 72 cm3), LD with high WBMTV (≥ 72 cm3), ED with low WBMTV (< 230 cm3) and ED with high WBMTV (≥ 230 cm3), respectively (p < 0.001, Table 3). These results were also replicated in the PFS analysis (p < 0.001).
TNM staging with WBMTV
Incorporation of TNM staging and WBMTV could classify the entire cohort into six subgroups with different prognoses (p < 0.001, Fig. 4). The median WBMTV was 49 cm3 in patients with stage I–II, 95 cm3 in patients with stage III and 230 cm3 in patients with stage IV cancer. The number of deaths was 1/5 (20%), 2/6 (33%), 9/18 (50%), 14/18 (78%), 25/29 (86%) and 29/30 (97%) with median OS of 32.3 (75% of survival), 21.1 (75% of survival), 22.1, 15.8, 11.7 and 8.0 months in stage I–II with low WBMTV (< 49 cm3), stage I–II with high WBMTV (≥ 49 cm3), stage III with low WBMTV (< 95 cm3), stage III with high WBMTV (≥ 95 cm3), stage IV with low WBMTV (< 230 cm3) and stage IV with high WBMTV (≥ 230 cm3), respectively (p < 0.001, Table 4). These results were also replicated in the PFS analysis (p < 0.001).
Discussion
In the present study, we demonstrate that the WBMTV, which is a systemic tumour burden encompassing the primary tumour, regional lymph node and all distant metastases, is a strong independent prognostic factor for death and progression after controlling for conventional staging, LDH, ECOG performance status and SUVmax in patients with SCLC. Incorporation of WBMTV with TNM staging can provide a more detailed prediction of prognosis than WBMTV with conventional staging as well as tumour staging alone.
The IASLC has established a database containing staging, treatment and follow-up data for more than 12,000 patients with SCLC, including 349 cases treated by surgery [2]. They proposed that the TNM staging system appears to be of relevance for SCLC, and thus it became the standard for all cases of SCLC and that the use of LD and ED staging be discouraged in the future. In accordance with these results, we identified that TNM classification could classify three subgroups with different prognoses in the entire cohort, although a limited number of patients with early TNM stage (I and II) were included (Fig. 2). A limited number of investigators have validated the use of more stratified classification combining metabolic parameters and conventional staging for SCLC so far [11, 16]. A recent study performed by Lee et al. showed that incorporation of SUVmax into a conventional staging system could identify three subgroups with different prognoses in all patients with SCLC [16]. More recently, Zhu et al. demonstrated that MTV combined with conventional staging could more appropriately reflect the tumour burden and predict survival of SCLC patients. Our results are consistent with those of Zhu et al. [11]. However, we also found that incorporation of WBMTV with TNM staging better predicts outcome than WBMTV combined with conventional staging.
Although suitability for concurrent radiation therapy is a strong prognostic indicator for SCLC, it gives limited prognostic information for some patients. It has been suggested that TNM staging can identify subgroups of patients with a distinct prognosis from within the broad definition of LD [2, 14]. The beneficial effect of radiotherapy for intrathoracic tumour and cranial metastatic sites for patients with ED has also been postulated by several studies [17, 18]. The Radiation Therapy Oncology Group (RTOG) 0937 phase II trial proposes to determine the role of consolidation radiotherapy for extracranial metastatic sites alongside prophylactic cranial irradiation (PCI) after a response to systemic chemotherapy (Gore, personal communication, http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0937). Because a conventional staging system classifies a wide range of patients into only two categories, more discriminative prediction of prognosis for appropriate therapy would be needed for SCLC. Patients with lesser WBMTV can be good candidates for additional radiotherapy to thoracic, cranial and potentially extracranial metastatic sites for better treatment response and longer survival. Our results may mean that FDG PET/CT scans have an important role in helping to reach treatment decisions by providing more detailed prognostic information in the initial staging for SCLC.
MTV is defined as the volume of tumour tissues with increased FDG uptake which is a recently investigated index in FDG PET. Because it represents the extent of FDG uptake by tumour tissues beyond the intensity of FDG uptake (SUVmax), it has been suggested for a better prognostic indicator than SUVmax for clinical outcomes in certain malignancies: pharyngeal cancer [8], cervical cancer [19], oesophageal cancer [9] and malignant pleural mesothelioma [20]. However, almost all previous studies treating MTV measured the tumour volume only in primary mass or regional lymph nodes. Very recently, Liao et al. investigated the prognostic value of whole-body metabolic tumour burden on FDG PET/CT in non-surgical patients with NSCLC [12]. They concluded that the baseline whole-body metabolic tumour burden was a prognostic measure independent of clinical stage and may be used to further stratify non-surgical patients with NSCLC. They also concluded that MTV is a better predictor of survival than SUVmax. In accordance with previous studies, WBMTV also showed better prognostic value than SUVmax in patients with SCLC. Tumour stage is known to be the most important prognostic factor of SCLC. ECOG performance status, age, gender and LDH are also known to correlate with the prognosis for SCLC [21–23]. Along with WBMTV, tumour staging (both conventional and TNM staging) and LDH level were also significant prognostic factors for poor prognosis in this study. High WBMTV was also associated with high LDH and high tumour staging. However, multivariate analysis revealed that WBMTV is a significant independent prognostic factor regardless of tumour staging, LDH, ECOG performance status and SUVmax.
Metabolic imaging using FDG facilitates the quantification of the volume of all malignant lesions in cancer patients. Metabolic tumour delineation is conventionally obtained applying a threshold of 40% of SUVmax or using a fixed SUV threshold of 2.5 [24]. However, no standardized method to estimate the systemic tumour burden has been established yet. We applied a 3.0 threshold of SUV for automatic delineation of tumour volume. Although it is variable according to tumours, an SUV of 3.0 is a general cut-off set for differentiating between malignant and benign lesions [25]. Moreover, in our experience, a threshold of 3.0 can minimize unwanted physiological FDG uptake in normal tissue during estimation of WBMTV and thereby shorten the processing time in almost all patients. Correlation between WBMTV and gross tumour volume (GTV) was not evaluated in this study. A previous study already demonstrated that MTV and GTV were highly correlated with a correlation coefficient of 0.93 (p < 0.001) [11]. However, some gross tumours have a necrotic portion without definitive FDG uptake and some metastatic lesions detected by PET/CT cannot be delineated by conventional imaging. MTV may reflect the biologically activated tumour cells more accurately than GTV.
The present study also has several limitations. First, because of the retrospective nature of the study design, heterogeneity of detailed treatment modalities could affect the treatment outcomes. However, WBMTV was a significant prognostic factor regardless of initial treatment regimen and number of lines of chemotherapy in this cohort. Second, not all concerned lesions were histopathologically confirmed. Instead of that, all possible clinical assays identifying the metastases, such as other imaging modalities including CT and MRI, follow-up PET/CT and response to therapy, were utilized for confirming metastasis. Third, MTV derived from a fixed threshold of SUV could be entirely influenced by the compounding factors for SUV including partial volume effect, time between tracer injection and imaging, and plasma glucose level. More controlled prospective studies with a larger numbers of patients will be needed to confirm this promising strategy to improve the prediction of prognosis in patients with SCLC.
Conclusion
WBMTV, which represents systemic tumour burden in the entire body, can accurately predict the prognosis in patients with SCLC. Incorporation of WBMTV with either conventional staging or TNM staging can provide more detailed prognostic information than tumour staging alone. WBMTV with TNM staging may be a more appropriate strategy to predict outcome than WBMTV with conventional staging, thereby helping to make optimized treatment decisions for patients with SCLC.
References
Simon GR, Wagner H, American College of Chest Physicians. Small cell lung cancer. Chest 2003;123:259S–71S.
Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049–59.
Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004;22:3248–54.
Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging 2004;31:1614–20.
Fischer BM, Mortensen J, Langer SW, Loft A, Berthelsen AK, Petersen BI, et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol 2007;18:338–45.
Kamel EM, Zwahlen D, Wyss MT, Stumpe KD, von Schulthess GK, Steinert HC. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med 2003;44:1911–7.
Seo YS, Kwon SY, Jeong SY, Song HC, Min JJ, Kim KS, et al. Correlation of pre-treatment FDG uptake to therapeutic response and relapse in patients with small cell lung cancer. Nucl Med Mol Imaging 2007;41:538–45.
Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009;15:5861–8.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 2010;17:115–22.
Yan H, Wang R, Zhao F, Zhu K, Jiang S, Zhao W, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced non-small cell lung cancer treated by non-surgical therapy. Acta Radiol 2011;52:646–50.
Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 2011;73:332–7.
Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on (18)F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:27–38. doi:10.1007/s00259-011-1934-6.
Stahel RA, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J, et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer 1989;5:119–26.
Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067–77.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al., editors. AJCC cancer staging manual. Chicago: Springer; 2010. p. 253–70.
Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res 2009;15:2426–32.
Bayman NA, Sheikh H, Kularatne B, Lorigan P, Blackhall F, Thatcher N, et al. Radiotherapy for small-cell lung cancer-where are we heading? Lung Cancer 2009;63:307–14.
Slotman BJ. Radiotherapy for extensive stage small cell lung cancer. Front Radiat Ther Oncol 2010;42:187–92.
Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol 2011;120:270–4.
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787–94.
Byhardt RW, Hartz A, Libnoch JA, Hansen R, Cox JD. Prognostic influence of TNM staging and LDH levels in small cell carcinoma of the lung (SCCL). Int J Radiat Oncol Biol Phys 1986;12:771–7.
Ludbrook JJ, Truong PT, MacNeil MV, Lesperance M, Webber A, Joe H, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys 2003;55:1321–30.
Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, et al. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer 2000;89:523–33.
Greco C, Rosenzweig K, Cascini GL, Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for non-small cell lung cancer (NSCLC). Lung Cancer 2007;57:125–34.
Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun 2004;25:651–6.
Acknowledgement
This research was supported by a grant from the Institute of Medical System Engineering (iMSE) of Gwangju Institute of Science and Technology (GIST), Republic of Korea.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, JR., Seo, JH., Chong, A. et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 39, 925–935 (2012). https://doi.org/10.1007/s00259-011-2059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-2059-7