Abstract
Methylglyoxal (MG) is a key signaling molecule resulting from glycolysis and other metabolic pathways. During abiotic stress, MG levels accumulate to toxic levels in affected cells. However, MG is routinely detoxified through the action of DJ1/PARK7/Hsp31 proteins that are highly conserved across kingdoms and mutations in such genes are associated with neurodegenerative diseases. Here, we report for the first time that, similar to abiotic stresses, MG levels increase during biotic stresses in plants, likely contributing to enhanced susceptibility to a wide range of stresses. We show that overexpression of yeast Heat shock protein 31 (Hsp31), a DJ-1 homolog with robust MG detoxifying capabilities, confers dual biotic and abiotic stress tolerance in model plant Nicotiana tabacum. Strikingly, overexpression of Hsp31 in tobacco imparts robust stress tolerance against diverse biotic stress inducers such as viruses, bacteria and fungi, in addition to tolerance against a range of abiotic stress inducers. During stress, Hsp31 was targeted to mitochondria and induced expression of key stress-related genes. These results indicate that Hsp31 is a novel attractive tool to engineer plants against both biotic and abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants being sessile are constantly challenged by various abiotic and biotic stresses in nature. These stresses usually lead to a series of morphological, physiological, molecular and biochemical changes in plants that collectively affect plant growth and development significantly (Tardieu and Tuberosa 2010; Peleg and Blumwald 2011; Roy et al. 2011; Furtado Macedo 2012). These changes are drastic and sometimes result in low productivity. However, during their extraordinary evolutionary path, plants have developed unique adaptations and mechanisms to counter variety of stresses. Every plant species often has its own array of mechanisms to cope with the unfavorable conditions imposed on them (Griffiths et al. 2002; Ahuja et al. 2010; Pareek et al. 2010; Nakaminami et al. 2012). There are also many stress-related mechanisms that are common across plants. One such mechanism includes evolution of enzymes to cope with metabolic and xenobiotic toxic substances and byproducts that result from various pathways (Bohnert et al. 2006; Szabados et al. 2011; Obata and Fernie 2012). Many enzymes also counter reactive oxygen species (ROS) that are routinely generated during stresses. Irrespective of the nature of the stress, the final toxic products and their chemical nature are often very similar. Hence, molecular and biochemical responses of diverse plants are often alike to counter different stresses (Urano et al. 2010; Luo et al. 2012; Nakaminami et al. 2012).
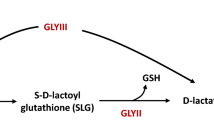
One such toxic product often generated within the cell is methylglyoxal (MG). MG is produced not just in plants, but also across all organisms and generally associated with pathological conditions in humans and other animals. Multiple metabolic pathways produce MG, and the most common biochemical reactions are associated with the glycolysis (Yadav et al. 2005a). Accumulation of these glyoxals causes various damages to cellular constituents. For instance, advanced glycated end products (AGEs) react with functional groups of many cellular enzymes and render them non-functional (Thornalley et al. 1999; Allaman et al. 2015). Among these targets, there are several key enzymes that are capable of ROS elimination, such as anti-oxidant enzymes, catalase, glutathione reductases and peroxidases that are routinely modified during MG accumulation (Shangari and O’Brien 2004). All these enhance the risk of oxidative stress during MG accumulation. In addition, the adverse effect of MG is not just limited to the proteome, but also to other key molecules such as lipids and nucleic acids (Thornalley 1996; Kasai et al. 1998). Therefore, regular detoxification of these toxic glyoxals is critical for the maintenance of cellular health in any organism. That is likely why routine MG neutralization is part of the physiological function, usually employing two enzymes that carry out important steps in eliminating accumulation of toxic intermediates, namely, glyoxalase-I and glyoxalase-II. These are two independent mechanisms in which the first step is performed by ‘methylglyoxal reductase’ and second is by ‘glyoxalase’ (Thornalley 1990). Although, MG detoxification through this mechanism is very successful in reducing their toxicity, the disadvantage in this process is the utilization of cellular glutathione (GSH) as a co-factor, which is metabolically expensive and limiting under stress conditions (Dixon et al. 1998; May et al. 1998; Szalai et al. 2009; Gill et al. 2013).
The ThiJ/DJ-1/PfpI family member proteins are widely distributed in nature and are represented in all major taxa. Despite their wide occurrence, significant studies were performed on human DJ-1 protein due to its association with neurodegenerative diseases (Lee et al. 2012). Members of these DJ-1 family proteins were identified as additional GSH independent glyoxalases present in diverse organisms. Initially, it was Hsp31 from E. coli, which was shown as a glyoxalase-III involved in converting cellular methylglyoxal into d-lactic acid (Subedi et al. 2011). A recent study demonstrated that E. coli YhbO and YajL proteins that belong to DJ-1 family, repair proteins from glycation induced by methylglyoxal and glyoxal (Abdallah et al. 2016). Saccharomyces cerevisiae encodes four members of DJ-1 proteins, namely Hsp31, 32 33 and 34, of which Hsp31 is the closest homolog of human DJ-1 and was shown to have robust methylglyoxalase activity (Bankapalli et al. 2015). Additionally, overexpression of Hsp31 enhanced stress resistance against both MG and H2O2 in S. cerevisiae. Robust methylglyoxalase activity of Hsp31 is critical in protecting yeast cells from glyoxal and oxidative stresses (Bankapalli et al. 2015). Recent findings highlight that S. cerevisiae Hsp31 exhibits chaperone activity and can prevent early stages of protein aggregation and associated cellular toxicities (Aslam and Hazbun 2016). Similarly, a DJ-1 family protein (Glx3) played an important role in conferring protection to the cells against methylglyoxal stress in a fungus, Candida albicans, indicating conservation of DJ-1 functions across major kingdoms (Hasim et al. 2014).
Human DJ-1 was shown to be effective in reducing toxic methylglyoxal levels in cells. Mutations in DJ-1 homolog is associated with onset of familiar form of Parkinson Disease (PD), a pathological condition which is characterized by progressive loss of dopaminergic neurons (Davie 2008) as well in Cruzfelt Jacob, Alzheimer and other diseases (Choi et al. 2006). These reports indicate that glyoxalase activity of DJ-1 is important for the cells to cope with glyoxal stress, and DJ-1 is critical to protect the cells from these pathological conditions. Indeed, overexpression of human DJ-1 could completely complement the function of Hsp31 in yeast indicates an unusual conservation of functions of DJ-1 family members (Bankapalli et al. 2015). Interestingly, multiple copies of these DJ-1 family proteins exist in plants, namely; DJ-1 A to F (A, B, C, D, E, and F), but their role in stress tolerance is not well understood. Out of these six members, DJ-1C in Arabidopsis thaliana was recently shown to be involved in chloroplast development (Lin et al. 2011). Furthermore, the loss of function of DJ-1A in A. thaliana was shown to cause cell death in plants, highlighting the importance of these proteins in protecting cells against various stresses (Xu et al. 2010).
Although involvement of MG in abiotic stresses in both plants and animals are well known, MG has not been implicated in biotic stresses so far. In our present study, we show that MG accumulated to higher levels during biotic stress, very similar to abiotic stress, indicating that this is a general phenomenon during stresses. Here, we report that expression of yeast Hsp31 in plants could provide extremely robust protection to cells under both abiotic and biotic stresses. Convincingly, we highlight that Hsp31 expressing transgenic plants survived better and had a higher amount of chlorophyll during various stresses tested. Expression of Hsp31 in tobacco plants resulted in striking tolerance against oxidative stress in protecting cells from lipid peroxidation and other damages to the cells. During stresses, Hsp31 was re-localized into the mitochondria, similar to human PARK7 (Junn et al. 2009), indicating functional overlaps across eukaryotes in providing cytoprotection. Together, our results provide evidence for the first time that, transgenic expression of Hsp31 in plants strongly confers protection against various cellular stresses induced by abiotic and biotic factors.
Materials and methods
Plant material and growth condition
Tobacco wild-type plants (Nicotiana tabacum cv. Wisconsin) and transgenic plants were grown under aseptic conditions on agar-solidified (MS) medium (Murashige and Skoog 1962) containing 30 g/L sucrose. Tobacco plants were maintained in the tissue culture room with 200 mmol photons/s/m under cycling light conditions (16 h light, 8 h dark).
Vector construction and transformation of plants
The transformation constructs pBIN-ScHsp31, pBIN-ScHsp31-eGFP, pBIN-ScHsp31-m1, pBIN-ScHsp31-m2 and pBIN-ScHsp31-m3 were constructed as follows. Hsp31, eGFP fused Hsp31 and Hsp31 mutants (m1, m2 and m3) genes were amplified from the pRS415 vector previously cloned by Bankapalli et al. (2015) using the following primer pairs: for the amplification of Hsp31 (720 bp) and Hsp31 mutants, Hsp31-F (5′-GCCGGGATCCATGGCCCCAAAAAAAG-3′, BamHI site in bold) and Hsp31-R (5′-GGGCGAGCTCTCAGTTTTTTAAAGCGTCG-3′, SacI site in bold) were used. For the amplification Hsp31-eGFP fusion (1.4 kb), Hsp31-F and, Hsp31-eGFP-R (5′-GGGCGAGCTCTTATTTGTATAGTTCATCC-3′, SacI site in bold) were used. The DNA fragments generated by PCR were digested with BamHI and SacI and introduced into the pBIN binary vector (Bevan 1984) which was linearized using the same restriction enzymes. DNA sequences of the recombinants were confirmed by sequencing. Transformation of tobacco plants (N. tabacum cv. Wisconsin) was performed using Agrobacterium tumefaciens (LBA4404 with pSB1 strain) as described previously (Shivaprasad et al. 2006). Transgenic plants were regenerated from kanamycin-resistant callii and maintained in the tissue culture facility for 8 weeks and later planted into soil and maintained in a green house.
RNA extraction and semi-quantitative RT-PCR analysis
Total RNA was isolated using the TRIzol® Reagent (Thermo scientific) according to the manufacturer’s instructions. For cDNA synthesis, 1 µg of RNA was treated with DNase I (Thermo scientific) to eliminate any DNA contamination, and cDNA was generated using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen).
A semi-quantitative RT-PCR analysis for the Hsp31 and Hsp31-eGFP expression was performed using the primer pairs, 5′-GCCGGGATCCATGGCCCCAAAAAAAG-3′, and 5′-GGGCGAGCTCTCAGTTTTTTAAAGCGTCG-3′. For the amplification of Hsp31-eGFP, primers Hsp31-F; 5′-GCCGGGATCCATGGCCCCAAAAAAAG-3′ and Hsp31-eGFP-R; 5′-GGGCGAGCTCTTATTTGTATAGTTCATCC-3′ were used. The semi-quantitative RT-PCR experiments were repeated twice with cDNA prepared from different batches of plant leaves.
Protein isolation and western blotting
Total protein extracts were prepared by grinding leaf material in liquid nitrogen, followed by resuspension in twofold Laemmli buffer [100 mM Tris–HCl pH 6.8, 4% SDS, 20% glycerol, 2 mM dithiothreitol (DTT), 0.01% bromophenol blue]. Before loading onto SDS–polyacrylamide gels, samples were incubated at 95 °C for 10 min. SDS-PAGE separation was performed using 12% acrylamide mini-gels. Subsequently, proteins were transferred onto nitrocellulose membranes (GE healthcare life sciences) using a Bio-Rad transfer apparatus. Membranes were probed using Hsp31 specific antibody (Bankapalli et al. 2015). Amersham ECL Prime Western Blotting Detection Reagent (GE healthcare life sciences) was used for signal detection.
Chlorophyll estimation
Chlorophyll content of the transgenic plants was determined according to Shivaprasad et al. (2011). One gram of fresh leaves from tobacco plants were taken and ground with 10 ml of 80% acetone and centrifuged at 5000 rpm for 10 min at 4 °C. The absorbance of the extract was measured at 645 and 663 nm against the solvent (80% acetone) control.
Leaf disc chlorophyll retention assay
To evaluate the stress tolerance potential of the plants over-expressing Hsp31, Hsp31 mutants and control pBIN-GFP plants, leaf disc assay was performed according to the method described by Veena and Sopory (1999). Briefly, leaf discs from transgenic plants were prepared using sterile cork borer and were incubated in the tissue culture room with appropriate concentration of stress inducer and observed after 3–4 days of incubation. The images were photographed and leaf discs were subjected to fresh weight measurement, chlorophyll estimation, methylglyoxalase activity and lipid peroxidation assay.
Estimation of MG
MG from tobacco plants (with and without stress) were estimated according to the method of Yadav et al. (2005a, b). Approximately 100 mg of leaf tissue was homogenised in 1 ml of 0.5 M perchloric acid. Extract was incubated on ice for 15 min and centrifuged at 4 °C for 20 min at 13,000 rpm. The supernatant was neutralized by incubating with saturated solution of potassium carbonate for 15 min at RT and the mixture was centrifuged at 13,000 rpm for 10 min. The neutralized supernatant was used for MG assay. In a total volume of 1 ml, 250 µl of 7.2 mM 1,2-diaminobenzene, 100 μl of 5 M perchloric acid, and 650 µl of the neutralized supernatant were added in that order. The absorbance at 335 nm of the MG derivative was recorded in a spectrophotometer. The final concentration of MG was calculated from the standard curve and expressed in terms of μmol/g fresh weight (FW).
Measurement of MDA content
Approximately 100 mg of tobacco leaves were ground to a fine powder under liquid nitrogen. 1 ml of 10% trichloroacetic acid was added to the powder and left at 4 °C overnight. The extract was centrifuged at 13,000 rpm for 20 min at 4 °C and the supernatant was transferred to a new tube. Equal volume of 0.6% thiobarbituric acid (TBA) was added to the supernatant, and the mixture was vortexed, heated in a boiling waterbath for 15 min, cooled immediately and centrifuged (13,000 rpm, 15 min at 4 °C). Absorbance of the supernatant was recorded at wavelengths of 532 and 450 nm using water as blank. The formula for the calculation of MDA content was:
Transient expression and subcellular localization of Hsp31
To examine the transient expression of GFP tagged Hsp31, Agrobacterium strain having Hsp31-eGFP construct was infiltrated into 4 week old N. benthamiana leaves and the expression was observed under UV light after 3–4 days of infiltration.
Subcellular localization of Hsp31-eGFP was observed using fluorescence microscopy. The epidermal peelings were obtained from the transgenic plants overexpressing Hsp31-eGFP subjected to 5 mM H2O2 treatment. Leaves from water treated plants served as control. The sections were observed with following set filters: DAPI (EX 340–380 nm, DM 400 nm, BA 435–485 nm) for blue fluorescence of DNA with Hoechst 33258 dye, FITC (EX 465–495 nm, DM 505 nm, BA 515–555 nm) for green fluorescence of Hsp31-eGFP, TRITC (EX 475–490 nm, DM 500–540 nm, BA 503–530 nm) for Mitotracker red (EX-excitation filter, DM-diachronic mirror, BA-absorption filter) under Delta Vision Elite Fluorescence Microscope (GE Healthcare) using 40× and 60× objective lens.
DAB Staining for H2O2
Eight-week old Hsp31, Hsp31-m1 and pBIN-GFP expressing transgenic plants were treated with 300 mM NaCl for 24 h and fourth leaf of the plant from tip was collected for DAB (3,3′-diaminobenzidine) staining as described (Daudi and O’Brien 2012). NaCl and water treated control leaves were immersed with DAB solution (1 mg/ml) and incubated in the dark for 6 h with shaking at 100 rpm. After incubation, the DAB solution was replaced with bleaching solution (ethanol:acetic acid:glycerol at a ratio of 3:1:1). The mixture was boiled for 20 min in a waterbath set at 95 °C. The bleaching solution was replaced by fresh solution and allowed to stand for 30 min. H2O2 was visualized as reddish-brown colouration in the leaf surface.
Biotic stress assays
Viral infection
Infection of Mungbean yellow mosaic virus (MYMV) in tobacco was carried out as described previously (Shivaprasad et al. 2006). Briefly, Agrobacterium strains harboring partial tandem repeats of MYMV DNA and DNA B were incubated with leaf discs derived from control and transgenic plants. Leaf discs were incubated for 10 days in MS medium before extracting nucleic acids for analysis.
Fungal infection
Alternaria alternata spores were grown on the potato dextrose agar media. The conidia of A. alternata were harvested to prepare suspensions with a concentration of 1 × 106 conidia/ml. About 5 ml of suspension was used to inoculate the leaves from aseptic seedlings of Hsp31, Hsp31-m1 and vector control pBIN-GFP. Inoculated plants were kept for 72 h to monitor the success of the inoculations. Leaf tissues from healthy aseptic tobacco seedling were the controls throughout the experiment. The symptoms were documented and infected leaves were used for the measurement of methylglyoxal.
Bacterial infection of tobacco with Pseudomonas syringae
Pseudomonas syringae pv tomato (strain DC3000) was grown at 28 °C in King’s B medium. Bacterial cells were collected by centrifugation at 4000×g for 15 min and resuspended in 10 mM MgCl2. The density of bacterial cell suspensions was adjusted to 4 × 107 colony forming units (CFU)/ml. Bacterial suspension was infiltrated into 4 week old tobacco leaves. Symptoms were recorded with a digital camera after 3 days.
Bacterial density in infected tissues were estimated in two leaf discs (1 cm in diameter) taken from infected leaf areas of two different plants at the indicated time points post infection. The discs were homogenized in 1 ml of sterile water, thoroughly mixed, and serially diluted (10−2 to 10−6) and were plated on King’s B agar. After incubation at 28 °C for 2 days, colonies were counted and represented as CFU/cm2 of the infected tissue.
Quantification of antioxidant genes
In order to validate the expression of antioxidant genes (Table 1) in the tobacco plants overexpressing Hsp31, Hsp31-m1 and pBIN-GFP under stress conditions, semi-quantitative PCR was performed using RNA samples. Total RNA extraction and cDNA preparation was discussed in the previous section. For each sample, 25 ng of cDNA was used as a template in a 20 μl PCR reaction containing 0.5 μl forward and 0.5 μl reverse primers (10 µM). PCR reactions were performed as follows: denaturation at 95 °C for 7 min, followed by 35 cycles at 95 °C for 1 min, 60 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 7 min. The PCR amplified products were separated on a 1% agarose gel and visualized. Tobacco GAPDH was used as endogenous control for the experiment.
Statistical analysis
Data were analysed separately for each experiment and were subjected to Arcsine transformation and analysis of variance (ANOVA). Further, the experimental results were subjected to Duncan’s multiple range test (DMRT) at P < 0.05 [SPSS tool (version 8)].
Results
Biotic stress induces MG accumulation in plants to levels similar to abiotic stresses
We hypothesized that levels of MG, being a product of glycolysis and other metabolic pathways, might get induced during biotic stress similar to abiotic stress. This hypothesis is largely driven by the observations that rate of glycolysis increases during stress (Kaur et al. 2014; Allaman et al. 2015). In order to explore this possibility, we infected tobacco plants with Mungbean yellow mosaic virus (MYVV) (Shivaprasad et al. 2006) and measured MG levels in tissues. High accumulation of MG was seen in tissues infected with MYVV but not in control tobacco inoculated with water control (Fig. 1a). Similarly, there was accumulation of MG during fungal infection (Alternria alternata) and during bacterial infection with Pseudomonas spp. up to several folds when compared to uninfected control. MG measurements were performed to compare the levels with abiotic stress inducers such as H2O2, NaCl and CuSO4 (Fig. 1b). Accumulation of MG was maximum during NaCl and CuSO4 induced stress.
Estimation of methylglyoxal (MG) levels in plants during biotic stress. a Determination of MG content in tobacco plants under biotic stresses. Eight-week old tobacco plants were infected with pathogens for 48 h and MG was estimated from leaf extracts. b Determination of MG content in tobacco plants under abiotic stress conditions. Eight-week old tobacco plants were treated with 300 mM NaCl, 250 μM CuSO4, 10 mM H2O2 for 48 h and MG was estimated from leaf extracts. Please refer “Materials and methods” section for details. c MG accumulation in mungbean in response to fungal, viral and bacterial infection. The data are means of three independent experiments. Bars indicate ± SE. Means designated with the asterisks (*) are significantly different according to DMRT at P < 0.05
In order to check if the accumulation of MG is true for plants other than the model plant tobacco, we used mungbean plants naturally infected with an yellow mosaic virus, a fungus (Colletotrichum Spp. causing anthracnose), and bacterial spot (Cercospora Spp.) (Fig. 1c). In agreement with the observations made in tobacco, MG levels accumulated highly during the bacterial, fungal and viral diseases in mungbean, suggesting that MG accumulation during biotic stress is likely a universal phenomenon across plants. It is important to note that mungbean plants were naturally infected with the pathogens, and hence level of MG was understandably lesser than artificially inoculated tobacco plants. Together, our results suggest that, MG accumulation is likely to be a universal phenomenon across the plants during the biotic stresses such as bacterial, fungal and viral diseases.
Transgenic tobacco expressing Hsp31 are tolerant to a wide variety of abiotic stresses
Plants harbor PARK7/Hsp31 family of proteins, but their functions in MG detoxification are less well understood. Model plant A. thaliana harbors six members of DJ-1 family (Supplementary Fig. 1) unlike a single copy of this gene in mammals. Three members of DJ-1, namely, DJ1-D, E and F, fall into yeast Hsp31 clade, while the remaining three (DJ1-A, B and C) fall into mammalian PARK7 clade. Amino acid sequence comparisons indicated that plant DJ members have less than 26–31% identity with yeast Hsp31. Since abiotic and biotic stresses in earlier experiments resulted in high accumulation of MG, we took the advantage of expressing a proven methylglyoxalase in plants and test whether this can provide cytoprotection to cells against MG toxicity induced by biotic and abiotic factors. It is also important to note that expression of yeast Hsp31 in heterologous plant systems is beneficial in avoiding issues related to stability of transgenes. Usually, introduction of genes into a plant as a transgene that has a similar homolog, generally silences one or both genes through RNA silencing (Baulcombe 2004).
Strategies involving expression of yeast proteins in transgenic plants to engineer various useful traits has been employed routinely (Eswaran et al. 2010; Padilla-Chacón et al. 2010; Ali et al. 2012; Nakahara et al. 2015). Expression of Hsp31 in yeast was very effective in offering abiotic stress tolerance, specifically against H2O2 and MG (Bankapalli et al. 2015). We explored if transgenic plants expressing Hsp31 can offer H2O2 and MG tolerance. In order to perform these experiments, we generated transgenic tobacco plants by introducing Hsp31 driven by Cauliflower mosaic virus 35 S promoter (Fig. 2a). Tobacco transformation was performed using well-established Agrobacterium-mediated leaf disc method. Multiple transgenic plants expressing high levels of Hsp31 were obtained (Fig. 2b). These transgenic plants also expressed Hsp31 transgene RNA and protein as expected (Fig. 2c, d). Transgenic plants derived from vector control were generated and used for comparison in all further experiments. Strikingly, transgenic plants expressing Hsp31 were healthier than the vector control transgenic plants. In support to this observation, Hsp31 expressing transgenic plants showed higher accumulation of chlorophyll (Fig. 2e).
Generation of transgenic tobacco plants expressing Hsp31. a Schematic representation of the T-DNA region of pBIN binary vector construct used for Agrobacterium-mediated transformation. LB left border, RB right border; 35S Pro, CaMV 35S promoter; Pnos, Nopaline synthase promoter; Tnos, Nos terminator; nptII, Kanamycin resistance gene; Hsp31, Saccharomyces cerevisiae heat shock protein 31. b Phenotype of control and Hsp31 transgenic plants. c Transgene expression analysis for Hsp31 transgenic plants. GAPDH was used as loading control. d Immuno-blot analysis of Hsp31 protein accumulation in tobacco plants. About 40 μg of total protein from each sample were separated in a 12% SDS-PAGE gel and blot was probed with polyclonal anti-Hsp31 antibody (1:2000 dilution) and detected as described in methods. e Determination of total chlorophyll content of the tobacco plants. The data are means of three independent experiments. Bars indicate ± SE. Means designated with the asterisks (*) are significantly different according to DMRT at P < 0.05
Leaf discs derived from transgenic and control plants (transformed with vector control, pBIN19) were incubated with various concentrations of H2O2. As reported previously, H2O2 levels of 10–50 mM is toxic to tobacco plants (Willekens et al. 1997). Leaf discs derived from Hsp31 alone provided tolerance against H2O2 (Fig. 3a). This is confirmed by comparing fresh weight (Fig. 3b) and taking measurements of chlorophyll in stress treated leaf discs (Fig. 3c). In all parameters, Hsp31 overexpressing plants were capable of offering tolerance against stress inducers, while control transgenic plants were susceptible. Similarly, Hsp31 expressing plants offered tolerance against MG as seen in leaf disc assays (Fig. 3d), dry weight (Fig. 3e) and chlorophyll measurements (Fig. 3f). These results confirm that Hsp31 expression, similar to yeast complementation experiments as described previously (Bankapalli et al. 2015), offer H2O2 and MG stress tolerance in plants.
Oxidative stress tolerance in Hsp31 transgenic tobacco plants. a Leaf disc senescence assay under H2O2 stress. Leaf discs were incubated with different concentrations of H2O2 (0, 10, 20, 30 and 50 mM). b Fresh weight of the tobacco plant leaf discs exposed to H2O2 stress. c Total chlorophyll content of the tobacco plant leaf discs exposed to H2O2 stress. d Phenotype of leaf discs under MG stress. e Fresh weight of the tobacco plant leaf discs exposed to MG stress. f Total chlorophyll content of the tobacco plant leaf discs exposed to MG stress. Chlorophyll retention assays were performed as described in “Materials and methods” section. Rest of the details are similar to Fig. 2 legend
Since Hsp31 expressing transgenic plants are tolerant to H2O2 and MG, we explored if they can offer tolerance to other abiotic stress inducers such as salt, CuSO4 and osmotic stress inducers. In experiments involving stress treatments using NaCl and CuSO4, Hsp31 offered high tolerance compared to vector control transgenic plants, as seen in the unbleached phenotypes of leaf discs (Fig. 4a, d), dry weight measurements (Fig. 4b, e) and chlorophyll estimation (Fig. 4c, f). Similarly, during osmotic stress, Hsp31 transgenic plants offered significant tolerance when compared to control plants (Supplementary Fig. 2). We utilized two osmotic stress inducers, namely, mannitol and polyethylene glycol. In stress treatments involving both osmotic stress inducers, Hsp31 transgenic plants offered high tolerance to stresses as quantified using fresh weight (Supplementary Fig. 2B and E) and chlorophyll estimations (Supplementary Fig. 2C and F). Multiple, independently generated transgenic plants expressing Hsp31 also exhibited tolerance against range of abiotic stresses (Supplementary Fig. 3). These results conclusively indicate that expression of Hsp31 offers robust abiotic stress tolerance in transgenic plants.
Salinity and heavy metal stress tolerance in Hsp31 transgenic plants. a Leaf discs derived from control and transgenic plants under salt stress. Fresh weight b and chlorophyll content c of leaf discs exposed to salt stress. d Leaf discs under CuSO4 stress. e Fresh weight of the tobacco plant leaf discs exposed to CuSO4. f Total chlorophyll content of the leaf discs exposed to CuSO4 stress. The values are mean ± SD from three independent experiments. Refer Fig. 2 legend for further details
Mutations in catalytic domain abolish stress tolerance activity of Hsp31 in plants
It has been observed that catalytic triad of Hsp31 (Bankapalli et al. 2015), PARK7 (Lee et al. 2012) and its homologs in bacteria (Subedi et al. 2011) have conserved catalytic domain that is essential for the MG detoxification activity (Bankapalli et al. 2015). In experiments involving PARK7 and Hsp31, mutants in the catalytic triad are ineffective in offering stress tolerance against H2O2. To study if the mutations in Hsp31 result in abolishing the activity, we raised transgenic plants with three mutant versions of Hsp31 (Fig. 5a, c). These mutations (C138A, H139A and E170A) were generated in the catalytic domain of Hsp31 (Fig. 5b). Unlike the Hsp31 overexpressing transgenic plants, mutants had chlorophyll levels comparable to control vector transformed and wild type untransformed plants (Fig. 5c, e). These plants expressed transgene mRNAs and proteins to levels similar to wild type Hsp31 transgenic plants, indicating that mutants are stable (Fig. 5d and Supplementary Fig. 4). Leaf discs derived from one of the Hsp31 mutant did not offer tolerance against a range of abiotic stress inducers (Fig. 5f). These results indicate that catalytic triad of Hsp31 are indeed essential, and the mechanism of stress tolerance offered by Hsp31 in plants is similar to yeast and other organisms.
Mutations in catalytic triad of Hsp31 abolishes stress tolerance. a Schematic representation of T-DNA region of binary vectors generated with Hsp31 mutants. Three mutants are Hsp31-m1 (C138A), Hsp31-m2 (H139A) and Hsp31-m3 (E170A). b Structure of Hsp31 indicating catalytic Cysteine, Histidine and Glutamine residues. c Phenotype of transgenic tobacco plants expressing Hsp31 mutants. d RT-PCR analysis confirming expression of mutant transgenic plants. GAPDH serves as internal control. e Comparison of total chlorophyll content between wild type, mutants and Hsp31 transgenic plants. f Leaf disc senescence assay for Hsp31 mutants under stress. Stress conditions used are H2O2 (10 mM), MG (10 mM), CuSO4 (250 µM) and NaCl (300 mM)
Transgenic Hsp31 expressing plants offer biotic stress tolerance in tobacco
Since MG levels are unregulated during diverse biotic stresses induced by fungal, bacterial and viral pathogens, we explored if Hsp31 expressing transgenic tobacco plants offers stress tolerance against these pathogens. To test this possibility, transgenic and control tobacco plants were exposed to Alternaria Spp. Three days of post-inoculation with the conidia, control untransformed as well as the transgenic vector transformed plants exhibited typical leaf spot symptoms accompanied with yellowing. Strikingly, upon inoculation, Hsp31 expressing plants but not the control plants remained healthy for more than 10 days, with occasional leaf spots that are too small while the leaves remained green (Fig. 6a and Supplementary Fig. 5). MG measurements from these plants also indicated that level of MG was much lower in Hsp31 transgenic plants when compared to control plants that are challenged with the fungus (Fig. 6b). We next infected tobacco plants with a member of Geminiviridae, with a ssDNA genome. Inoculation of infectious clones of MYMV to Hsp31 transgenic plants showed resistance to the pathogen, while control plants indicated successful infection as quantified with an RT-PCR analysis for the viral gene AC2 (Fig. 6c). Additionally, we infected tobacco plants with a bacterial pathogen, P. syringae pv tomato DC3000. Typical hypersensitive response was observed in control plants and Hsp31-m1, while plants harboring wild type Hsp31 offered extreme resistance to the pathogen (Fig. 6d). Density of bacteria was correspondingly much lower in Hsp31 overexpressing plants than in control plants (Fig. 6e, f). These experiments clearly indicate that Hsp31 overexpression in transgenic plants can provide wide-spectrum resistance to diverse stress inducers.
Hsp31 overexpressing tobacco plants offer biotic stress tolerance. a Symptoms of the brown spot disease in transgenic and control plants after 7 days after infection with Alternaria alternata, the characteristic necrosis symptom was visible on the infected tobacco leaves (arrow). b MG content in the tobacco leaves after the fungal infection. c RT-PCR analysis for MYMV early gene AC2 in transgenic and control plants. MYMV was introduced to leaf discs as described in “Materials and methods” section. d Phenotypes of the tobacco leaves derived from pBIN-GFP, Hsp31 and Hsp31-m1 plants inoculated with P. syringae. e Density of P. syringae in transgenic and control tobacco plants. Tissue homogenate from the lesion area of the infected leaves were serially diluted and spread on King’s B agar plates after 2 days of incubation. f Estimation of bacterial density on the infected tobacco leaves (10−6 dilution). The data are means of three independent experiments. Bars indicate ± SE. Means designated with the asterisks (*) are significantly different according to DMRT at P < 0.05
Mechanism of Hsp31 action in protecting transgenic plants from abiotic and biotic stresses
In order to understand the ability of Hsp31 to suppress biotic and abiotic stresses of seemingly diverse nature, we measured if indeed Hsp31 could reduce MG levels in transgenic plants. In leaf discs derived from three stress treatments, MG levels were much lower in transgenic plants expressing Hsp31 when compared to control plants indicating that the mechanism may be similar to yeast (Bankapalli et al. 2015) and humans (Lee et al. 2012) (Supplementary Fig. 6). In all tested conditions, Hsp31 mutant was unable to detoxify MG and did not offer tolerance to stresses. In order to obtain further insight about the mechanism of action, we measured lipid peroxidation in the stress-treated plants. Lipid peroxidation is a well-established mechanism of cellular injury and is routinely used as an indicator of oxidative stress in cells and tissues (Niki et al. 2005). It was previously known that increased MG stress induces oxidative stress in the cells that can further lead to membrane peroxidation (Yadav et al. 2005a, b; Thornalley 2008). Therefore, to examine oxidant status and lipid peroxidation in tobacco plants treated with stress inducers, levels of malondialdehyde (MDA), an end-product of lipid peroxidation, were measured. Interestingly, a three-fold-lower MDA level were seen in transgenic plants expressing Hsp31 when compared to control plants and Hsp31 mutant plants (Supplementary Fig. 7A and B). As expected, H2O2 treatment generally produced higher levels of MDA in cells when compared to salt and MG stress. Staining for 3,3-diaminobenzidine (DAB) to detect the presence and distribution of H2O2 in the salt treated plants indicated that Hsp31 overexpressing transgenic plants accumulated lower levels of H2O2 during stress (Supplementary Fig. 8). Together, these observations reveal a broad-spectrum multi-faceted property of Hsp31 in providing stress tolerance.
Exogenous application of MG led to widespread changes in gene expression in rice seedlings (Kaur et al. 2015). Several of these genes were stress-related indicating a cross talk between MG responsive and stress-related genes. Our results also suggested a collective stress response mediated by Hsp31 in the transgenic plants, and we hypothesized that these may include changes in expression of known stress-related genes. RNA expression analysis using Hsp31 expressing transgenic plants revealed that expression of key genes such as NtCAT1 (N. tabacum Catalase 1), NtPER12 (N. tabacum Peroxidase 12) and NtCOX1 (N. tabacum Cytochrome C oxidase 1) were induced during stress (Supplementary Fig. 9). Surprisingly, Hsp31 expressing transgenic plants also exhibited upregulation of GST indicating a feedback mechanism that is not well known previously. GST levels were not upregulated in control plants lacking Hsp31 during stress indicating that GST upregulation is specific to Hsp31 expressing plants. This is very surprising since GST is also involved in MG detoxification as suggested by the previous reports (Dixon et al. 1998; Hossain et al. 2012; Hasim et al. 2014). GST is also a stress marker that catalyzes the conjugation of glutathione (GSH) to electrophilic compounds through thioether linkages (Boyland and Chasseaud 1967). It has also been reported that glutathionylation by GST is necessary for the activation of certain redox related enzymes for the maintenance of intracellular H2O2 (Rhee et al. 2005). In addition, expression levels of few superoxide dismutases were reduced in Hsp31 overexpressing transgenic plants during stresses and implication of this is not clearly understood.
In yeast, Hsp31 is redirected to mitochondria during H2O2 stress, and this was a critical event influencing protection of the organelles during stress conditions (Bankapalli et al. 2015). Similarly, PARK7 is redirected to mitochondria during oxidative stress in humans (Canet-Avilés et al. 2004). Various studies have indicated that mitochondrial targeting of DJ-1 protein is integral part of the stress tolerance mechanism (Morcos et al. 2008; Hao et al. 2010; Wang et al. 2012). This is consistent with our RNA analysis where mitochondrial COX1 was upregulated during stress in Hsp31 transgenic plants (Supplementary Fig. 9). In order to explore if Hsp31 is redirected to mitochondria during stress in transgenic plants, we used a GFP fusion construct of Hsp31 and introduced into plants as a reporter (Supplementary Fig. 10A and B). GFP fusion did not destabilize Hsp31, and the protein expressed at high levels in plant leaves (Supplementary Fig. 4). We subjected transgenic tobacco tissues to abiotic stresses and tracked localization of Hsp31-GFP fusion protein (Fig. 7 and Supplementary Fig. 10C). As expected, control plants lacking GFP did not show any signal, while GFP protein was localized everywhere in the cell in GFP expressing plants. On the other hand, Hsp31-GFP fusion protein was expressed both in nucleus and cytoplasm. Interestingly, during stress, Hsp31 was re-localized into mitochondria. Mitotracker-Red was used as an indicator for the visualization of mitochondria. These results indicated that Hsp31 localization is altered during stress as shown previously in yeast and humans, and validates cross-kingdom conservation of functions of DJ-1 family proteins.
Subcellular localization of Hsp31 during stress. Fluorescence images of the subcellular localization of Hsp31 by GFP tagging. The sections were made from the transgenic Hsp31, pBIN-GFP vector control and wild-type tobacco plants that were treated with and without 5 mM H2O2 for 24 h. DAPI and Mitotracker red were used for the labeling of nucleus and mitochondria respectively. Scale 50 μm
Discussion
Numerous studies have shown the detrimental effects of biotic and abiotic stresses, however, most of those studies have largely focused on abiotic stress and its consequence in plants. Few studies have focused on finding of common changes that are detrimental to plants during biotic stress induced by diverse pathogens. These effects are mostly intervened by abundant metabolic changes in cells and accumulation of these metabolic intermediates are normally harmful in nature. In our present study, we demonstrate that, apart from many abiotic stresses, biotic stresses such as viral, fungal and bacterial infections also cause enhanced accumulation of toxic metabolites in the affected cells leading to disease. It is possible to envision that accumulation of such harmful intermediates might further dampen the tolerance and resistance mechanisms of the host cells, thereby leading to additional susceptibility to a wide range of stresses. Even though conventional mechanisms have been described to cope with a plethora of stresses, there is also an unmet demand for advanced mechanisms and candidates to engineer multiple stresses in plants. In addition, pyramiding of multiple genes and mechanisms that can effectively equip plants against multiple stresses through traditional and genetic engineering approaches is cumbersome. Here we show, upon transgenic expression of a single-gene Hsp31, which was reported as a robust methylglyoxalase, plants showed extraordinary tolerance to both abiotic and biotic stresses.
Our approach of using Hsp31 to regulate toxic MG is on par with the examples that involved manipulation of abiotic and biotic stress regulators in plants such as those involved in boosting flavonoid biosynthesis (Treutter 2005; Nakabayashi et al. 2014) and polyamine biosynthesis (Gill and Tuteja 2010; Hussain et al. 2011). These substances are well known mediators of abiotic stress tolerance; however, recent results indicate their involvement in biotic stress tolerance as well (Hussain et al. 2011). Priming against both biotic and abiotic stress involves accumulation of similar metabolic intermediates and cellular byproducts (Conrath et al. 2006). Priming has been recently proposed as a novel idea to engineer biotic and abiotic stress tolerance. In addition, heterologous expression of proteins from non-plant sources has been employed with adequate success in plants (Padilla-Chacón et al. 2010; Ali et al. 2012). A targeted approach to engineer both biotic and abiotic stress tolerance was achieved through introduction of a heterologous modified gene coding for an antimicrobial peptide in potato (Goyal et al. 2013). Heterologous expression of fungal chitinase in transgenic tobacco plants also conferred abiotic and biotic stress tolerance through enhanced peroxidase activity (de las Mercedes Dana et al. 2006). Our study indicates that manipulating levels of MG, which also acts as a signal molecule, is able to provide general stress tolerance against abiotic and biotic stresses and thus offers a new strategy for genetic engineering and plant breeding.
Hsp31 is a homodimeric protein structurally similar to human PARK7 (DJ-1 member), a PD-linked protein, and both are members of the DJ-1/ThiJ/PfpI superfamily. An emerging view is that Hsp31 and its family members in diverse organisms have multiple, often overlapping functions in regulating various types of cellular stresses. Hsp31 has several biochemical activities, including chaperone and detoxifying activities that modulate at different points of a stress pathway such as toxicity associated with protein misfolding (Tsai et al. 2015; Aslam and Hazbun 2016). Due to wide-spread interest in managing human neurodegenerative diseases that are linked to MG and DJ-1 proteins, it has been proposed that research using plant models such as Arabidopsis could play a vital role in understanding functions of these proteins (Xu and Møller 2010). So far, a clear understanding of the functions of DJ-1 family members in plants is lacking and it will be interesting to understand how plants have evolved mechanisms of their own to counter MG accumulation during stresses. In the current study, we show that yeast Hsp31 can provide diverse stress tolerance capabilities in plants. Convincingly, these outcomes are consistent with the earlier observations where human DJ-1 completely complemented the functions of Hsp31 in maintaining ROS homeostasis in yeast (Bankapalli et al. 2015). In our present report, we also found that, expression of Hsp31 in plants induced few stress responsive genes such as CAT1, GST21, which are indeed critical in protection against stresses. Cellular GSTs are multifunctional enzymes that are major players in cellular detoxification against superoxide radicals and other endogenous toxic metabolites (Allocati et al. 2009). Furthermore, differential localization of GSTs in the subcellular compartments is a phenomenon by which cell maintains redox status of its organelles (Raza 2011). Our observations are also consistent with the previous studies where expression of DJ-1 resulted in enhancement of cellular GSH levels under oxidative stress conditions (Zhou and Freed 2005). These results strongly suggest that heterologous expression of yeast Hsp31 in plants is able to provide severe metabolic stress protection by enhancing cellular GST levels as one of the mechanisms. Since very few candidate genes in plants are associated with both biotic and abiotic stress tolerance; we believe Hsp31 could be an ideal and a potential candidate to cope with these stresses. Further, studies on plant DJ-1 members might unravel even potent candidates to engineer plants and use in breeding strategies, especially if there is diversity in the sequences of genes so that silencing of transgenes could be avoided.
Based on homology, it is known that plant DJ-1 proteins resemble partially with yeast Hsp31 and human DJ-1 (nearly 26 to 31% homology, depending on the DJ-1 member). Intriguingly, both human and yeast DJ-1 homologs share a common feature in the formation of homo-dimerization of protein (Choi et al. 2006; Wei et al. 2007; Tsai et al. 2015). Unlike human and yeast DJ-1 proteins, plant DJ-1 members are bigger proteins, roughly double in size. We envision that plant DJ-1 proteins might bypass homo-dimerization since they are already pseudo-dimers. Such a strategy might help plants to respond to stress in a robust way, perhaps by bypassing requirements of potential cofactors. Several studies have shown that protective role by DJ-1 against oxidative stress is due to the presence of free cysteine residue (Taira et al. 2004; Wilson 2011). Strikingly, oxidation of cysteine in human DJ-1 is known to be associated with numerous functions (Zhou et al. 2006). Previous studies with yeast Hsp31 has also proven that the free cysteine residue is well conserved and is essential for its enzymatic activity (Bankapalli et al. 2015). These reports highlight the importance of free cysteine residue present in DJ-1 homologs, whose activity significantly depends on the modification of this conserved residue. Interestingly, most of the plant DJ-1 homologs also harbor this free cysteine in their sequences. Based on the structural and sequential similarities as detailed above, we presume plant DJ-1 family proteins perhaps work in a similar or even superior manner in protecting cells against various stresses.
References
Abdallah J, Mihoub M, Gautier V, Richarme G (2016) The DJ-1 superfamily members YhbO and YajL from Escherichia coli repair proteins from glycation by methylglyoxal and glyoxal. Biochem Biophys Res Commun 470:282–286. doi:10.1016/j.bbrc.2016.01.068
Ahuja I, de Vos RCH, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674. doi:10.1016/j.tplants.2010.08.002
Ali W, Isner JC, Isayenkov SV et al (2012) Heterologous expression of the yeast arsenite efflux system ACR3 improves Arabidopsis thaliana tolerance to arsenic stress. New Phytol 194:716–723. doi:10.1111/j.1469-8137.2012.04092.x
Allaman I, Bélanger M, Magistretti PJ (2015) Methylglyoxal, the dark side of glycolysis. Front Neurosci. doi:10.3389/fnins.2015.00023
Allocati N, Federici L, Masulli M, Di Ilio C (2009) Glutathione transferases in bacteria. FEBS J 276:58–75. doi:10.1111/j.1742-4658.2008.06743.x
Aslam K, Hazbun TR (2016) Hsp31, a member of the DJ-1 superfamily, is a multitasking stress responder with chaperone activity. Prion 10:103–111. doi:10.1080/19336896.2016.1141858
Bankapalli K, Saladi S, Awadia SS et al (2015) Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae. J Biol Chem 290:26491–26507. doi:10.1074/jbc.M115.673624
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363. doi:10.1079/IVP2004619
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721. doi:10.1093/nar/12.22.8711
Bohnert HJ, Gong Q, Li P, Ma S (2006) Unraveling abiotic stress tolerance mechanisms-getting genomics going. Curr Opin Plant Biol 9:180–188. doi:10.1016/j.pbi.2006.01.003
Boyland E, Chasseaud LF (1967) Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem J 104:95–102
Canet-Avilés RM, Wilson MA, Miller DW et al (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 101:9103–9108. doi:10.1073/pnas.0402959101
Choi J, Sullards MC, Olzmann JA et al (2006) Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem 281:10816–10824. doi:10.1074/jbc.M509079200
Conrath U, Beckers GJM, Flors V, et al (2006) Priming: getting ready for battle. Mol Pant-Microbe Interact 19:1062–1071. doi:10.1094/MPMI-19-1062
Daudi A, O’Brien JA (2012) Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-protocol 2:e263. doi:10.1007/BF00139728.5. http://www.bio-protocol.org/e263
Davie CA (2008) A review of Parkinson’s disease. Br Med Bull 86:109–127. doi:10.1093/bmb/ldn013
de las Mercedes Dana M, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730. doi:10.1104/pp.106.086140
Dixon DP, Cummins I, Cole DJ, Edwards R (1998) Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol 1:258–266. doi:10.1007/s00299-002-0545-x
Eswaran N, Parameswaran S, Sathram B et al (2010) Yeast functional screen to identify genetic determinants capable of conferring abiotic stress tolerance in Jatropha curcas. BMC Biotechnol 10:23. doi:10.1186/1472-6750-10-23
Furtado Macedo A (2012) Abiotic stress responses in plants. Springer, Berlin. doi:10.1007/978-1-4614-0634-1
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33. doi:10.4161/psb.5.1.10291
Gill SS, Anjum NA, Hasanuzzaman M et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212. doi:10.1016/j.plaphy.2013.05.032
Goyal RK, Hancock REW, Mattoo AK, Misra S (2013) Expression of an engineered heterologous antimicrobial peptide in potato alters plant development and mitigates normal abiotic and biotic responses. PLoS ONE. doi:10.1371/journal.pone.0077505
Griffiths H, Parry MAJ, Hsiao T (2002) Plant responses to water stress. Annu Rev Plant Physiol 89:801–802
Hao L-Y, Giasson BI, Bonini NM (2010) DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci USA 107:9747–9752. doi:10.1073/pnas.0911175107
Hasim S, Hussin NA, Alomar F et al (2014) A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in candida albicans. J Biol Chem 289:1662–1674. doi:10.1074/jbc.M113.505784
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37. doi:10.1155/2012/872875
Hussain SS, Ali M, Ahmad M, Siddique KHM (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311. doi:10.1016/j.biotechadv.2011.01.003
Junn E, Jang WH, Zhao X et al (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 87:123–129. doi:10.1002/jnr.21831
Kasai H, Iwamoto-Tanaka N, Fukada S (1998) DNA modifications by the mutagen glyoxal: adduction to G and C, deamination of C and GC and GA cross-linking. Carcinogenesis 19:1459–1465. doi:10.1093/carcin/19.8.1459
Kaur C, Singla-Pareek SL, Sopory SK (2014) Glyoxalase and Methylglyoxal as Biomarkers for Plant Stress Tolerance. Crit Rev Plant Sci 33:429–456. doi:10.1080/07352689.2014.904147
Kaur C, Kushwaha HR, Mustafiz A et al (2015) Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front Plant Sci 6:682. doi:10.3389/fpls.2015.00682
Lee JY, Song J, Kwon K et al (2012) Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet 21:3215–3225. doi:10.1093/hmg/dds155
Lin J, Nazarenus TJ, Frey JL et al (2011) A plant DJ-1 homolog is essential for Arabidopsis thaliana chloroplast development. PLoS ONE. doi:10.1371/journal.pone.0023731
Luo M, Liu X, Singh P, et al. (2012) Chromatin modifications and remodeling in plant abiotic stress responses. Biochim Biophys Acta 1819:129–136. doi:10.1016/j.bbagrm.2011.06.008
May MJ, Vernoux T, Leaver C et al (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667. doi:10.1093/jxb/49.321.649
Morcos M, Du X, Pfisterer F et al (2008) Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 7:260–269. doi:10.1111/j.1474-9726.2008.00371.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nakabayashi R, Yonekura-Sakakibara K, Urano K et al (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379. doi:10.1111/tpj.12388
Nakahara Y, Sawabe S, Kainuma K et al (2015) Yeast functional screen to identify genes conferring salt stress tolerance in Salicornia europaea. Front Plant Sci 6:920. doi:10.3389/fpls.2015.00920
Nakaminami K, Matsui A, Shinozaki K, Seki M (2012) RNA regulation in plant abiotic stress responses. Biochim Biophys Acta 1819:149–153. doi:10.1016/j.bbagrm.2011.07.015
Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun 338:668–676. doi:10.1016/j.bbrc.2005.08.072
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69:3225–3243. doi:10.1007/s00018-012-1091-5
Padilla-Chacón D, Cordoba E, Olivera T et al (2010) Heterologous expression of yeast Hxt2 in arabidopsis thaliana alters sugar uptake, carbon metabolism and gene expression leading to glucose tolerance of germinating seedlings. Plant Mol Biol 72:631–641. doi:10.1007/s11103-010-9602-y
Pareek A, Sopory SK, Bohnert HJ (2010) Abiotic stress adaptation in plants. Physiol Mol Genom Found. doi:10.1007/978-90-481-3112-9
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295. doi:10.1016/j.pbi.2011.02.001
Raza H (2011) Dual localization of glutathione S-transferase in the cytosol and mitochondria: Implications in oxidative stress, toxicity and disease. FEBS J 278:4243–4251. doi:10.1111/j.1742-4658.2011.08358.x
Rhee SG, Yang K-S, Kang SW et al (2005) Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal 7:619–626. doi:10.1089/ars.2005.7.619
Roy SJ, Tucker EJ, Tester M (2011) Genetic analysis of abiotic stress tolerance in crops. Curr Opin Plant Biol 14:232–239. doi:10.1016/j.pbi.2011.03.002
Shangari N, O’Brien PJ (2004) The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol 68:1433–1442. doi:10.1016/j.bcp.2004.06.013
Shivaprasad PV, Thillaichidambaram P, Balaji V, Veluthambi K (2006) Expression of full-length and truncated Rep genes from Mungbean yellow mosaic virus-Vigna inhibits viral replication in transgenic tobacco. Virus Genes 33:365–374. doi:10.1007/s11262-006-0077-5
Shivaprasad PV, Dunn RM, Santos BA et al (2011) Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. EMBO J 31:257–266. doi:10.1038/emboj.2011.458
Subedi KP, Choi D, Kim I et al (2011) Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol Microbiol 81:926–936. doi:10.1111/j.1365-2958.2011.07736.x
Szabados L, Kovács H, Zilberstein A, Bouchereau A (2011) Plants in extreme environments: importance of protective compounds in stress tolerance. Adv Bot Res. doi:10.1016/B978-0-12-387692-8.00004-7
Szalai G, Kellos T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80. doi:10.1007/s00344-008-9075-2
Taira T, Saito Y, Niki T et al (2004) DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 5:213–218. doi:10.1038/sj.embor.7400074
Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Curr Opin Plant Biol 13:206–212. doi:10.1016/j.pbi.2009.12.012
Thornalley PJ (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269:1–11
Thornalley PJ (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification: a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol 27:565–573. doi:10.1016/0306-3623(95)02054-3
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol Drug Interact 23:125–150. doi:10.1515/DMDI.2008.23.1-2.125
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344:109–116. doi:10.1042/bj3440109
Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7:581–591. doi:10.1055/s-2005-873009
Tsai CJ, Aslam K, Drendel HM et al (2015) Hsp31 is a stress response chaperone that intervenes in the protein misfolding process. J Biol Chem 290:24816–24834. doi:10.1074/jbc.M115.678367
Urano K, Kurihara Y, Seki M, Shinozaki K (2010) “Omics” analyses of regulatory networks in plant abiotic stress responses. Curr Opin Plant Biol 13:132–138. doi:10.1016/j.pbi.2009.12.006
Veena RVS, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395. doi:10.1046/j.1365-313X.1999.00390.x
Wang X, Petrie TG, Liu Y et al (2012) Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem 121:830–839. doi:10.1111/j.1471-4159.2012.07734.x
Wei Y, Ringe D, Wilson MA, Ondrechen MJ (2007) Identification of functional subclasses in the DJ-1 superfamily proteins. PLoS Comput Biol 3:0120–0126. doi:10.1371/journal.pcbi.0030010
Willekens H, Chamnongpol S, Davey M et al (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816. doi:10.1093/emboj/16.16.4806
Wilson MA (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal 15:111–122. doi:10.1089/ars.2010.3481
Xu XM, Møller SG (2010) ROS removal by DJ-1: Arabidopsis as a new model to understand Parkinson’s Disease. Plant Signal Behav 5:1034–1036. doi:10.4161/psb.5.8.12298
Xu XM, Lin H, Maple J et al (2010) The Arabidopsis DJ-1a protein confers stress protection through cytosolic SOD activation. J Cell Sci 123:1644–1651. doi:10.1242/jcs.063222
Yadav SK, Singla-Pareek SL, Ray M et al (2005a) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337:61–67. doi:10.1016/j.bbrc.2005.08.263
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005b) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271. doi:10.1016/j.febslet.2005.10.006
Zhou W, Freed CR (2005) DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem 280:43150–43158. doi:10.1074/jbc.M507124200
Zhou W, Zhu M, Wilson MA et al (2006) The oxidation state of DJ-1 regulates its chaperone activity toward α-synuclein. J Mol Biol 356:1036–1048. doi:10.1016/j.jmb.2005.12.030
Acknowledgements
The authors acknowledge access to Imaging, greenhouse and sequencing facilities from their respective institutions. Thanks to Prof. K. Veluthambi for pBIN19 vector, viral clones and Agrobacterium strain LBA4404 (pSB1), Dr. Radhika Venkatesan for P. syringae DC3000, Prof. Janardhana for Alternaria Spp., Swetha Chenna for help in structure prediction and N. D. Sunitha for comments.
Author contributions
PVS and PD designed all experiments, discussed results and wrote the manuscript. MP performed most of the experiments. KB designed constructs and performed microscopy.
Funding
PVS acknowledges support from Ramanujan Fellowship (SR/S2/RJN-109/2012; Department of Science and Technology, Government of India). PVS lab is supported by NCBS-TIFR core funding and a grant (BT/PR12394/AGIII/103/891/2014) from Department of Biotechnology, Government of India. PDS acknowledges support from Swarnajayanti Fellowship (DST/SJF/LS-01/2011–2012), DBT-IISc partnership program (DBT/BF/PR/INS/2011-12/IISc) and UGC-CAS SAP-II program (UGC LT. No. F. 5-2/2012. SAP-II). KB acknowledges research fellowship from CSIR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Melvin, P., Bankapalli, K., D’Silva, P. et al. Methylglyoxal detoxification by a DJ-1 family protein provides dual abiotic and biotic stress tolerance in transgenic plants. Plant Mol Biol 94, 381–397 (2017). https://doi.org/10.1007/s11103-017-0613-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0613-9