Abstract
Glutaminase 1 (GLS) is a therapeutic target for breast cancer; although GLS inhibitors have been developed, only a few subjects responded well to the therapy. Considering that the expression of histone H3 lysine 27 trimethylation (H3K27me3) and menopausal status was closely linked to GLS, we examined the effects of H3K27me3 and menopausal status on GLS to breast cancer prognosis. Data for 962 women diagnosed with primary invasive breast cancer were analyzed. H3K27me3 and GLS expression in tumors were evaluated with tissue microarrays by immunohistochemistry. Hazard ratios (HRs) and their 95% confidence intervals (CIs) for overall survival and progression-free survival were estimated using Cox regression models. Statistical interaction was assessed on multiplicative scale. There was a beneficial prognostic effect of GLS expression on overall survival for those with low H3K27me3 level (HR = 0.50, 95% CI: 0.20–1.28) but an adverse prognostic effect for those with high H3K27me3 level (HR = 3.90, 95% CI: 1.29–11.78) among premenopausal women, and the statistical interaction was significant (Pinteraction = 0.003). Similar pattern was further observed for progression-free survival (HR = 0.44, 95% CI: 0.20–0.95 for low H3K27me3 level, HR = 1.35, 95% CI: 0.74–2.48 for high H3K27me3 level, Pinteraction = 0.024). The statistical interaction did not occur among postmenopausal women. Our study showed that the prognostic effects of GLS on breast cancer correlated to the expression level of H3K27me3 and menopausal status, which would help optimize the medication strategies of GLS inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutaminase 1 (GLS) is a key enzyme in glutamine catabolism which is critical for the proliferation of various tumors [1,2,3]. Many studies have found that GLS overexpression was associated with the poor prognostic characteristics of breast cancer [4,5,6]. Thereafter, GLS inhibitors have emerged as a therapeutic avenue for breast cancer [7,8,9]. However, the results of clinical trials were not promising [10]: the objective response rates were only 22% in the Phase I study and 6% in the Phase II study [11, 12]. Actually, the findings of the association between GLS expression and breast cancer prognosis were not consistent. For example, a higher expression of GLS was observed to be related to a better prognosis [13]. Therefore, we hypothesize that the effects of GLS or the inhibitors on breast cancer may be affected by other factors.
It was found that breast cancer cells which resisted GLS inhibitors mobilized more fatty acids into mitochondria for oxidation [14]. Moreover, the increased fatty acid catabolism was associated with a decrease of histone H3 lysine 27 trimethylation (H3K27me3) [15, 16] which was also an independent prognostic factor for breast cancer [17, 18]. Furthermore, many studies have found that the decreased level of H3K27me3 suppressed the transcription of oncogene MYC which was essential for the expression of GLS [19, 20]. Thus, the relationship between GLS and H3K27me3 was intriguing, which may affect the prognostic role of GLS on breast cancer.
In addition, it was found that women with high progesterone level have higher level of H3K27me3 [21, 22] and estrogen has the effect of up-regulating GLS [23], which suggested that the relationship between GLS and H3K27me3 may be differentiated by menopause. Therefore, in the present study, we examined the effects of H3K27me3 on GLS to breast cancer prognosis by menopausal status.
Materials and methods
Study population
A total of 1062 female patients with pathologically diagnosed primary invasive breast cancer and > 1 cm of tumor size in diameter between January 2008 and December 2015 were recruited from the Cancer Center of Sun Yat-sen University in Guangzhou, China. Patients with metastatic tumor and missing information of H3K27me3 and GLS (N = 88) were excluded. Most (98.8%) of the included patients were successfully followed up until Dec 31, 2019. This study was approved by the Ethics Committee of the School of Public Health at Sun Yat-sen University. Informed consent was obtained from each participant.
Baseline data collection
Information on demographic and clinicopathologic characteristics was collected at diagnosis using structured questionnaire and from patients’ medical records, including age, menopausal status, body mass index (BMI), family history of breast cancer, clinical stage, histological grade, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) status, and proliferation index factor Ki67 (Ki67). The definition of ER, PR, and HER2 status was described in detail previously [24].
Tissue microarray and immunohistochemistry
The expression levels of H3K27me3 and GLS were evaluated with tissue microarrays (TMAs) by immunohistochemistry (IHC). TMAs were constructed as previously described [25]. The TMAs were baked at 60 °C for 2 h and then dewaxed with xylene and ethanol. Then, antigen retrieval was accomplished using EDTA (PH 9.0) in super-pressure kettle and endogenous peroxide was blocked using 3% H2O2. Antigen–antibody reactions for H3K27me3 and GLS were performed separately. For H3K27me3, slides were incubated in mouse monoclonal to H3K27me3 [mAbcam 6002]-ChIP Grade (ab6002, diluted 1:100, Abcam) and then labeled with the EnVision Detection System (Peroxidase/DAB, Rabbit/Mouse) (Dako K5007). For GLS, slides were incubated in rabbit monoclonal to GLS [EP7212] (ab156876, diluted 1:100, Abcam). Then, slides were developed by diaminobenzidine (DAB) and counterstained by hematoxylin. These slides were finally dehydrated and mounted.
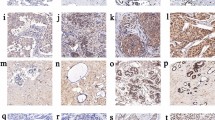
IHC-stained sections were digitally imaged using Pannoramic Scanner and CaseViewer software. IHC staining was analyzed by an experienced pathologist and scored for staining intensity (0-no staining, 1-weak, 2-moderate, and 3-strong) and percentage of tumor cell staining (0–100). Representative immunohistochemical staining of H3K27me3 and GLS is shown in Fig. 1. IHC scoring was done by H-score which was calculated by multiplying the staining intensity by the percentage of positive cells. Thus, the minimal H-score was 0, whereas the maximum H-score was 300. To avoid the observation variability, the mean value of duplicate scores was adapted for further analysis.
Representative immunohistochemical staining of H3K27me3 and GLS. a–d The staining of H3K27me3 in different intensity (a, no staining; b, weak; c, moderate; d, strong). e–h The staining of GLS in different intensity (e, no staining; f, weak; g, moderate; h, strong). GLS glutaminase 1, H3K27me3 histone H3 lysine 27 trimethylation
Follow-up and outcomes
Patients were followed up by phone calls or out-patient visits every 3 months in the first year, every 6 months in the second and third year after diagnosis, and annually thereafter. Outcomes of interest were overall survival (OS) and progression-free survival (PFS). OS was defined as the time from diagnosis to death and PFS was the time from diagnosis to disease progression including recurrence, metastasis, and death. Survival status was censored at the latest follow-up date or Dec 31, 2019.
Statistical analysis
The expressions of H3K27me3 and GLS were treated as binary variables. The optimal cut-off value of H3K27me3 was determined by the minimum P value from log-rank chi-square statistics based on PFS using the X-tile 3.6.1 software (Yale University, New Haven, CT, USA) [26]. For GLS, in fact, nearly half (44.2%) of the H-score of GLS was 0. Therefore, the cut-off value of GLS was determined by the H-score = 0 or not. Next, GLS H-score = 0 was considered negative and GLS H-score > 0 was considered positive. Frequency distribution was used to compare demographic and clinicopathologic characteristics according to H3K27me3 and GLS category. Kaplan–Meier method was used to estimate the 5-year survival. Cox proportional hazard model was used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the associations between demographic and clinicopathologic characteristics and survival (OS and PFS) and the associations between H3K27me3 and GLS and survival.
The statistical interaction between H3K27me3 and GLS on survival was evaluated on the multiplicative scale. HRs (95% CIs) for the association between GLS and survival within H3K27me3 strata were calculated. Furthermore, the statistical interaction was separately tested in premenopausal and postmenopausal patients. All the analyses were conducted using R 3.6.3 and a two-sided P-value below 0.05 was considered statistically significant.
Results
Demographic and clinicopathological characteristics and the associations with breast cancer prognosis and the expression levels of H3K27me3 and GLS
Of 974 eligible women, 962 (98.8%) were included in the statistical analysis after excluding women with loss of follow-up (Fig. 2). Almost all (99.2%) of them were pathologically diagnosed with invasive ductal carcinoma (IDC). The median age at diagnosis was 48 years (interquartile range: 42–56). More than half (57.9%) of the women were premenopausal and 56.1% of them had a BMI between 18.5 and 23.9 kg/m2. The majority of the women were diagnosed with low histological grade (grade I/II: 73.1%), early clinical stage (stage I/II: 71.7%), ER-positive (73.1%), PR-positive (72.2%), or HER2-negative (66.5%) (Table 1).
The optimal cut-off value of H3K27me3 H-score was 175 according to the X-tile plot (Online Supplementary Information, Supplementary Fig. S1). A great part (71.5%) of the women had the H-score > 175 of H3K27me3 and the percentage of GLS negativity was 44.2%. For H3K27me3, women with the H-score ≤ 175 were more likely to be premenopausal and have grade III, ER-negative, PR-negative, and Ki67 > 14% tumors than the subjects with H-score > 175. For GLS, women with GLS negativity were more likely to have grade I/II, ER-positive, and Ki67 ≤ 14% tumors than the subjects with GLS positivity (Table 1). Univariable analysis showed that age, BMI, histological grade, clinical stage, and ER were associated with OS and clinical stage and ER were associated with PFS (Online Supplementary Information, Supplementary Table S1).
Prognostic effects of H3K27me3 and GLS on breast cancer
Of the 962 eligible women, 102 died and 187 experienced disease progression with a median follow-up time of 70.6 months (interquartile range: 45.6–103.9). Five-year OS rate and PFS rate were 91.4% and 84.8%, respectively. A consistent relation between H3K27me3 and survival (OS and PFS) was observed from both univariable and multivariable analyses (Table 2). After adjustment for confounders, women with H3K27me3 H-score > 175 (HR = 0.61, 95% CI: 0.39–0.95) had a better OS compared to H-score ≤ 175. A similar pattern of association was observed for PFS (HR = 0.67, 95% CI: 0.48–0.94) (Table 2). For GLS, no significant association with the prognosis was observed in both univariate analysis (HR = 1.24, 95% CI: 0.83–1.86 for OS; HR = 1.05, 95% CI: 0.79–1.40 for PFS) and multivariate analysis (HR = 1.06, 95% CI: 0.69–1.62 for OS; HR = 0.92, 95% CI: 0.68–1.26 for PFS) (Table 2).
Statistical interaction
The statistical interaction between H3K27me3 and GLS was significant among premenopausal women (Pinteraction = 0.003 for OS; Pinteraction = 0.024 for PFS) but not postmenopausal women (Pinteraction = 0.730 for OS; Pinteraction = 0.720 for PFS) (Table 3). GLS positivity was significantly associated with a poorer OS compared to GLS negativity (HR = 3.90, 95% CI: 1.29–11.78) in patients with H3K27me3 H-score > 175, while it was related to a better OS (HR = 0.50, 95% CI: 0.20–1.28) in patients with H3K27me3 H-score ≤ 175 among premenopausal women. Similar pattern was also observed for PFS.
Discussion
In the present study, we found that a higher GLS expression level was associated with more aggressive characteristics of breast cancer, such as higher histological grade, ER negative, and Ki67 overexpression. There was also a statistical interaction between H3K27me3 and GLS on breast cancer prognosis, particularly among premenopausal women. Compared with the GLS negativity, GLS positivity was associated with a protective effect on the survival of patients with a lower H3K27me3 expression, while it was associated with an adverse effect on the survival of patients with a higher level of H3K27me3 expression among premenopausal women. Our results suggested that the prognostic effects of GLS on breast cancer may correlate to the H3K27me3 expression level in the tumor tissues and the menopausal status.
In consistent with our study, several previous population studies have also found that the level of GLS expression was higher in TNBC than in other subtypes of breast cancer and GLS tended to be negativity in ER and PR-positive tumors [4, 27]. Cellular experiments revealed that levels and activity of GLS were significantly increased in TNBC and HER2 positive breast cancer cell lines [28]; in our study, the HER2 positive patients also tended to be GLS positivity. In terms of breast cancer outcomes, the prognostic role of GLS in the total patients was not significant in these studies [4, 27], which was also consistent with our findings.
It was interesting and pragmatic that there was an opposite prognostic effect of GLS among premenopausal women with high and low levels of H3K27me3 expression. Previous studies have found that a low level of H3K27me3 expression was associated with an increased fatty acid catabolism [15, 16]. Considering that fatty acids were utilized as an alternative energy source after glutamine deprivation in breast cancer cells [29], the low level of H3K27me3 suggested the deprivation of glutamine and the effect of GLS on catalyzing glutamine catabolism (which was recognized to be related to a poor prognosis) would be depressed, inducing a better prognosis of breast cancer. In turn, among women with a higher H3K27me3 expression, GLS would largely play its role with sufficient glutamine and led a poor prognosis. Moreover, it has been found that the breast cancer cells which resisted GLS inhibitors mobilized more fatty acids to catabolize [14], which also supported our hypothesis. In addition, compared with postmenopausal women, premenopausal women had a significantly lower serum concentration of glutamine [30], which would accelerate the deprivation of glutamine, supporting that the statistical interaction between H3K27me3 and GLS was evident among premenopausal women. Furthermore, it may also partially explain why the statistical interaction only existed in premenopausal women that the overall metabolism capacity of premenopausal women was higher than postmenopausal women [31, 32].
Our study has several limitations that need to be taken into consideration. First, we were unable to separately evaluate the three isoforms of GLS (KGA, GAC, and GAM) expression [6, 33] and their associations with breast cancer prognosis. However, these three isoforms of GLS play the same role in breast cancer, so it is feasible to assess its relationship with breast cancer prognosis using the total GLS protein expression, as has been done in previous literatures [1, 6]. Second, only patients with tumor > 1 cm were included, which may lead to selective bias. However, GLS expression was independent of tumor size in this study and the selection may not affect our findings. Finally, we did not collect the information of treatment which was associated with the outcomes. However, since the treatment was determined according to the clinicopathological characteristics, adjustment of these characteristics in the analysis was able to largely control the confounding effects of the treatment.
In conclusion, this study firstly demonstrated that the prognostic roles of GLS in breast cancer correlated to the level of H3K27me3 expression and this statistical interaction occurred only if the patients were premenopausal rather than postmenopausal. When the premenopausal women express a low H3K27me3 level, the overexpression of GLS may be a protective factor according to this study; thus, these patients may not be suitable for the treatment of GLS inhibitors. In turn, GLS inhibitors may be more effective for premenopausal women with the high H3K27me3 expression. Therefore, it is necessary to consider the level of H3K27me3 expression and the menopausal status when applying GLS inhibitors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The R code used during the current study is available from the corresponding author on reasonable request.
References
Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, Pereira HM, Garratt RC, Dias SM, Ambrosio AL (2012) Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci U S A 109:1092–1097. https://doi.org/10.1073/pnas.1112495109
Yu D, Shi X, Meng G, Chen J, Yan C, Jiang Y, Wei J, Ding Y (2015) Kidney-type glutaminase (GLS1) is a biomarker for pathologic diagnosis and prognosis of hepatocellular carcinoma. Oncotarget 6:7619–7631. https://doi.org/10.18632/oncotarget.3196
Cao J, Zhang C, Jiang GQ, Jin SJ, Gao ZH, Wang Q, Yu DC, Ke AW, Fan YQ, Li DW, Wang AQ, Bai DS (2019) Expression of GLS1 in intrahepatic cholangiocarcinoma and its clinical significance. Mol Med Rep 20:1915–1924. https://doi.org/10.3892/mmr.2019.10399
Kim JY, Heo SH, Choi SK, Song IH, Park IA, Kim YA, Park HS, Park SY, Bang WS, Gong G, Lee HJ (2017) Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch 470:381–389. https://doi.org/10.1007/s00428-017-2083-5
Saha SK, Islam S, Abdullah-Al-Wadud M, Islam S, Ali F, Park KS (2019) Multiomics analysis reveals that GLS and GLS2 differentially modulate the clinical outcomes of cancer. J Clin Med 8. https://doi.org/10.3390/jcm8030355
Masisi BK, El AR, Alfarsi L, Rakha EA, Green AR, Craze ML (2020) The role of glutaminase in cancer. Histopathology 76:498–508. https://doi.org/10.1111/his.14014
Budczies J, Pfitzner BM, Gyorffy B, Winzer KJ, Radke C, Dietel M, Fiehn O, Denkert C (2015) Glutamate enrichment as new diagnostic opportunity in breast cancer. Int J Cancer 136:1619–1628. https://doi.org/10.1002/ijc.29152
Huang Q, Stalnecker C, Zhang C, McDermott LA, Iyer P, O’Neill J, Reimer S, Cerione RA, Katt WP (2018) Characterization of the interactions of potent allosteric inhibitors with glutaminase C, a key enzyme in cancer cell glutamine metabolism. J Biol Chem 293:3535–3545. https://doi.org/10.1074/jbc.M117.810101
Zimmermann SC, Duvall B, Tsukamoto T (2019) Recent progress in the discovery of allosteric inhibitors of kidney-type glutaminase. J Med Chem 62:46–59. https://doi.org/10.1021/acs.jmedchem.8b00327
Grinde MT, Hilmarsdottir B, Tunset HM, Henriksen IM, Kim J, Haugen MH, Rye MB, Maelandsmo GM, Moestue SA (2019) Glutamine to proline conversion is associated with response to glutaminase inhibition in breast cancer. Breast Cancer Res 21:61. https://doi.org/10.1186/s13058-019-1141-0
Kalinsky K, Harding JJ, DeMichele A, Infante JR, Gogineni K, Owonikoko TK, Isakoff S, Iliopoulos O, Patel MR, Munster P, Telli ML, Jenkins Y, Fiji GP, Whiting SH, Meric-Bernstam F (2018) Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Cancer Res 78S
Vidal G, Kalinsky K, Stringer-Reasor E, Lynce F, Cole J, Valdes-Albini F, Soliman H, Nikolinakos P, Silber A, DeMichele A, Ali H, Graham D, Giguere J, Brufsky A, Liang Y, Holland S, Fiji G, O’Keeffe B, Gogineni K (2019) Efficacy and safety of CB-839, a small molecule inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer (TNBC): initial findings from a multicenter, open-label phase 2 study. Cancer Res 79S. https://doi.org/10.1158/1538-7445.SABCS18-P6-20-07
Petovari G, Danko T, Tokes AM, Vetlenyi E, Krencz I, Raffay R, Hajdu M, Sztankovics D, Nemeth K, Vellai-Takacs K, Jeney A, Kulka J, Sebestyen A (2020) In situ metabolic characterisation of breast cancer and its potential impact on therapy. Cancers (Basel) 12. https://doi.org/10.3390/cancers12092492
Reis L, Adamoski D, Ornitz OSR, Rodrigues AC, Sousa DOK, Correa-da-Silva F, Malta DSPF, Meira DM, Consonni SR, Mendes DMP, Silber AM, Dias S (2019) Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells. J Biol Chem 294:9342–9357. https://doi.org/10.1074/jbc.RA119.008180
Zha L, Li F, Wu R, Artinian L, Rehder V, Yu L, Liang H, Xue B, Shi H (2015) The histone demethylase UTX promotes brown adipocyte thermogenic program via coordinated regulation of H3K27 demethylation and acetylation. J Biol Chem 290:25151–25163. https://doi.org/10.1074/jbc.M115.662650
Seok S, Kim YC, Byun S, Choi S, Xiao Z, Iwamori N, Zhang Y, Wang C, Ma J, Ge K, Kemper B, Kemper JK (2018) Fasting-induced JMJD3 histone demethylase epigenetically activates mitochondrial fatty acid beta-oxidation. J Clin Invest 128:3144–3159. https://doi.org/10.1172/JCI97736
Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, Albarracin C, Yu D, Abbruzzese JL, Mills GB, Bast RJ, Hortobagyi GN, Hung MC (2008) Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog 47:701–706. https://doi.org/10.1002/mc.20413
Fontes-Sousa M, Lobo J, Lobo S, Salta S, Amorim M, Lopes P, Antunes L, de Sousa SP, Henrique R, Jeronimo C (2020) Digital imaging-assisted quantification of H3K27me3 immunoexpression in luminal A/B-like, HER2-negative, invasive breast cancer predicts patient survival and risk of recurrence. Mol Med 26:22. https://doi.org/10.1186/s10020-020-0147-5
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105:18782–18787. https://doi.org/10.1073/pnas.0810199105
Bialopiotrowicz E, Noyszewska-Kania M, Kachamakova-Trojanowska N, Loboda A, Cybulska M, Grochowska A, Kopczynski M, Mikula M, Prochorec-Sobieszek M, Firczuk M, Graczyk-Jarzynka A, Zagozdzon R, Zabek A, Mlynarz P, Dulak J, Gorniak P, Szydlowski M, Pyziak K, Martyka J, Sroka-Porada A, Jablonska E, Polak A, Kowalczyk P, Szumera-Cieckiewicz A, Chapuy B, Rzymski T, Brzozka K, Juszczynski P (2020) Serine biosynthesis pathway supports MYC-miR-494-EZH2 feed-forward circuit necessary to maintain metabolic and epigenetic reprogramming of Burkitt lymphoma cells. Cancers (Basel) 12. https://doi.org/10.3390/cancers12030580
Izzo F, Mercogliano F, Venturutti L, Tkach M, Inurrigarro G, Schillaci R, Cerchietti L, Elizalde PV, Proietti CJ (2014) Progesterone receptor activation downregulates GATA3 by transcriptional repression and increased protein turnover promoting breast tumor growth. Breast Cancer Res 16:491. https://doi.org/10.1186/s13058-014-0491-x
Xiong Y, Wang J, Liu L, Chen X, Xu H, Li TC, Wang CC, Zhang S (2017) Effects of high progesterone level on the day of human chorionic gonadotrophin administration in in vitro fertilization cycles on epigenetic modification of endometrium in the peri-implantation period. Fertil Steril 108:269–276. https://doi.org/10.1016/j.fertnstert.2017.06.004
Zhou WJ, Zhang J, Yang HL, Wu K, Xie F, Wu JN, Wang Y, Yao L, Zhuang Y, Xiang JD, Zhang AJ, He YY, Li MQ (2019) Estrogen inhibits autophagy and promotes growth of endometrial cancer by promoting glutamine metabolism. Cell Commun Signal 17:99. https://doi.org/10.1186/s12964-019-0412-9
He JR, Tang LY, Yu DD, Su FX, Song EW, Lin Y, Wang SM, Lai GC, Chen WQ, Ren ZF (2011) Epstein-Barr virus and breast cancer: serological study in a high-incidence area of nasopharyngeal carcinoma. Cancer Lett 309:128–136. https://doi.org/10.1016/j.canlet.2011.05.012
Chen QX, Yang YZ, Liang ZZ, Chen JL, Li YL, Huang ZY, Weng ZJ, Zhang XF, Guan JX, Tang LY, Yun JP, Ren ZF (2021) Time-varying effects of FOXA1 on breast cancer prognosis. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-021-06125-7
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713
Kim S, Kim DH, Jung WH, Koo JS (2013) Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer 20:339–348. https://doi.org/10.1530/ERC-12-0398
Craze ML, Cheung H, Jewa N, Coimbra N, Soria D, El-Ansari R, Aleskandarany MA, Wai CK, Diez-Rodriguez M, Nolan CC, Ellis IO, Rakha EA, Green AR (2018) MYC regulation of glutamine-proline regulatory axis is key in luminal B breast cancer. Br J Cancer 118:258–265. https://doi.org/10.1038/bjc.2017.387
Guo X, Wang A, Wang W, Wang Y, Chen H, Liu X, Xia T, Zhang A, Chen D, Qi H, Ling T, Piao HL, Wang HJ (2021) HRD1 inhibits fatty acid oxidation and tumorigenesis by ubiquitinating CPT2 in triple-negative breast cancer. Mol Oncol 15:642–656. https://doi.org/10.1002/1878-0261.12856
Auro K, Joensuu A, Fischer K, Kettunen J, Salo P, Mattsson H, Niironen M, Kaprio J, Eriksson JG, Lehtimaki T, Raitakari O, Jula A, Tiitinen A, Jauhiainen M, Soininen P, Kangas AJ, Kahonen M, Havulinna AS, Ala-Korpela M, Salomaa V, Metspalu A, Perola M (2014) A metabolic view on menopause and ageing. Nat Commun 5:4708. https://doi.org/10.1038/ncomms5708
Deming SL, Nass SJ, Dickson RB, Trock BJ (2000) C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. Br J Cancer 83:1688–1695. https://doi.org/10.1054/bjoc.2000.1522
Barger JF, Plas DR (2010) Balancing biosynthesis and bioenergetics: metabolic programs in oncogenesis. Endocr Relat Cancer 17:R287–R304. https://doi.org/10.1677/ERC-10-0106
Elgadi KM, Meguid RA, Qian M, Souba WW, Abcouwer SF (1999) Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics 1:51–62. https://doi.org/10.1152/physiolgenomics.1999.1.2.51
Acknowledgements
We sincerely thank the patients who participated in this study, the staff who conducted the baseline and the follow-up data collection, and the medical staff in the breast departments of the First Affiliated Hospital, and the Cancer Center of Sun Yat-Sen University.
Funding
This study was funded by the National Natural Science Foundation of China (81773515 and 81973115) and the Science and Technology Planning Project of Guangdong Province, China (2019B030316002). The founders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Meng Zhou, Ze-fang Ren, and Qian-xin Chen designed and directed the study, wrote and/or revised the manuscript. Yuan-zhong Yang and Yue-lin Li constructed the TMAs. Yuan-zhong Yang contributed to the IHC. Zi-jin Weng, Xiao-fang Zhang, Jie-xia Guan, and Lu-ying Tang contributed to digital imaging of IHC-stained sections and the assessment of immunohistochemical expression. Meng Zhou, Qian-xin Chen, Zhuo-zhi Liang, Zi-yi Huang, and Yue-lin Li contributed to clinical data collection and curation. Meng Zhou, Qian-xin Chen, Zhuo-zhi Liang, and Zi-yi Huang participated in the statistical analysis plan and interpretation of results. Ze-fang Ren provided administrative support and supervision for the study. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethics committee of School of Public Health, Sun Yat-sen University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, M., Chen, Qx., Yang, Yz. et al. Prognostic value of glutaminase 1 in breast cancer depends on H3K27me3 expression and menopausal status. Virchows Arch 480, 259–267 (2022). https://doi.org/10.1007/s00428-021-03210-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03210-6