Abstract
Main conclusion
Overexpression of OsDJ-1C in rice improves root architecture, photosynthesis, yield and abiotic stress tolerance through modulating methylglyoxal levels, antioxidant defense, and redox homeostasis.
Abstract
Exposure to abiotic stresses leads to elevated methylglyoxal (MG) levels in plants, impacting seed germination and root growth. In response, the activation of NADPH-dependent aldo-keto reductase and glutathione (GSH)-dependent glyoxalase enzymes helps to regulate MG levels and reduce its toxic effects. However, detoxification may not be carried out effectively due to the limitation of GSH and NADPH in plants under stress. Recently, a novel enzyme called glyoxalase III (GLY III) has been discovered which can detoxify MG in a single step without needing GSH. To understand the physiological importance of this pathway in rice, we overexpressed the gene encoding GLYIII enzyme (OsDJ-1C) in rice. It was observed that OsDJ-1C overexpression in rice regulated MG levels under stress conditions thus, linked well with plants' abiotic stress tolerance potential. The OsDJ-1C overexpression lines displayed better root architecture, improved photosynthesis, and reduced yield penalty compared to the WT plants under salinity, and drought stress conditions. These plants demonstrated an improved GSH/GSSG ratio, reduced level of reactive oxygen species, increased antioxidant capacity, and higher anti-glycation activity thereby indicating that the GLYIII mediated MG detoxification plays a significant role in plants' ability to reduce the impact of abiotic stress. Furthermore, these findings imply the potential of OsDJ-1C in crop improvement programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can adapt to fluctuating environmental conditions and accordingly, they adjust their growth and developmental processes. However, the manifold array of abiotic stresses often triggers plant’s innate stress tolerance mechanisms. The prolonged activation of this system can alter the delicate balance between source and sink, ultimately leading to an imbalanced state that ultimately manifests as a reduction in plant yield (as reviewed by Nutan et al. 2020; Hamdan et al. 2022). These changes invariably disrupt cellular homeostasis, resulting in an accumulation of reactive carbonyl species (RCS) such as methylglyoxal (MG) to a deleterious level (Hoque et al. 2016; Gupta et al. 2018). These metabolites can alter cellular activities such as lipid peroxidation, protein, and fatty acid oxidation (Sankaranarayanan et al. 2017; Biswas and Mano 2021). The biological impact of RCS is that these are strong electrophiles, that target a functional group of many macromolecules which results in the loss or decrease of enzyme activity, mutagenicity, genotoxicity, and formation of dysfunctional forms such as advanced glycation end products (AGEs) (Kalapos 1994; Thornalley 2008; Semchyshyn 2014; Chaudhuri et al. 2018; Reddy et al. 2022).
MG is the most reactive metabolite among the reactive carbonyl species (RCS) that needs detoxification to maintain proteins in their biologically active conformation, ensuring efficient cell survival under stress conditions. MG is naturally produced in various metabolic pathways, particularly linked to glycolysis and photosynthesis (Li et al. 2018b). Its function in living organisms depends on its concentration in plant cells. A low level of MG in plants plays a significant role as a signaling molecule, initiating signaling cascades through Ca2+, ROS, K+, and abscisic acid leading to stomatal movement, plant growth, and development (Kaur et al. 2015; Li et al. 2018b; Askari-Khorasgani and Pessarakli 2019; Li 2020). The higher accumulation of MG forms complexes with large biological molecules such as DNA and proteins, leading to the disruption of various physiological processes (Li et al. 2018b; Nowicka et al. 2023). The regulation of MG levels is critical for maintaining cellular homeostasis (Singla-Pareek et al. 2020). MG accumulation in plants is primarily induced by a range of biotic and abiotic stresses, including drought, salinity, extreme temperatures, oxidative stress, and pathogen attack, which ultimately lead to altered carbon metabolism (Mostofa et al. 2018; Proietti et al. 2019). In different crop systems, plants exposed to chromium toxicity exhibited significant levels of oxidative stress, as evidenced by elevated levels of MG in wheat and rice (Alam et al. 2023; Pan et al. 2023). Furthermore, the accumulation of MG in tobacco has been observed as a result of salinity stress, while similar accumulation of MG have been reported in tomato under conditions of salinity, osmotic stress, and cold stress (Ghosh et al. 2022; Gambhir et al. 2023).
There exist multiple positive and negative regulators that exert an influence on the accumulation of MG in plants. The presence of elevated glucose levels and mitochondrial dysfunction during stress condition enhance the levels of MG in a positive manner (Mostofa et al. 2018). The higher levels of MG are negatively regulated by the enhanced expression of stress-related genes. This regulation is facilitated by the efficient glyoxalase system, improved antioxidant system, and protein homeostasis through heat shock proteins. In addition, the indirect effects of plant phytohormones such as abscisic acid and salicylic acid also contribute to this negative regulatory effect (Askari-Khorasgani and Pessarakli 2019; Ghosh et al. 2022). Several reports have suggested that the exposure to methylglyoxal has a negative impact on the growth of rice seedlings, particularly in terms of root elongation and yield (Kaur et al. 2015). The reduction of root length, surface area, and volume due to MG stress results in the inhibition of root growth and a decrease in crop yield, ultimately impacting the quantity and quality of grains produced (Kaur et al. 2015). In Arabidopsis, methylglyoxal hinders in the germination of seeds and elongation of roots and concurrently upregulates the transcription of stress-responsive genes through an ABA-dependent pathway (Hoque et al. 2012). Plants resist MG-associated cellular toxicity by activating the glyoxalase detoxification mechanism, which converts the toxic form of MG into d-lactate, a non-toxic molecule (Ghosh et al. 2016; Gupta et al. 2018).
The glyoxalase detoxification mechanism is evolutionarily conserved and consists of two enzymes, glyoxalase I (GLYI) and glyoxalase II (GLYII) that work in series to convert MG to d-lactate using glutathione (GSH) as the cofactor (Singla-Pareek et al. 2003). In brief, toxic MG undergoes spontaneous interaction with GSH, resulting in the formation of hemithioacetal. This molecule is then metabolized by GLYI, leading to the production of S-d-lactoylglutathione as an intermediate product. Subsequently, GLYII facilitates the transformation of S-d-lactoylglutathione into d-lactate, thereby releasing GSH molecule back into the cellular system (Singla-Pareek et al. 2003). Several reports have demonstrated the pivotal role played by GLYI and GLYII in regulation of stress tolerance in crop plants. The amélioration of salinity tolerance has been observed through the overexpression of Brassica juncea GLYI gene in tobacco and rice (Veena and Sopory 1999; Gupta et al. 2018). Furthermore, the ectopic expression of GLYI from rice and tobacco has exhibited enhanced tolerance to heavy metal toxicity and various abiotic stressors (Singla-Pareek et al. 2006; Mustafiz et al. 2014). Similarly, the overexpression of rice GLYII has been found to confer higher tolerance to exogenous MG and salt stress in rice (Singla-Pareek et al. 2008; Gupta et al. 2018). Moreover, simultaneous overexpression of GLYI and GLYII genes has been demonstrated to promote greater adaptability under salt and heavy metal stress in comparison to plants transformed with a single gene (Singla-Pareek et al. 2006; Gupta et al. 2018). Apart from these two glyoxalases, our group has discovered glyoxalase III (GLYIII) in rice, which possesses DJ-1/PfpI domain(s). Out of the six genes responsible for encoding OsDJ-1, OsDJ-1C demonstrates a distinctive enzymatic activity that facilitates the conversion of MG into non-toxic d-lactate through a single step reaction, without necessitating glutathione as a cofactor (Ghosh et al. 2016). These proteins containing DJ-1/pfp1 domain have also been identified in E. coli (Sastry et al. 2002), Arabidopsis thaliana (Xu et al. 2010), humans (Lee et al. 2012), Candida albicans (Hasim et al. 2014), Saccharomyces cerevisiae (Bankapalli et al. 2015), Oryza sativa (Ghosh et al. 2016), Medicago truncatula (Ghosh 2017), Nicotiana tabacum (Melvin et al. 2017), Glycine max (Islam and Ghosh 2018), sugarcane (Mohanan et al. 2020), date palm (Jana et al. 2021) and tomato (Gambhir et al. 2023). The promoter region of OsDJ-1 genes comprises certain hormone responsive elements such as CGTCA box, TGACG motif, TATC box, TCA element, and TGA element. These elements may detect different signaling responses and govern the expression of corresponding genes in reaction to both stress and developmental cues (Ghosh et al. 2016). There is a lack of information pertaining the signaling players that specifically govern the function of OsDJ-1C. Nevertheless, numerous reports have indicated the involvement of signaling players in regulating the function of glyoxalase enzymes (GlyI and GlyII), either positively or negatively. Nahar and his colleagues have demonstrated the protective effects of exogenous GSH application in mung bean seedlings against damages caused by salinity, drought, and high temperature through a positive regulation of glyoxalase enzymes and antioxidant systems (Nahar et al. 2015a, b, c). Another study supports the notion that either GSH or GSH–NaHS (H2S donor) play a positive role in co-regulating glyoxalase enzymes and alleviating salinity stress in Capsicum plants (Kaya et al. 2020). Moreover, phytohormones such as salicylic acid, auxin, cytokinin, brassinosteroids, gibberellic acid etc. are also involved in regulating the function of glyoxalase enzymes either positively or negatively (Garai et al. 2021). Gambhir et al. (2023) observed elevated MG levels in the 2-week-old tomato seedlings subjected to hormone treatments such as 2,4-D and ABA within 12 h compared to their untreated controls. Previous research has demonstrated that overexpression of AtDJ-1a improves tolerance to abiotic stresses in Arabidopsis plants exposed to environmental challenges, and loss-of-function of this gene promoted death in aging plants (Xu et al. 2010). Loss of AtDJ-1C function leads to non-viable albino seedlings, suggesting its role in chloroplast formation (Lin et al. 2011). Under abiotic stress conditions, date palm PdDJ-1 protein plays a crucial role in the detoxification of MG and in preserving the redox equilibrium (Jana et al. 2021). Transgenic tobacco plants overexpressing E. coli GLY III gene (EcGLY-III) showed improved tolerance to salinity stress during both the seedling and reproductive phases (Ghosh et al. 2022). Glyoxalase III is also involved in fruit ripening and the regulation of ethylene production in tomatoes. Recently, a glutathione‐independent tomato glyoxalaseIII2, SlGLYIII2, has been shown to confer improved tolerance under osmotic and salt stresses (Gambhir et al. 2023). OsGLYI3, a gene encoding glyoxalase, has also been reported to express in the rice grains and plays a significant role in enhancing the longevity of seeds and improving tolerance to salt stress (Liu et al. 2022). In the present study, we have carried out the detailed functional characterization of OsDJ-1C, concerning its role in abiotic stress tolerance in rice. We found that increased levels of OsDJ-1C in rice positively links with tolerance to several abiotic stresses as determined by morphological, physiological, and agronomic traits. The result reported here uncovers the crucial role of GLYIII protein in the detoxification of MG and ROS levels in rice which will help to develop a stress-resilient rice genotype in the future.
Materials and methods
Plant material and their growth condition
Oryza sativa L. cv. IR64 has been used for the development of transgenic plants. Seeds of IR64 and OsDJ-1C overexpression rice lines were surface sterilized with 0.2% bavistin, thoroughly rinsed with distilled water and then germinated in a hydroponics system under controlled growing conditions of 16/8 h day/night cycles, 28/23 °C day/night temperature and 60% relative humidity in a plant growth chamber.
Construct preparation and generation of OsDJ-1C overexpression lines
To generate OsDJ-1C overexpression lines, the coding sequence of OsDJ-1C (1164 bp) was amplified from rice (IR64) cDNA and was cloned into the TOPO-TA vector for stabilization and sequencing. After sequencing, full-length OsDJ-1C gene was further cloned into pRT101 shuttle vector. The whole cassette (CaMV35S promoter, OsDJ-1C gene, and the CaMV polyadenylation site) was amplified from pRT101 and was subcloned into pCAMBIA1300 plant expression vector. Cloning into pCAMBIA1300 was confirmed by colony PCR, restriction digestion, and sequencing. Primers used in various steps of cloning have been listed in Suppl. Table S1. This overexpression construct was used for rice transformation through Agrobacterium-mediated transformation (Sahoo et al. 2011).
Salinity and drought stress treatment at the seedling stage
Seven-day-old rice seedlings were used for the treatment of drought and salinity stress. Seedlings were then kept in ½ strength Yoshida medium (Yoshida et al. 1976) containing 200 mM NaCl for salinity and 20% PEG-6000 for drought for 6 days. Simultaneously, control seedlings were kept in 1/2 strength Yoshida media. Stress-treated and control seedlings were harvested to evaluate various biochemical and physiological parameters such as seedling fresh weight (FW), methylglyoxal content, glyoxalase III activity, reduced and oxidized glutathione (GSH:GSSG) ratio, total antioxidant capacity, lipid peroxidation, and AGEs estimation.
Visualization of Na+ by utilizing CoroNa Green dye
Sodium ions (Na+) present inside the root cells at a given point of time were visualized by staining with CoroNa Green dye, as previously described by Gupta et al. (2018). Root samples of wild‐type (WT) and OsDJ-1C overexpression lines (exposed with 200 mM salt for 6 days) were utilized for this analysis. The visualization of CoroNa Green-dependent fluorescence was done using a confocal laser scanning microscope (Nikon) with 488 nm excitation and 505–525 nm emission wavelength.
Methylglyoxal (MG) estimation
MG level was measured in the leaf samples of WT and transgenic plants using the method described previously (Ghosh et al. 2016). One ml of reaction mixture contained 250 μl of 7.2 mM 1,2-diaminobenzene, 100 μl of 5 M perchloric acid, 10 μl of 100 mM NaN3 (to inhibit peroxidase) and 650 μl of the neutralized supernatant. The blank absorbance (at 336 nm) of the reaction mixture was taken at 0 h. The similar reaction mixture was incubated at room temperature for 3 h and then optical density (O.D.) was measured at 336 nm. The MG content was measured using a standard curve and was represented as μg/mg FW.
GlyIII activity and reduced and oxidized glutathione measurement
Glyoxalase III activity was determined according to Kwon et al. (2013). A decrease in the absorbance of MG at 540 nm was monitored to determine the specific enzyme activity in nmol−1 min−1 mg protein−1. Protein extract from leaves was added to the reaction mixture containing 10 mM MG, and 20 mM sodium phosphate buffer (pH 6.8), followed by incubation at 37 °C for 30 min. 30 μl of this solution was mixed with 90 μl of a solution containing 2 M HCl, 0.1% 2,4-dinitrophenylhydrazine, and 210 μl distilled water. The reaction was terminated by adding 420 μl of 10% NaOH. The remaining MG was measured by taking the absorbance at 540 nm (UV Ultraspec 2100 pro spectrophotometer). The ratio of GSH/GSSG was measured using a glutathione GSH/GSSG colorimetric assay kit (Sigma-Aldrich) following the user manual.
Total antioxidant capacity measurement
The total antioxidant capacity of the protein extracts was measured by utilizing phosphomolybdenum method, as described by Prieto et al. (1999). To initiate the reaction, a mixture of 3 ml of reagent solution containing 0.6 M sulfuric acid, 28 mM sodium phosphate buffer, and 4 mM ammonium molybdate was combined with 0.3 ml of protein extract. The reaction mixture was incubated at 95 °C for 90 min followed by cooling at room temperature and the absorbance was measured at 695 nm (UV Ultroapec 2100 pro). Methanol (0.3 ml) was used as a blank instead of the extract. Total antioxidant activity is measured in gram equivalents of ascorbic acid. A calibration curve was generated using methanol with varying concentrations of ascorbic acid (1000, 500, 250, 125, 62.5, and 31.25 g/ml).
Antioxidant enzyme assay protocol
Extraction of plant protein
Fresh leaf tissue was weighed (100 mg) and homogenized to a fine powder using liquid nitrogen. The fine powder was resuspended in 2 ml of protein extraction buffer containing 0.1 M sodium phosphate buffer (pH 7), 10 mM MgSO4, 0.2% PVPP, and 0.2 mM PMSF, 50% glycerol, followed by two rounds of centrifugation at 12,000g for 30 min at 4 °C and the supernatant was used as the total protein extract. The total protein content was quantified following the Bradford’s method (Bradford 1976) and used for assaying various antioxidant enzyme activities.
Catalase (CAT) assay
Catalase activity was measured following the method of Beers and Sizer (1952), with slight modifications. The reaction mixture (1.0 ml) contained 100 mM sodium phosphate buffer (pH 7.0), 1 mM PMSF, 0.1 μM EDTA, 20 mM H2O2, and 5 µg enzyme extract. The reaction was initiated by adding 10 mM H2O2 and absorbance was measured at 240 nm for 5 min at every 30 s interval. The reduction in H2O2 was quantified using molar extinction coefficient (0.0436 mM−1 cm-1). One unit of catalase is the amount that decomposes 1 μM of H2O2 to O2 and H2O per min at 25 °C, pH 7.0, at a substrate concentration of 10 mM H2O2.
Superoxide dismutase (SOD) assay
SOD activity was estimated according to the protocol developed by Giannopolitis and Ries (1977). The 1.5 ml of total reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 0.1 μM EDTA, 2 μM riboflavin, 75 μM nitroblue tetrazolium (NBT), and 5 µg enzyme extract. Riboflavin was then added just before illumination to start the reaction and allowed to proceed for reaction by irradiating for 15 min followed by measuring the absorbance at 560 nm. Enzyme activity (units/ml) was calculated as one unit of SOD activity is the amount of enzyme essential for inhibiting the reduction of NBT by 50%. All the experiments related to physiological and biochemical analysis were conducted three times using three biological replicates from each line along with WT.
Ascorbate peroxidase (APX) assay
Approximately 100 mg leaf tissue from the control, drought, and salinity-treated plants were harvested and grinded in ice-cold 50 mM K2PO4 buffer (pH 7.5) containing 2 mM EDTA, 0.1 mM phenyl-methylsulfonyl fluoride (PMSF), and 2 mM of Na-ascorbate. After centrifugation at 12,000g for 10 min at 4 °C, the supernatant was utilized for enzyme assay. The Bradford (1976) protocol was used to measure the total protein content in the supernatant. The determination of APX activity was carried out according to the protocol described by Nakano and Asada (1981). The 1 ml of total reaction mixture consisted of 50 mM (pH 7.5) K2PO4 buffer, 0.25 mM ascorbate, 0.1 mM EDTA, 10 mM H2O2, and 5 µg enzyme extract. H2O2 based oxidation of ascorbate was measured through spectrophotometer by recording the decrease in absorbance at 290 nm (D = 2.8 mM−1 cm−1). The slope value of absorbance at 290 nm was considered for the calculation of the rate.
Assessment of morphological, physiological and yield traits during drought and salinity stress treatment at the pre-flowering stage
For the salinity stress treatment, approximately two-month-old WT and OsDJ-1C overexpression lines were subjected to a mixture of salts containing of 6.25 mM CaCl2, 15 mM MgSO4, 3.75 mM MgCl2, and 50 mM NaCl with EC of 10 dS/m at the pre‐flowering stage (Singh and Flowers 2010). The plant’s performance was consistently observed and their morphological, physiological and yield related parameters were recorded after 45 days exposure of salinity stress. For drought stress treatment, at pre‐flowering stage (approximately 2-month-old) WT and OsDJ-1C overexpression lines were not watered for 18 days until soil moisture capacity reduced to 30%. Subsequently, the plants were recovered by re‐watering and physiological parameters were recorded after 3 days of recovery, and all the parameters related to yield were measured upon plant maturity (Gupta et al. 2018).
Measurement of leaf gas exchange parameters
For control, drought recovery, and salinity stress conditions, the chlorophyll (Chl) a fluorescence levels of the WT and OsDJ-1C lines were measured by Handy-PEA fluorimeter (Hansatech Instruments Ltd). The measurements were conducted after a 10 min period of dark adaption of the leaves as described by Mishra et al. (2021). The Handy-PEA parameters were double normalized at Fo and Fm, and the OJIP test was performed for analysing the photosynthetic parameters. This methodology has been previously described by Kalaji et al. (2017) and Anwar et al. (2022). Further, the calculation of energy trapping, also known as the quantum yield of PSII (ΦP), electron transport after the reduction of QA to QA_ (ΦE), reduction of end electron acceptor molecules (ΦR), performance index based on absorption (PIABS), and performance index of overall photochemistry (PItotal) was carried out as per the methodology described by Strasser et al. (2010).
The assessment of relative water content (RWC), electrolyte leakage (EL), and K+/Na+ ratio
The method described by Reguera et al. (2013) was employed to assess RWC. The leaf samples were obtained from both the control and stress conditions, and the FW of leaves was recorded. The samples were then soaked in water for 6 h to determine their turgid weight (TW). Thereafter, the samples were subjected to 24 h of drying at a temperature of 80 °C in an oven to obtain their dry weight (DW). The RWC was subsequently calculated using the formula presented below:
The endogenous EL levels were assessed in accordance with Gupta et al. (2018). Specifically, 100 mg of leaf tissue was placed in 20 ml of deionized water and incubated at 37 °C for a duration of 2 h. The solution’s E1 was then measured using a conductivity meter manufactured by Eleins Inc. Following this, the mixture was autoclaved at 121 °C for 15 min. After cooling down the samples, the E2 was measured. The EL was calculated using the formula provided below.
The levels of endogenous K+ and Na+ content in both OsDJ-1C overexpression lines and WT grown under control and salinity stress condition were assessed using the methodology described by Mishra et al. (2021). Briefly, 100 mg of leaf tissue was collected and subjected to digestion with 0.1% HNO3 in a boiling water bath for 30 min in double‐distilled water with the purpose of extracting ions. Atomic Absorption Spectroscopy (Thermo Fisher Ice3000 Series) was used for the determination of ions in the samples.
Anti-glycation activity
The protocol described by Peng et al. (2008) was used to measure the inhibition of AGEs formation. The protein extract was prepared and quantified as per the protocol described above. Briefly, MG and bovine serum albumin (BSA) were combined in a 100 mM potassium phosphate (pH 7.4) buffer to attain a final concentration of 5 mM and 1 mg ml−1, respectively. Sodium azide (0.2 g l−1) was added to the mixture and incubated at 37 °C with or without total plant protein extract (20 µg) for the duration of five days. A positive control reaction was setup by adding 5 mM aminoguanidine in place of protein extract. The fluorescence of the reaction mixture was recorded at an excitation wavelength of 340 nm and an emission wavelength of 420 nm, respectively, and percentage AGEs inhibition was calculated as per the formula given below. Phosphate buffer was used as the blank.
The fluorescence intensities of the sample and blank are represented by Fs and Fb, respectively.
Measurement of lipid peroxidation
The malondialdehyde (MDA) content was measured to evaluate the degree of lipid peroxidation as described by Zhou and Leul (1998). Leaf tissue weighing around 100 mg was ground in 0.15 (w/v) trichloroacetic acid (TCA) and then the sample was centrifuged at 15,000g for 10 min at 4 °C. The supernatant was blended with 0.5% thiobarbituric acid (TBA) that was diluted in 20% TCA and incubated in a hot water bath for 25 min. The samples were then centrifuged for 5 min at 15,000g and the supernatant’s absorbance was measured at 532 and 600 nm (the value of the extinction coefficient of MDA at 532 nm was taken as 155 mM−1 cm−1). Measurements were corrected for nonspecific turbidity by deducting the absorbance at 600 nm.
Reactive oxygen species (ROS) staining
For the staining of O2−, we used NBT (Sigma-Aldrich) as described by Ramu et al. (2002) with some changes. The roots were kept in 10 mM sodium phosphate buffer (pH 7.8) with 1 mg ml−1 NBT and 10 mM NaN3. Following this, vacuum infiltration was carried out for 10 min and then incubation was done for 30 min at 37 °C. The reaction was terminated by removing the NBT staining solution and roots were washed twice in 80% (v/v) ethanol. Stained roots were then used for imaging.
Statistical analyses
The statistical analysis was carried out and bar graphs were created with GraphPad prism9 scientific software. Significance levels of variation were determined with a one-way ANOVA test of P < 0.05 in all experiments. The comparison was done between WT and OsDJ-1C overexpression lines.
Results
Cloning of OsDJ-1C into plant expression vector and generation of transgenic rice plant
The coding sequence of OsDJ-1C was amplified from IR64 rice cDNA using DJ-1CXhoI forward and DJ-1C XbaI reverse primers. The amplified fragment was cloned into pRT101 (shuttle vector) at XhoI and XbaI restriction sites. After confirmation through sequencing, the entire cassette (CaMV35S promoter + OsDJ-1C + PolyA terminator) was amplified using CaMV35SPstI forward and PolyAPstI reverse primers and was cloned into the pCAMBIA1300 plant expression vector at the PstI site. The further confirmation of the cloning was done through restriction digestion and sequencing. The T-DNA map showing the OsDJ-1C expression cassette in the pCAMBIA1300 vector is represented in Fig. 1a. The pCAMBIA1300_OsDJ-1C construct was then transformed into rice (cv. IR64) via Agrobacterium-mediated transformation (Sahoo et al. 2011). The putative transgenic plants were screened initially through genomic DNA PCR using gene-specific internal primers (Fig. 1b). The PCR-positive lines were further checked for the OsDJ-1C transcript abundance using qRT-PCR. An approximately 4.5–5.5-fold increase in the OsDJ-1C transcript was observed in overexpression lines than WT with L-4, L-8, and L-11 lines showing the highest transcript level (Fig. 1c). Primers used for cloning, screening, and qRT-PCR are listed in Suppl. Table S1.
Generation of gene construct and analysis of rice plants overexpressing OsDJ-1C. a Schematic depiction of OsDJ-1C overexpression construct generated in pCAMBIA1300 plant expression vector. This overexpression construct was used for transformation into rice cv. IR64. b Screening of putative OsDJ-1C transformed lines (L-1 to L-12) for the presence of transgene by genomic DNA PCR using gene-specific primers. Arrow indicates the presence of desired amplicon of the OsDJ-1C. WT: Wild type, M: Marker. c PCR-positive lines were further checked for OsDJ-1C transcript levels by qRT-PCR in WT and OsDJ-1C overexpression lines. Data is presented as mean ± SD, n = 3. The asterisk mark * indicates values that are significantly different from the WT. ****P < 0.0001 (the significance of variation was calculated using one-way ANOVA)
Assessment of OsDJ-1C overexpression lines for their abiotic stress tolerance and redox homeostasis at the seedling level
To further assess the role of OsDJ-1C in stress tolerance, WT and OsDJ-1C overexpression lines were grown under control conditions for 7 days. The 7-day old seedlings (Fig. 2a) were then exposed to drought stress (20% PEG) (Fig. 2b) and salinity stress (200 mM NaCl) (Fig. 2c), resulting in the appearance of severe stress symptoms after 6 days. Although stress-induced injury was observed in both transgenic and WT plants under both stress conditions but the severity was higher in WT than transgenic lines. The overexpression lines maintained better growth as evident by the extensive root development at seedling level (Fig. 2d) implying that overexpression of OsDJ-1C improves root growth and development under stress conditions. In agreement with the better root growth, the relative Na+ content in the roots of WT and OsDJ-1C overexpressing lines was determined using CoroNa Green fluorescence dye. Fluorescence imaging revealed a significantly much higher accumulation of Na+ in the roots of WT in comparison to the overexpression lines (Fig. 2e). Root elongation is inhibited by reactive oxygen species (ROS) levels in the presence of drought and salinity stress. To test whether the roots of OsDJ-1C lines have altered ROS levels, we used the ROS reactive dyes NBT to detect the levels of O2– in the roots of WT and OsDJ-1C overexpression lines. Further, staining of roots with NBT dye showed more intense staining in the roots of WT under both stresses as compared to the WT (Fig. 2d) indicating lower superoxide accumulation and therefore lower impact of oxidative stress in the roots of transgenic plants as compared to the WT. Along with the enhanced root growth, overexpression lines exhibited superior overall growth than WT as indicated by seedling FW (Fig. 2f). Under drought stress, 63% reduction in FW was observed in WT, whereas, 33% decrease was observed in overexpression lines. Under salinity stress, 56% decrease in FW was observed in WT, whereas, 33.7% decrease was observed in overexpression lines. Several other parameters were also examined to assess the impact of both stresses on WT and overexpression lines such as methylglyoxal (MG) content, GlyIII activity, GSH:GSSG ratio, total antioxidant capacity, MDA content (membrane stability index), and AGEs inhibition. We observed a significant increase in MG levels in WT (50% and 58% under drought and salinity stress, respectively) compared to overexpression lines (20% and 42% under drought and salinity stress, respectively) whereas, under control condition, overexpression lines have less accumulation of MG (20%) as compared to WT lines (Fig. 2g). Since GlyIII is involved in MG detoxification and thus provides tolerance against multiple stresses, the analysis was extended to investigate the GlyIII activity in WT and overexpression lines under both stresses. Measurement of GLYIII enzyme activity indicates that overexpression lines maintained higher GLYIII activity than WT (Fig. 2h), because reduced GSH is another substrate of the MG detoxifying mechanism via glyoxalases and its ratio with oxidized glutathione (GSSG) is widely employed as a measure of oxidative stress.We found a better GSH/GSSG ratio in overexpression lines compared to WT upon encountering drought and salinity stress (Fig. 2i). During stressful situations, ROS are a vital element that are produced within the system and have the potential to harm various cellular components. The ability of a plant to scavenge damaging ROS is connected to its antioxidant capability. We found that the overexpression lines were able to maintain higher antioxidant capacity (24% and 26% under drought and salinity, respectively) than the WT plants (16% and 18% under drought and salinity, respectively) enabling overexpression lines to thrive well under these stresses (Fig. 2j). Because ROS accumulation causes membrane damage, the MDA concentration in root samples from both the WT and OsDJ-1C overexpression lines was measured to calculate the membrane stability index (MSI), which determines the turgidity and stability of the plasma membrane under salt and drought conditions. In both WT and OsDJ-1C overexpression lines, we found that salt and drought dramatically increase lipid peroxidation, with WT having greater MDA content than OsDJ-1C overexpression lines. In WT roots, the MDA content increased by 70% under drought and by 90% under salinity, but in OsDJ-1C overexpression lines, the MDA level increased by 50% under drought and by 60% under salinity (Fig. 2k). Excess level of MG and ROS start to oxidize proteins in different ways, leading to the formation of AGEs. As a result, the total protein extracts from both WT and overexpression lines were examined for the ability to suppress AGEs production using a conventional BSA-MGO experiment (Fig. 2l). When compared to WT, transgenic plants had much stronger anti-glycation activity under both stress and normal conditions. Thus, it may be concluded that overexpression plants not only resist the buildup of MG and ROS during stress but also limit the development of AGEs. Finally, we conclude that overexpression of OsDJ-1C in rice leads to better stress tolerance and redox homeostasis at the seedling level. Overexpression lines were found to be capable of maintaining ion homeostasis and ROS levels under drought and salinity stress, enhancing plants' ability to cope with stress while maintaining growth and yield.
OsDJ-1C overexpression improves tolerance towards drought and salinity stresses at the early vegetative stage. Seeds of WT and OsDJ-1C overexpression lines were germinated for 7 days and maintained either a under control conditions or subjected to b drought stress (20% PEG), and c salinity (200 mM NaCl) for six days. d NBT staining of superoxide (O2−) in the roots of WT and OsDJ-1C overexpressing lines after stress treatment. e The Na+ accumulation in the root of both WT and transgenic plants that overexpress OsDJ-1C during the seedling stage under salinity stress was examined by staining with CoroNa Green fluorescent dye. The root tip was visualized using confocal microscopy. Several parameters were evaluated to check the performance of WT and OsDJ-1C overexpressing seedlings under stress conditions such as f seedling FW, g methylglyoxal (MG) content, h GlyIII activity (i) GSH:GSSG ratio, j total antioxidant capacity, k MDA content, and l AGEs (% inhibition). For the transgenic lines, data shown is the average of all three transgenic lines (L-4.1, L-8.3 and L-11.2) with three biological replicates. Data is presented as mean ± SD, n = 9. The asterisk mark * indicates values that are significantly different from the WT. *P < 0.05; **P < 0.01 (the significance of variation was calculated using one-way ANOVA)
Growth and physiological performance of WT and OsDJ-1C overexpressing rice plants under drought and salinity stress conditions at the reproductive stage
The response of WT and OsDJ-1C overexpressing plants to drought (water withheld till soil moisture content reduced to 30%) and salinity (EC 10 dS m–1) was examined during pre-flowering (reproductive) stage. Under conditions of drought, both the WT and transgenic lines exhibited a decrease in growth following 18 days of stress. It is noteworthy that within 3 days of rewatering, transgenic plants recovered quickly and unfolded their leaves (Fig. 3a), while the WT plants did not recover to the same extent even after 30 days of rewatering. Similarly, when subjected to salinity stress, WT plants experienced a significant decline in growth after 45 days, while the transgenic plants continued to grow with higher greenness in leaf and were able to withstand the stress until maturity (Fig. 3b). These results revealed that the OsDJ-1C overexpressing plants exhibited higher stress tolerance in comparison to WT. Root biomass was also found to be higher in OsDJ-1C overexpression lines under control, drought and salinity stress conditions than in WT (Fig. 3c, d), which may be related to increased root length to absorb enough amounts of water under stress conditions. Assessment of the overall yield of WT and overexpression lines indicated that under salinity stress, overexpression lines showed a reduction in total grain yield by 53.8%, whereas WT showed a decline of 99% (Fig. 3e). Drought stress resulted in a loss of 96.3% in the total yield of WT and a decline of 43.5% in the overexpression lines (Fig. 3e). Estimation of the antioxidant enzyme activity (ascorbate peroxidase, catalase, and superoxide dismutase), which was essential for maintaining redox balance, reveals that transgenic lines exhibit 2–3 times higher antioxidant enzyme activity under stress conditions compared to WT lines (Fig. 3f–h), suggesting that OsDJ-1C overexpression improves the antioxidant defense machinery which further improves the survival of plants under abiotic stress conditions.
Performance of WT and OsDJ-1C overexpression rice plants at the reproductive stage under drought and salinity stress as well as after recovery from drought stress conditions. Approximately 2-month-old WT and OsDJ-1C overexpression lines (L-4.1, L-8.3, L-11.2) at their pre-flowering stage were a subjected to drought stress by withholding water for 18 d followed by rewatering and b subjected to salinity stress by fortnightly irrigation with a mixture of salt solution having electrical conductivity (EC) of 10 dS/m. Growth of plants was regularly monitored and were photographed on days as mentioned. Various physiological and antioxidant enzyme related parameters were recorded after exposure to drought and salinity stress, such as c root morphology, d root biomass, e total grains/plant, f ascorbate peroxidase (APX), g catalase, and h superoxide dismutase (SOD) activity in WT and OsDJ-1C overexpression lines. Data is presented as mean ± SD, n = 3. The asterisk mark * indicates values that are significantly different from the WT. *P < 0.05; **P < 0.01 (the significance of variation was calculated using one-way ANOVA)
OsDJ-1C overexpressing rice plants exhibited improved chlorophyll fluorescence and leaf gas exchange parameters during salinity and drought stresses
To further investigate the effect of drought and salinity on photosynthetic efficiency, various photosynthetic parameters were measured after 3 days of drought stress recovery and 45 days of salinity stress. The overall quantum yield efficiency of photosystem II (PSII) was determined using Handy-PEA after exciting the plants with 3100 µmol photons m−2 s−1 and 2700 µmol photons m−2 s−1. To measure the qualitative parameters of PSII, we checked the OJIP phase of Chl a fluorescence rise in WT and OsDJ-1C overexpression lines under these stress conditions (Fig. 4). Distinct OJIP patterns and associated metrics, including initial slope, the time taken to achieve J, I, and P levels, and the area under the curve were observed in both WT and transgenic plants under conditions of drought and salinity stress. The area of OJIP transient was reduced in WT plants compared to OsDJ-1C overexpression plants under drought and salinity stress conditions which indicate reduction in the number of electrons passing through the electron transport chain (Fig. 4a). The subsequent variables were further measured. The measurement of ΦP (also referred as PSII quantum yield) defines the total energy trapped in PSII reaction centres (Fig. 4b), ΦE describes the overall energy utilized in driving electron transport chain from the trapped energy (Fig. 4c) and the total energy utilized in reducing the end electron receptors in PSI is defined by ΦR (Fig. 4d). PIABS defines the overall performance of PSII based on photon absorption (Fig. 4e) and PItotal defines the overall performance of the photosynthetic electron transport chain (Fig. 4f). During the periods of drought and salinity stress, the overexpression lines of OsDJ-1C exhibited a higher Φp, ΦE, ΦR, PIABS, and PItotal than the WT (Fig. 4b) which indicated that the OsDJ-1C overexpression lines exhibit a remarkable ability to maintain chlorophyll fluorescence under both drought and salinity stress and therefore they have enhanced photosynthetic quantum yield compared to WT (Fig. 4c–f). This suggests that the OsDJ-1C overexpression lines possess a greater capacity to withstand stress conditions and maintain optimal photosynthetic performance.
Photosynthetic efficiency of WT and OsDJ-1C transgenic lines during drought and salinity stress. a Chl a fluorescence of dark-adapted WT and OsDJ-1C transgenic plants during drought and salinity stress. The O, J, I, and P transients are noticeable in the curve. b ΦP, energy trapping (also termed as PSII quantum yield), c ΦE (also termed as electron transport after the reduction of QA to QA_), d ΦR (also termed as reduction of end acceptor molecules), e PIABS denoted as performance index based on absorption, f PItotal denoted as the performance index of overall photochemistry for WT and OsDJ-1C overexpression lines in presence of drought and salinity stress condition. For the transgenic lines, data shown here is the average of all three transgenic lines (L-4.1, L-8.3 and L-11.2) and three biological replicates for each line. Data is presented as mean ± SD, n = 9. The asterisk mark * indicates values that are significantly different from the WT. *P < 0.05; **P < 0.01; ***P < 0.001 (the significance of variation was calculated using one-way ANOVA)
Discussion
Plants are frequently subjected to extreme environmental conditions in their natural habitat, which significantly impact their cellular machinery, growth, and development, ultimately leading to a decrease in their yield. In response to these hostile conditions, plants exhibit morphological, physiological, molecular, and biochemical changes. Although much is known about how environmental factors affect development, decoding their molecular reactions to the stage of phenotypic manifestation remains a major challenge for molecular biologists. It is worth noting that the information encoded in the plant genome and its regulation in response to various stresses are crucial for the adaptability of a genotype. In addition, plants have developed unique adaptations and mechanisms to combat unfavorable conditions, including ubiquitous stress-related mechanisms across the plant kingdom. One such mechanism is driven by specific enzymes to counteract toxic compounds that arise from various cellular pathways during severe stress conditions. Methylglyoxal, an important signaling molecule, has been observed to accumulate to levels that are detrimental to cellular homeostasis. This accumulation adversely affects macromolecules such as proteins, lipids, and nucleic acids. Consequently, stress-induced mechanisms for detoxifying excess MG play a critical role in maintaining cellular homeostasis and enhancing stress tolerance. Two glyoxalase detoxification pathways exist to balance cellular MG levels: a glutathione-dependent bi-enzymatic system (GLYI and GLYII) and a glutathione-independent single enzyme (GLYIII). In the present study, we have explored the role of glutathione-independent GLYIII/OsDJ-1C through a gene overexpression approach in rice to understand the involvement of OsDJ-1C in abiotic stress tolerance.
OsDJ-1C overexpression improves abiotic stress tolerance at the seedling stage mainly by activating MG detoxification via the GSH-independent glyoxalase pathway
The seedling stage is the most sensitive stage to abiotic stresses in higher plants and various regulatory pathways are known to provide protection against these stresses (Guan et al. 2017). In order to determine the possible role of OsDJ-1C in the stress physiology of rice seedlings, a qRT-PCR based expression analysis of OsDJ-1C was carried out under different concentrations of MG and observed that OsDJ-1C showed a high level of expression at all MG concentrations (Suppl. Fig. S1) indicating that toxic metabolites act as an inducer of OsDJ-1C protein (Mustafiz et al. 2014; Ghosh et al. 2016). We also observed that OsDJ-1C overexpressing seedlings were less affected by drought and salinity stress as evident by the seedlings FW (Fig. 2f). Seedling’s FW is the major parameter indicating the overall growth of overexpressing lines compared to WT under abiotic stresses (Mishra et al. 2021). The abiotic stresses stimulate MG accumulation, which directly or indirectly interacts with and inactivates cellular micromolecules and macromolecules imposing detrimental effect on their function (Melvin et al. 2017). We advanced our study through measuring the level of MG in both overexpression and WT plants. As previously stated, the GlyIII enzyme irreversibly detoxifies MG in a glutathione-independent way (Ghosh et al. 2022; Gambhir et al. 2023). Therefore, due to enhanced GLYIII activity, MG accumulation was observed to be lower in the overexpression lines than WT under both control and stress conditions (Fig. 2g, h). The levels of reduced glutathione inside the plant cell is another vital criteria for plants to survive in harsh environmental conditions (Hasanuzzaman et al. 2017; Gupta et al. 2018). Reduced GSH with an increase in GSH/GSSG ratio has long been recognized as critical in protecting plants against a wide spectrum of heavy metals and xenobiotics (Singla-Pareek et al. 2003, 2006; Khan et al. 2020). As a result, maintaining cellular GSH range under stress could be another effective strategy to tackle diverse stress conditions. Here, GSH/GSSG ratio was also observed to be higher in overexpression lines compared to WT (Fig. 2i) suggesting the superiority of GSH-independent GLYIII pathway in alleviating stress responses in overexpression lines. According to several reports, higher levels of MG accumulation might raise levels of cellular ROS in cells and leading to oxidative stress (Xu et al. 2010; Gupta et al. 2018; Ghosh et al. 2022; Gambhir et al. 2023). Maintaining optimal ROS levels in the cell allows proper redox reactions and the regulation of several plant functions such as growth and development (Mittler 2017). Concerning this, we measured the total antioxidant capacity of overexpression lines and observed a substantial increase during both stressed conditions (Fig. 2j). Several studies have shown a considerable rise in the activity of antioxidant enzymes such as catalase, peroxidase, superoxide dismutase in response to abiotic stressors, correlating plant antioxidant capability with ROS accumulation (Singla-Pareek et al. 2008; Melvin et al. 2017; Mohanan et al. 2020, 2021). The accumulation of ROS additionally leads to membrane damage; therefore, we measured the MDA concentration in order to indirectly evaluate the degree of lipid peroxidation. MDA is a derivative of polyunsaturated fatty acids that is primarily formed during the peroxidation of membrane lipids and its levels significantly increase under stress conditions. According to Grotto et al. (2009), the greater buildup of MDA content is an indication that free radicals are being produced in more amounts. Both, the WT and OsDJ-1C overexpression lines, showed increase in MDA concentrations, as seen in Fig. 2k. However, OsDJ-1C overexpression lines had lower levels of MDA than WT, indicating less lipid peroxidation in the later. Higher levels of MG accumulation glycate proteins, lipids, and nucleic acids, resulting in the production of anti-glycation end products (AGEs) (Thornalley 2008; Gupta et al. 2018). Transgenic plants were shown to better resist MG and ROS accumulation and possessed significantly higher anti-glycation activity (Fig. 2l). Thus, we can conclude that overexpression of OsDJ-1C can improve stress tolerance in rice seedlings by increasing MG detoxification via the glyoxalase pathway in GSH-independent manner via maintaining better antioxidant machinery.
OsDJ-1C overexpression improves root architecture and reduces stress-induced cell damage
Most investigations concerning the mechanisms of abiotic stress tolerance tend to concentrate on aerial organs due to the challenges of examining roots in their natural habitat. It has been noted that aboveground and belowground organs exhibit distinct responses, and the physiological and molecular pathways leading to stress tolerance may not necessarily be identical across various tissues and organs (Ambroise et al. 2020). Taking this into consideration, we also observed the impact of abiotic stresses on the root morphology of WT and overexpression lines. Excess ions accumulate in salinity, causing ion toxicity, which causes DNA damage and therefore cell death. Uncontrolled cell death caused by long-term and adverse stress causes a significant reduction in crop productivity. Several groups have reported a correlation between Na+ buildup and cell death (Kavitha et al. 2012; Gupta et al. 2018; Lakra et al. 2019; Mishra et al. 2021). Staining of roots with CoroNa Green dye has revealed that the OsDJ-1C overexpression lines had reduced cellular Na+ ions accumulation and therefore reduced cell death in the roots compared to WT (Fig. 2e). The efficient utilization of limited resources can be possible through a healthier and more robust root system (Hannan et al. 2020). Extensive root biomass at seedling levels contributes towards the efficient utilization of soil nutrient resources that ultimately affect salinity and drought stress tolerance. OsDJ-1C overexpressing lines showed expanded root system under these stress conditions which might also be due to the adaptive response of root development responsive genes under these stresses such as ROS scavengers (Bhaskarla et al. 2020) which subsequently activate antioxidant defense system and confers stress tolerance (Zhang et al. 2014). NBT staining of roots of OsDJ-1C overexpression lines has revealed a noteworthy observation, which highlights the reduced accumulation of superoxide radicals within their roots indicating their effectively combat and counteract drought and salinity tolerance stress via combating oxidative stress (Fig. 2d).
OsDJ-1C overexpression improves plant performance under drought and salinity stress at the reproductive stage
Together with the seedling stage, the reproductive stage is equally sensitive to abiotic stresses, and the degree of damage increases as the stress intensity and duration increases (Guan et al. 2017; Salgotra and Chauhan 2023). These stresses can substantially decrease crop productivity, particularly during the reproductive stage (Salgotra and Chauhan 2023). ROS accumulation is one of the major consequences of abiotic stresses and glyoxalase pathway is the main way of ROS detoxification, as evidenced by the several reports. EcGLY-III expressing transgenic tobacco plants exhibited enhanced tolerance to several abiotic stresses than WT plants by preventing chlorophyll loss and intracellular accumulation of H2O2 (Ghosh et al. 2022). In addition, the overexpression of AtDJ-1a in Arabidopsis resulted in enhanced resistance to oxidative stress (Xu et al. 2010). According to Ghosh et al. (2016), OsDJ-1C, a member of the OsDJ-1 family, exhibited significant constitutive expression under drought and salinity stress conditions, encompassing all developmental stages and tissues of rice. Therefore, it is worthwhile to further investigate the involvement of OsDJ-1C transgenic lines during the reproductive stages in terms of their ability to withstand abiotic stress conditions and enhance crop yield. In the current study, OsDJ-1C overexpressing plants were able to grow normally and were able to better maintain their cellular machinery, resulting in enhanced tolerance to abiotic stresses at both the seedling and reproductive stages (Figs. 2, 3) which is directly connected to the higher root biomass (Fig. 3c, d) and better antioxidant enzymes activities of overexpressing lines than WT (Fig. 3f–h). Higher root biomass plays a crucial role in enhancing the ability of plants to withstand and adapt to abiotic stress tolerance. It enables plants to explore a larger volume of soil, increasing their chances of getting water in deeper soil layers (Gupta et al. 2018; van der Bom et al. 2023; Huang et al. 2023). This helps plants in surviving drought situations by ensuring enough water supply and reducing water stress (Li et al. 2023). It expands the accessible surface area for nutrient absorption, allowing plants to collect nutrients more efficiently (Yetgin 2023). This increased nitrogen absorption can assist plants in overcoming nutritional shortages induced by stress conditions. Plants with more root biomass can collect suitable solutes in their roots, such as proline or sugars, which aids in the maintenance of cellular water potential and osmotic balance during conditions of water deficiency or salinity stress (Dien et al. 2019; Yetgin 2023). Overall, increased root biomass gives plants an advantage in responding to abiotic stresses. ROS induce oxidative damage in various cellular components, including lipids, nucleic acids, and proteins, resulting in cell death and hindrance to plant growth. Antioxidant enzymes play a significant role in enhancing the ability of rice to tolerate abiotic stress by counteracting the adverse effects of ROS and the maintenance of cellular redox balance (Das et al. 2015; Singla-Pareek et al. 2003, 2006; Vijayaraghavareddy et al. 2022). In the present study, the activity of these antioxidant enzymes increases in both WT and overexpression lines in response to abiotic stresses, although the rise in activity was greater in overexpression lines than in the WT (Fig. 3f–h). Their enhanced expression and activity aid in the scavenging and detoxification of ROS, thus minimizing oxidative damage and preserving cellular redox equilibrium. This antioxidative defense mechanism is critical for rice abiotic stress tolerance because it protects cellular components, maintains membrane integrity, and maintains overall plant health under unfavorable environmental circumstances. Girotto et al. (2014) found that DJ-1 proteins from Arabidopsis and humans activate internal superoxide dismutase (SOD) to provide tolerance to oxidative stress. Upon the overexpression of Erianthus arundinaceus GLY III in sugarcane, a significant rise in SOD activity was noted in both leaf and root tissues under drought and salinity stress (Mohanan et al. 2020, 2021). Similarly, increased SOD, peroxidase, and catalase activity was detected in EcGLY-III transgenic plants under both normal and stress conditions (Ghosh et al. 2022). The overexpression of yeast Hsp31 in transgenic tobacco plants led to a remarkable rise in the expression of crucial oxidative stress genes, such as peroxidase, catalase, ascorbate peroxidase, glutathione S-transferase, manganese superoxide dismutase, and copper/zinc superoxide dismutase (Melvin et al. 2017). Thus, our findings of increased antioxidant enzyme activity are consistent with earlier research. Taken together, our findings suggest that OsDJ-1C plays a crucial role in enhancing rice plant ability to withstand the negative effect of salinity and drought stress.
OsDJ-1C overexpression lines exhibit higher energy trapping capability and regulated photochemistry under drought and salinity stress
Maximal quantum yield of PSII (Fv/Fm) is frequently utilized in numerous research studies to assess a plant’s photosynthesis-related abilities under normal and stressful conditions. This measure serves as an indirect method of quantifying photosynthesis efficiency (Kalaji et al. 2011; Stirbet et al. 2018). Studies suggest that tolerant genotypes can sustain high Fv/Fm ratios under any stressful situations (Kromdijk et al. 2016; Soda et al. 2018; Wungrampha et al. 2019). In the current study, we observed that the quantum yield of Chl a fluorescence was always maintained higher in overexpressing lines than in WT (Fig. 4). The electrons from the PSII are transported from QA to QA— under normal photosynthesis circumstances. However, under stress, such as salinity and drought in our instance, the pace of electron transport from QA to QA— is slower, that leads to the PSII reaction centres closure (Stirbet and Govindjee 2011). The restriction of electron flow during the electron transport reaction further narrows down the area of the OJIP transient rise even further (Strasser and Stirbet 2001). We have observed that the initial slope of the OJIP curve for both WT and overexpression lines declines under drought and salinity stress, indicating the closure of PSII reaction centres. Nonetheless, the rate of initial slope reduction is faster in WT than in overexpression lines, suggesting that the reaction centre closure is faster in WT than in overexpression lines (Fig. 4a). The JIP test was conducted after the fluorescence being double normalized at the Fo and Fm levels to thoroughly examine the photosynthetic parameters. All photosynthetic parameters determined using the JIP test showed that the overexpressing lines performed better than WT under drought and salinity stress conditions (Fig. 4b–f). Several articles have discussed the maximal quantum yield of PSII in the stress-tolerant genotype (Li et al. 2018a; Irakoze et al. 2019). Consequently, when all factors are taken into consideration, the overexpressing lines of OsDJ-1C demonstrated minimal yield penalty (Fig. 3e) under drought and salinity stress, as there exists a direct correlation between photosynthesis and crop yield (Long et al. 2006).
Conclusion
Taken together, OsDJ-1C from rice has emerged as a superior positive regulator of abiotic stress responses in rice. It uniquely detoxifies MG independently in a single step without the need for a cofactor and provides an alternative, fast, and glutathione-independent pathway for controlling cytotoxic MG levels. MG signaling negatively affects rice growth and root development by inhibiting various developmental and physiological processes such as seed germination, root elongation, and photosynthesis. MG accumulation correlate with enhanced ROS production and impairing the function of antioxidant enzymes in plants under abiotic stresses (Maeta et al. 2005; Yadav et al. 2005; Kalapos 1994; Gupta et al. 2018; Ghosh et al. 2022). In the present study, overexpression of OsDJ-1C in rice resulted in enhanced drought and salinity tolerance during both the seedling and reproductive stages. Under saline and drought stress, transgenic plants were able to set seeds (yield) with moderate penalty, but WT plants suffered significant loss of productivity. A thorough comparison of morphological, physiological and biochemical parameters between the WT and overexpression lines, under drought and salinity, confirmed the better performance of the OsDJ-1C overexpression plants. This advantage is attributed to their ability to effectively limit MG accumulation, maintain a more favorable GSH/GSSG ratio and higher antioxidant capacity. Transgenic plants finely balance several toxic metabolites, such as ROS and MG, and also exhibit greater anti-glycation activity. Furthermore, the overexpression lines exhibited a high level of tolerance towards abiotic stresses due to their strong and robust root system, effective regulation of ion homeostasis, controlled photosynthesis, and efficient functioning of PSII (Fig. 5). This research strongly supports the potential of OsDJ-1C in enhancing tolerance to various abiotic stresses. Such insights could pave the way for developing novel strategies for increasing agricultural output.
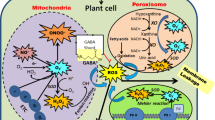
A model summarizing regulation of stress-related parameters in OsDJ-1C overexpression lines compared to the WT. Abiotic stresses lead to the accumulation of MG in higher amounts which lead to production of reactive oxygen species along with MG and Advanced Glycation End Products (AGEs) resulting in cellular toxicity and cell death. OsDJ-1C overexpression leads to efficient MG detoxification. This results in better photosynthesis, higher antioxidant capacity, better glutathione and K+/Na+ ratio, reduced ROS and AGEs accumulation together contributing to the improved plant health under abiotic stresses compared to the WT. The OsDJ-1C overexpression plants showed better root growth and maintained redox homeostasis leading to enhanced abiotic stress tolerance that ensures plant survival and boosts total yield of the plant
Data availability
The data reported in this study are contained within the manuscript and there is no associated data available.
Abbreviations
- AGEs:
-
Advanced glycation end products
- GSH/GSSG:
-
Reduced and oxidized glutathione
- MDA:
-
Malondialdehyde
- MG:
-
Methylglyoxal
- NBT:
-
Nitroblue tetrazolium
References
Alam R, Rasheed R, Ashraf MA, Hussain I, Ali S (2023) Allantoin alleviates chromium phytotoxic effects on wheat by regulating osmolyte accumulation, secondary metabolism, ROS homeostasis and nutrient acquisition. J Hazard Mater 458:131920. https://doi.org/10.1016/j.jhazmat.2023.131920
Ambroise V, Legay S, Guerriero G, Hausman JF, Cuypers A, Sergeant K (2020) The roots of plant frost hardiness and tolerance. Plant Cell Physiol 61(1):3–20. https://doi.org/10.1093/pcp/pcz196
Anwar K, Joshi R, Morales A, Das G, Yin X, Anten NP, Raghuvanshi S, Bahuguna RN, Singh MP, Singh RK, van Zanten M, Sasidharan R, Singla-Pareek SL, Pareek A (2022) Genetic diversity reveals synergistic interaction between yield components could improve the sink size and yield in rice. Food Energy Secur 11(2):e334. https://doi.org/10.1002/fes3.334
Askari-Khorasgani O, Pessarakli M (2019) Manipulation of plant methylglyoxal metabolic and signaling pathways for improving tolerance to drought stress. J Plant Nutr 42(10):1268–1275. https://doi.org/10.1080/01904167.2019.1589502
Bankapalli K, Saladi S, Awadia SS, Goswami AV, Samaddar M, D’Silva P (2015) Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae. J Biol Chem 290(44):26491–26507. https://doi.org/10.1074/jbc.M115.673624
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140. https://doi.org/10.1016/S0021-9258(19)50881-X
Bhaskarla V, Zinta G, Ford R, Jain M, Varshney RK, Mantri N (2020) Comparative root transcriptomics provide insights into drought adaptation strategies in chickpea (Cicer arietinum L.). Int J Mol Sci 21(5):1781. https://doi.org/10.3390/ijms21051781
Biswas MS, Mano J (2021) Lipid peroxide-derived reactive carbonyl species as mediators of oxidative stress and signaling. Front Plant Sci 12:720867. https://doi.org/10.3389/fpls.2021.720867
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P (2018) The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab 28(3):337–352. https://doi.org/10.1016/j.cmet.2018.08.014
Das P, Lakra N, Nutan KK, Singla-Pareek SL. Pareek A (2015) Pot level drought stress tolerance assay in tobacco through plant phenotyping and antioxidant assay. Bio-Protoc 5(19):e1605. http://www.bio-protocol.org/e1605
Dien DC, Mochizuki T, Yamakawa T (2019) Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Prod Sci 22(4):530–545. https://doi.org/10.1080/1343943X.2019.1647787
Gambhir P, Singh V, Raghuvanshi U, Parida AP, Pareek A, Roychowdhury A, Sopory SK, Kumar R, Sharma AK (2023) A glutathione-independent DJ-1/PfpI domain-containing tomato glyoxalaseIII2, SlGLYIII2, confers enhanced tolerance under salt and osmotic stresses. Plant Cell Environ 46(2):518–548. https://doi.org/10.1111/pce.14493
Garai S, Bhowal B, Kaur C, Singla-Pareek SL, Sopory SK (2021) What signals the glyoxalase pathway in plants? Physiol Mol Biol Plants 27(10):2407–2420. https://doi.org/10.1007/s12298-021-00991-7
Ghosh A (2017) Genome-wide identification of glyoxalase genes in Medicago truncatula and their expression profiling in response to various developmental and environmental stimuli. Front Plant Sci 8:836. https://doi.org/10.3389/fpls.2017.00836
Ghosh A, Kushwaha HR, Hasan MR, Pareek A, Sopory SK, Singla-Pareek SL (2016) Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci Rep 6:18358. https://doi.org/10.1038/srep18358
Ghosh A, Mustafiz A, Pareek A, Sopory SK, Singla-Pareek SL (2022) Glyoxalase III enhances salinity tolerance through reactive oxygen species scavenging and reduced glycation. Physiol Plant 174(3):e13693. https://doi.org/10.1111/ppl.13693
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol 59(2):315–318. https://doi.org/10.1104/pp.59.2.315
Girotto S, Cendron L, Bisaglia M, Tessari I, Mammi S, Zanotti G, Bubacco L (2014) DJ-1 is a copper chaperone acting on SOD1 activation. J Biol Chem 289(15):10887–10899. https://doi.org/10.1074/jbc.M113.535112
Grotto D, Maria LS, Valentini J, Paniz C, Schmitt G, Garcia SC, Pomblum VJ, Rocha JBT, Farina M (2009) Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova 32:169–174. https://doi.org/10.1590/S0100-40422009000100032
Guan Q, Liao X, He M, Li X, Wang Z, Ma H, Yu S, Liu S (2017) Tolerance analysis of chloroplast OsCu/Zn-SOD overexpressing rice under NaCl and NaHCO3 stress. PLoS ONE 12(10):e0186052. https://doi.org/10.1371/journal.pone.0186052
Gupta BK, Sahoo KK, Ghosh A, Tripathi AK, Anwar K, Das P, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL (2018) Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Environ 41(5):1186–1200. https://doi.org/10.1111/pce.12968
Hamdan MF, Karlson CKS, Teoh EY, Lau SE, Tan BC (2022) Genome editing for sustainable crop improvement and mitigation of biotic and abiotic stresses. Plants 11(19):2625. https://doi.org/10.3390/plants11192625
Hannan A, Hoque MN, Hassan L, Robin AHK (2020) Adaptive mechanisms of root system of rice for withstanding osmotic stress. In: Rahman Ansari M ur (ed) Recent advances in rice research. Intech Open, Rijeka, pp 161–180. https://doi.org/10.5772/intechopen.87472
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23(2):249–268. https://doi.org/10.1007/s12298-017-0422-2
Hasim S, Hussin NA, Alomar F, Bidasee KR, Nickerson KW, Wilson MA (2014) A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J Biol Chem 289(3):1662–1674. https://doi.org/10.1074/jbc.M113.505784
Hoque TS, Uraji M, Tuya A, Nakamura Y, Murata Y (2012) Methylglyoxal inhibits seed germination and root elongation and upregulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol 14:854–858. https://doi.org/10.1111/j.1438-8677.2012.00607.x
Hoque TS, Hossain MA, Mostofa MG, Burritt DJ, Fujita M, Tran LSP (2016) Methylglyoxal: an emerging signaling molecule in plant abiotic stress responses and tolerance. Front Plant Sci 7:1341. https://doi.org/10.3389/fpls.2016.01341
Huang L, Pei Y, Jia X, Tang X, Zhang Y, Pan Y (2023) Multi-species plantation intensifies soil water competition and groundwater depletion in a water-limited desert region. For Ecol Manag 537:120953. https://doi.org/10.1016/j.foreco.2023.120953
Irakoze W, Vanpee B, Rufyikiri G, Dailly H, Nijimbere S, Lutts S (2019) Comparative effects of chloride and sulfate salinities on two contrasting rice cultivars (Oryza sativa L.) at the seedling stage. J Plant Nutr 42(9):1001–1015. https://doi.org/10.1080/01904167.2019.1584222
Islam T, Ghosh A (2018) Genome-wide dissection and expression profiling of unique glyoxalase III genes in soybean reveal the differential pattern of transcriptional regulation. Sci Rep 8(1):4848. https://doi.org/10.1038/s41598-018-23124-9
Jana GA, Krishnamurthy P, Kumar PP, Yaish MW (2021) Functional characterization and expression profiling of glyoxalase III genes in date palm grown under abiotic stresses. Physiol Plant 172(2):780–794. https://doi.org/10.1111/ppl.13239
Kalaji HM, Govindjee BK, Kościelniak J, Żuk-Gołaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72. https://doi.org/10.1016/j.envexpbot.2010.10.009
Kalaji HM, Schansker G, Brestic M, Bussotti F, Calatayud A, Ferroni L, Goltsev V, Guidi L, Jajoo A, Li P, Losciale P, Mishra VK, Misra AN, Nebauer SG, Pancaldi S, Penella C et al (2017) Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res 132(1):13–66. https://doi.org/10.1007/s11120-016-0318-y
Kalapos MP (1994) Methylglyoxal toxicity in mammals. Toxicol Lett 73(1):3–24. https://doi.org/10.1016/0378-4274(94)90184-8
Kaur C, Kushwaha HR, Mustafiz A, Pareek A, Sopory SK, Singla-Pareek SL (2015) Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front Plant Sci 6:682. https://doi.org/10.3389/fpls.2015.00682
Kavitha PG, Miller AJ, Mathew MK, Maathuis FJM (2012) Rice cultivars with differing salt tolerance contain similar cation channels in their root cells. J Exp Bot 63(8):3289–3296. https://doi.org/10.1093/jxb/ers052
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater 399:123020. https://doi.org/10.1016/j.jhazmat.2020.123020
Khan MN, Siddiqui MH, AlSolami MA, Alamri S, Hu Y, Ali HM, Al-Amri AA, Alsubaie QD, Al-Munqedhi BMA, Al-Ghamdi A (2020) Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol Biochem 156:278–290. https://doi.org/10.1016/j.plaphy.2020.09.017
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354(6314):857–861. https://doi.org/10.1126/science.aai8878
Kwon K, Choi D, Hyun JK, Jung HS, Baek K, Park C (2013) Novel glyoxalases from Arabidopsis thaliana. FEBS J 280(14):3328–3339. https://doi.org/10.1111/febs.12321
Lakra N, Kaur C, Singla-Pareek SL, Pareek A (2019) Mapping the “early salinity response” triggered proteome adaptation in contrasting rice genotypes using iTRAQ approach. Rice 12(1):3. https://doi.org/10.1186/s12284-018-0259-5
Lee J, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C (2012) Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet 21(14):3215–3225. https://doi.org/10.1093/hmg/dds155
Li ZG (2020) Regulative role of calcium signaling on methylglyoxal-improved heat tolerance in maize (Zea mays L.) seedlings. Plant Signal Behav 15(9):1788303. https://doi.org/10.1080/15592324.2020.1788303
Li Y, Fei J, Li G, Xie H, Yang Y, Li J, Xu Y, Sun B, Xia J, Fu X, Li J (2018a) Supramolecular assembly of photosystem II and adenosine triphosphate synthase in artificially designed honeycomb multilayers for photophosphorylation. ACS Nano 12(2):1455–1461. https://doi.org/10.1021/acsnano.7b07841
Li ZG, Long WB, Yang SZ, Wang YC, Tang JH (2018b) Signaling molecule methylglyoxal-induced thermotolerance is partly mediated by hydrogen sulfide in maize (Zea mays L.) seedlings. Acta Physiol Plant 40:1–10. https://doi.org/10.1007/s11738-018-2653-4
Li PF, Ma BL, Palta JA, Wei XF, Guo S, Ding TT, Ma YQ (2023) Distinctive root system adaptation of ploidy wheats to water stress: A cue to yield enhancement. J Agron Crop Sci 209(4):566–577. https://doi.org/10.1111/jac.12641
Lin J, Nazarenus TJ, Frey JL, Liang X, Wilson MA, Stone JM (2011) A plant DJ-1 homolog is essential for Arabidopsis thaliana chloroplast development. PLoS ONE 6(8):e23731. https://doi.org/10.1371/journal.pone.0023731
Liu S, Liu W, Lai J, Liu Q, Zhang W, Chen Z, Gao J, Song S, Liu J, Xiao Y (2022) OsGLYI3, a glyoxalase gene expressed in rice seed, contributes to seed longevity and salt stress tolerance. Plant Physiol Biochem 183:85–95. https://doi.org/10.1016/j.plaphy.2022.04.028
Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29(3):315–330. https://doi.org/10.1111/j.1365-3040.2005.01493.x
Maeta K, Izawa S, Inoue Y (2005) Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 280(1):253–260. https://doi.org/10.1074/jbc.M408061200
Melvin P, Bankapalli K, D’Silva P, Shivaprasad PV (2017) Methylglyoxal detoxification by a DJ-1 family protein provides dual abiotic and biotic stress tolerance in transgenic plants. Plant Mol Biol 94(4–5):381–397. https://doi.org/10.1007/s11103-017-0613-9
Mishra M, Wungrampha S, Kumar G, Singla-Pareek SL, Pareek A (2021) How do rice seedlings of landrace Pokkali survive in saline fields after transplantation? Physiology, biochemistry, and photosynthesis. Photosynth Res 150(1–3):117–135. https://doi.org/10.1007/s11120-020-00771-6
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mohanan MV, Pushpanathan A, Sasikumar SPT, Selvarajan D, Jayanarayanan ANRAK, Ramalingam S, Karuppasamy SN, Subbiah R, Ram B, Chinnaswamy A (2020) Ectopic expression of DJ-1/PfpI domain containing Erianthus arundinaceus Glyoxalase III (EaGly III) enhances drought tolerance in sugarcane. Plant Cell Rep 39(11):1581–1594. https://doi.org/10.1007/s00299-020-02585-1
Mohanan MV, Pushpanathan A, Padmanabhan S, Sasikumar T, Jayanarayanan AN, Selvarajan D, Ramalingam S, Ram B, Chinnaswamy A (2021) Overexpression of Glyoxalase III gene in transgenic sugarcane confers enhanced performance under salinity stress. J Plant Res 134(5):1083–1094. https://doi.org/10.1007/s10265-021-01300-9
Mostofa MG, Ghosh A, Li ZG, Siddiqui MN, Fujita M, Tran LSP (2018) Methylglyoxal—a signaling molecule in plant abiotic stress responses. Free Radic Biol Med 122:96–109. https://doi.org/10.1016/j.freeradbiomed.2018.03.009
Mustafiz A, Ghosh A, Tripathi AK, Kaur C, Ganguly AK, Bhavesh NS, Tripathi JK, Pareek A, Sopory SK, Singla-Pareek SL (2014) A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J 78(6):951–963. https://doi.org/10.1111/tpj.12521
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015a) Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exper Bot 112:44–54. https://doi.org/10.1016/j.envexpbot.2014.12.001
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015b) Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant 59:745–756. https://doi.org/10.1007/s10535-015-0542-x
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015c) Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 7:plv069. https://doi.org/10.1093/aobpla/plv069
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nowicka B, Hoque TS, Khatun SM, Naim J, Hasan AK, Hossain MA (2023) Heavy‐metal‐induced reactive oxygen species and methylglyoxal formation and detoxification in crop plants: modulation of tolerance by exogenous chemical compounds. In: Hossain MA, Hossain AZ, Bourgerie S, Fujita M, Dhankher OP, Haris P (eds) Heavy metal toxicity and tolerance in plants: a biological, omics, and genetic engineering approach. Wiley, New York, pp 461–492. https://doi.org/10.1002/9781119906506.ch21
Nutan KK, Rathore RS, Tripathi AK, Mishra M, Pareek A, Singla-Pareek SL (2020) Integrating the dynamics of yield traits in rice in response to environmental changes. J Exp Bot 71(2):490–506. https://doi.org/10.1093/jxb/erz364
Pan X, Ullah A, Feng YX, Tian P, Yu XZ (2023) Proline-mediated activation of glyoxalase II improve methylglyoxal detoxification in Oryza sativa L. under chromium injury: clarification via vector analysis of enzymatic activities and gene expression. Plant Physiol Biochem 201:107867. https://doi.org/10.1016/j.plaphy.2023.107867
Peng X, Zheng Z, Cheng KW, Shan F, Ren GX, Chen F, Wang M (2008) Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem 106(2):475–481. https://doi.org/10.1016/j.foodchem.2007.06.016
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–341. https://doi.org/10.1006/abio.1999.4019
Proietti S, Falconieri GS, Bertini L, Baccelli I, Paccosi E, Belardo A, Timperio AM, Caruso C (2019) GLYI4 plays a role in methylglyoxal detoxification and jasmonate-mediated stress responses in Arabidopsis thaliana. Biomolecules 9(10):635. https://doi.org/10.3390/biom9100635
Ramu SK, Peng HM, Cook DR (2002) Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant Microbe Interact 15(6):522–528. https://doi.org/10.1094/MPMI.2002.15.6.522
Reddy VP, Aryal P, Darkwah EK (2022) Advanced glycation end products in health and disease. Microorganisms 10(9):1848. https://doi.org/10.3390/microorganisms10091848
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163(4):1609–1622. https://doi.org/10.1104/pp.113.227702
Sahoo KK, Tripathi AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods 7(1):49. https://doi.org/10.1186/1746-4811-7-49
Salgotra RK, Chauhan BS (2023) Ecophysiological responses of rice (Oryza sativa L.) to drought and high temperature. Agron 13(7):1877. https://doi.org/10.3390/agronomy13071877
Sankaranarayanan S, Jamshed M, Kumar A, Skori L, Scandola S, Wang T, Spiegel D, Samuel MA (2017) Glyoxalase goes green: The expanding roles of glyoxalase in plants. Int J Mol Sci 18(4):898. https://doi.org/10.3390/ijms18040898
Sastry MS, Korotkov K, Brodsky Y, Baneyx F (2002) Hsp31, the Escherichia coli yedU gene product, is a molecular chaperone whose activity is inhibited by ATP at high temperatures. J Biol Chem 277(48):46026–46034. https://doi.org/10.1074/jbc.M205800200
Semchyshyn HM (2014) Reactive carbonyl species in vivo: generation and dual biological effects. Sci World J 2014(10):417842. https://doi.org/10.1155/2014/417842
Singh RK, Flowers TJ (2010) The physiology and molecular biology of the effects of salinity on rice. In: Pessarakli M (ed) Handbook of plant and crop stress, 3rd edn. Taylor and Francis, Florida, pp 901–942
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Nat Acad Sci USA 100(25):14672–14677. https://doi.org/10.1073/pnas.2034667100
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140(2):613–623. https://doi.org/10.1104/pp.105.073734
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transl Res 17(2):171–180. https://doi.org/10.1007/s11248-007-9082-2
Singla-Pareek SL, Kaur C, Kumar B, Pareek A, Sopory SK (2020) Reassessing plant glyoxalases: large family and expanding functions. New Phytol 227(3):714–721. https://doi.org/10.1111/nph.16576
Soda N, Gupta BK, Anwar K, Sharan A, Govindjee S-P, Pareek A (2018) Rice intermediate filament, OsIF, stabilizes photosynthetic machinery and yield under salinity and heat stress. Sci Rep 8(1):4072. https://doi.org/10.1038/s41598-018-22131-0
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B Biol 104(1–2):236–257. https://doi.org/10.1016/j.jphotobiol.2010.12.010
Stirbet A, Lazár D, Kromdijk J, Govindjee (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 56:86–104. https://doi.org/10.1007/s11099-018-0770-3
Strasser RJ, Stirbet AD (2001) Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O-J–I–P: fitting of experimental data to three different PS II models. Math Comput Simul 56(4–5):451–462. https://doi.org/10.1016/S0378-4754(01)00314-7
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 1797(6–7):1313–1326. https://doi.org/10.1016/j.bbabio.2010.03.008
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems role in ageing and disease. Drug Metabol Drug Interact 23(1–2):125–150. https://doi.org/10.1515/dmdi.2008.23.1-2.125
van der Bom FJ, Williams A, Raymond NS, Alahmad S, Hickey LT, Singh V, Bell MJ (2023) Root angle, phosphorus, and water: interactions and effects on durum wheat genotype performance in drought-prone environments. Plant Soil. https://doi.org/10.1007/s11104-023-05966-z
Veena RVS, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17(4):385–395. https://doi.org/10.1046/j.1365-313x.1999.00390.x
Vijayaraghavareddy P, Lekshmy SV, Struik PC, Makarla U, Yin X, Sreeman S (2022) Production and scavenging of reactive oxygen species confer to differential sensitivity of rice and wheat to drought stress. Crop Environ 1(1):15–23. https://doi.org/10.1016/j.crope.2022.03.010
Wungrampha S, Joshi R, Rathore RS, Singla-Pareek SL, Govindjee PA (2019) CO2 uptake and chlorophyll a fluorescence of Suaeda fruticosa grown under diurnal rhythm and after transfer to continuous dark. Photosynth Res 142(2):211–227. https://doi.org/10.1007/s11120-019-00659-0
Xu XM, Lin H, Maple J, Björkblom B, Alves G, Larsen JP, Møller SG (2010) The Arabidopsis DJ-1a protein confers stress protection through cytosolic SOD activation. J Cell Sci 123(Pt 10):1644–1651. https://doi.org/10.1242/jcs.063222
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579(27):6265–6271. https://doi.org/10.1016/j.febslet.2005.10.006
Yetgin A (2023) Exploring the dynamic nature of root plasticity and morphology in the face of changing environments. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2023.07.008. (in Press)
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. Int Rice Res Ins, Philippines
Zhang H, Liu Y, Wen F, Yao D, Wang L, Guo J, Ni L, Zhang A, Tan M, Jiang M (2014) A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J Exp Bot 65(20):5795–5809. https://doi.org/10.1093/jxb/eru313
Zhou W, Leul M (1998) Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul 26:41–47. https://doi.org/10.1023/A:1006004921265
Acknowledgements
This work was supported by the internal funds of ICGEB, New Delhi. R.S.R. thanks Department of Science Technology (DST) for Inspire Fellowship (IF160321). The funding agency played no role in the design of the study or collection, analysis and interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, SLS-P. Data curation, RSR. Formal analysis, RSR and MM. Resources, SLS-P and AP. Supervision, SLS-P. Writing–original draft, RSR. Writing—review and editing, RSR, MM, AP, and SLS-P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathore, R.S., Mishra, M., Pareek, A. et al. A glutathione-independent DJ-1/Pfp1 domain containing glyoxalase III, OsDJ-1C, functions in abiotic stress adaptation in rice. Planta 259, 81 (2024). https://doi.org/10.1007/s00425-023-04315-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04315-9