Abstract
The bacterial quorum sensing signals N-acyl-l-homoserine lactones enable bacterial cells to regulate gene expression depending on population density, in order to undertake collective actions such as the infection of host cells. Only little is known about the molecular ways of plants reacting to these bacterial signals. In this study we show that the contact of Arabidopsis thaliana roots with N-hexanoyl-dl-homoserine-lactone (C6-HSL) resulted in distinct transcriptional changes in roots and shoots, respectively. Interestingly, unlike most other bacterial signals, C6-HSL influenced only a few defense-related transcripts. Instead, several genes associated with cell growth as well as genes regulated by growth hormones showed changes in their expression after C6-HSL treatment. C6-HSL did not induce plant systemic resistance against Pseudomonas syringae. The inoculation of roots with different types of AHLs led predominantly for short chain N-butyryl-dl-homoserine lactone and C6-HSL to root elongation. Determination of plant hormone concentrations in root and shoot tissues supported alterations of auxin to cytokinin ratio. Finally, we provide evidence that Arabidopsis takes up bacterial C6-HSL and allows systemic distribution throughout the plant. In sum, the bacterial quorum sensing signal C6-HSL does induce transcriptional changes in Arabidopsis and may contribute to tuning plant growth to the microbial composition of the rhizosphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria use signaling molecules for intercellular communication. The most common quorum sensing molecules found in Gram-negative bacteria are N-acyl-l-homoserine lactones (AHL). Excretion of these chemical signals occurs in a cell density and growth phase-dependent manner and facilitates their adaptation to changing environmental conditions (Whitehead et al. 2001; Hense et al. 2007). Bacterial quorum sensing coordinates gene expression in many physiological processes, such as symbiosis, virulence, EPS-production, resistance to oxidative stress, antibiotic production, motility and biofilm formation (Fuqua et al. 2001; Miller and Bassler 2001; Quinones et al. 2005). It is now evident that many pathogenic bacteria utilize this fact to control premature expression of virulence factors. This control is thought to decrease the probability that the host would detect the pathogen’s presence and activate its defense system. Therefore, it is of crucial importance to elucidate whether host organisms can detect bacterial AHLs and respond appropriately. Bacterial mutants that are defective in quorum sensing are usually avirulent or significantly reduced in virulence (Bauer and Mathesius 2004). AHL signaling in the opportunistic animal and plant pathogen Pseudomonas aeruginosa is a model for the relationship between quorum sensing, pathogenicity, and community behavior. In recent years, several studies demonstrated that eukaryotes respond to these bacterial quorum sensing signals albeit often in many different ways. N-3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL), an AHL from Pseudomonas aeruginosa (an opportunistic pathogen in cystic fibrosis patients) can stimulate various host signaling pathways and activate immune cell responses. AHLs at high concentrations may modulate T helper cell responses and they have been shown to affect IL-8 production in human cells through transcriptional regulation by NF-kB and activator protein-2 (Smith and Iglewski 2003; Wagner et al. 2006).
However, AHLs are not only produced by pathogenic bacteria but also orchestrate important processes of many beneficial rhizosphere colonizing bacteria generally called plant growth promoting rhizobacteria (PGPRs). In these PGPRs AHLs regulate the interaction between microbial populations and their plant hosts, including rhizosphere competence and plasmid conjugal transfer (Newton and Fray 2004) as well as swarming, production of proteolytic enzymes or biofilm formation. For example: deletion of the gene pcoI responsible for the production of the AHLs 3-oxo-C6-HSL and 3-oxo-C8-HSL in Pseudomonas fluorescens 2P24 left the mutant significantly defective in biofilm formation, colonization on wheat rhizosphere and biocontrol ability against wheat take-all, while complementation of pcoI restored the biocontrol activity to the wild-type level (Wei and Zhang 2006). However, while clearly bacteria highly depend on, and make use of, complex signaling pathways in the plant rhizosphere it is still largely unknown if plants functionally respond to those same signals as well. It has been hypothesized (Mathesius et al. 2003) that the information encrypted in AHL signaling could be sufficiently reliable for plants to have evolved the means to recognize such compounds in order to activate defenses during pathogen population-build-up ahead of infection. Experiments with Serratia liquefaciens MG1 producing AHL in the rhizosphere showed increased systemic resistance in tomato plants against the fungal leaf pathogen Alternaria alternata in comparison to the AHL-negative mutant MG44. Furthermore, macroarray experiments gave evidence that C6-HSL-molecules induce systemic accumulation of salicylic acid (SA)—and ethylene-dependent defense genes (Schuhegger et al. 2006). Application of a homoserine lactone, a breakdown product of AHL by means of soil bacteria, to bean roots leads to an increase of stomatal conductance and transpiration in shoots. This in turn is beneficial for both, the plant and its root colonizing bacteria, through an increased uptake of mineral nutrients (Joseph and Phillips 2003). Another study uses proteome analysis to investigate the reaction of plants to bacterial quorum sensing signals. The model legume Medicago truncatula responds to nanomolar to micromolar concentrations of two different AHL-types (3-oxo-C12-HSL and 3-oxo-C16:1-HL) and shows significant changes in the accumulation of over 150 proteins in axenically grown roots (Mathesius et al. 2003). These proteins were found to have functions in host defense, stress responses, energetic and metabolic activities, transcriptional regulation, protein processing, cytoskeletal activities and plant hormone responses. Furthermore, AHLs were found to induce changes in the plants’ secretion of compounds that mimic quorum sensing signals. Thus, plants have the ability to interfere with quorum sensing in root-associated bacteria (Gao et al. 2003; Mathesius et al. 2003; Bauer and Mathesius 2004).
In this study we investigated the response of the model plant Arabidopsis thaliana to AHLs of different side chain lengths. The effects of the short chain AHL, N-hexanoyl-dl-HSL (C6-HSL) (produced by the rhizosphere colonizing bacterium Serratia liquefaciens MG1) was examined in detail. Transcriptional regulation of many genes involved in hormonal signaling and growth, and associated alterations of plant hormone levels were monitored. Our findings indicate that AHLs are detected by plants and may reprogram some important metabolic processes.
Materials and methods
Plant growth conditions and treatments
Arabidopsis thaliana (L.) cv. Columbia 0 seeds (Lehle seeds, Round Rock, TX, USA) were surface-sterilized (5 min 70% ethanol, 5 min 1% NaClO incl. 0.2% Tween20, 5 × washed with sterile H2O) and grown on ½ Murashige and Skoog (MS) medium including vitamins, 1% sucrose and 0.25% Gelrite. Plants were grown in growth chambers at 18°C in the dark and 20°C in the light at 14-h light cycle. Seedlings were transplanted after 7 days in sterile Vitro Vent boxes (Duchefa Biochemie, Haarlem, The Netherlands) containing the same media as mentioned above and grown for 10 days. 17-day-old seedlings were transplanted in a floating hydroponic system (Battke 2003) in Vitro Vent boxes containing liquid ½ MS medium including vitamins and 1% sucrose. Each box contained nine plants, 300 ml medium and 250 ml polypropylene (PP) granulate as the floating material. Roots were treated with AHL at different concentrations. AHL was purchased from Sigma-Aldrich (Deisenhofen, Germany) stored dry and diluted as 10 mM stock solution in ethanol adjusted to pH 5 just prior to use. We used short chain N-hexanoyl-dl-homoserine-lactone (C6-HSL) (according to Schuhegger et al. 2006) as elicitor for all experiments. For root elongation experiments in addition to C6-HSL, several short- and long-chain AHLs were tested. Plant material (either whole roots or shoots including stems) was harvested at different timepoints after AHL treatment, frozen in liquid nitrogen and stored at −80°C. For each experiment data from one Vitro Vent box (corresponding to nine plants) per timepoint was used and pooled. All experiments were done three times as independent biological replicates and for each analysis mean values, standard deviations and appropriate statistics were calculated. Control plants were inoculated with an appropriate amount of ethanol. After harvest an aliquot of the growth medium was plated on different bacterial growth media (LB, NB, King’s B) and incubated at 37°C overnight and 28°C for 4 days, respectively, to check for contaminations that occurred during the experiment. Any contaminated samples were discarded.
RNA extraction
Total RNA from homogenized plant tissues was extracted using TRI reagent (Chomczynski and Sacchi 1987). 100–150 mg tissue material was mixed with 1 ml Tri-reagent, incubated for 5 min on a shaker (900 rpm) and supplemented with 200 μl chloroform. Samples were carefully mixed for 15 s, incubated for 2–3 min at room temperature and centrifuged for 15 min at 4°C (10,000g). Colorless upper aqueous phase was transferred to a fresh tube. RNA was precipitated by mixing with isopropanol and incubating for 45 min at 4°C. Samples were centrifuged (10 min, 4°C, 10,000g) and washed with 70% ethanol twice. The air-dried RNA pellet was dissolved in sterile water.
Microarray analyses
Plant material was harvested 4 h, 1 and 4 days after C6-HSL treatment. Target RNA from C6-HSL treated roots and leaves were extracted as described above. Probes were made using indirect aminoallyl labeling method (see http://www.tigr.org/tdb/microarray/protocolsTIGR.shtml). Control and treated mRNA samples were reverse transcribed with 5-(3-aminoallyl)-2′ deoxyuridine-5′ triphosphate (Sigma-Aldrich). Aminoallyl-labeled-cDNA was coupled to Cy3- and Cy5-dye esters (GE Healthcare, Chalfont St. Gile,UK) and purified according to standard protocol.
For whole genome microarray analysis the Arabidopsis 3 Oligo Microarray Kit was used (Agilent Technologies, Palo Alto, CA, USA). Hybridization of combined Cy3 and Cy5 labeled probes was performed according to the supplier’s instructions for cDNA labeled targets (Agilent 60-mer oligo microarray processing protocol/Surehyb enabled/SSPE Wash).
Arrays were scanned using an AXON GenePix 4000A Microarray Scanner (Molecular Devices, Union City, CA, USA) and GenPix Pro 6.0 software packages. Background fluorescence signal was calculated as the median fluorescence signal of no target pixels around each gene spot. Spots showing less than 50% difference between background and signal were excluded. Additionally only spots with a high-total signal (Sum of Medians 635/532 > 800) were analyzed. Presented results are median values of three independent biological replicates. Normalization was over all normalization features which are all features printed on the array that met the following quality criteria: at least 55% of the pixels in both signals (635 nm and 532 nm) of a given spot were stronger than the background, plus standard deviation. Feature of background uniformity: [Rgn R² (635/532)] was higher than 0.5; only spots with less than 3% saturated pixels were considered and undetected spots or weak signals (Sum of Medians > 500) were excluded. All results are indicated in lg2(~) ratios, i.e., a nominal value of 2 corresponds to an induction of 4. Gene expression was considered as induced or repressed if the transcript level showed a minimum of 1.6-fold change (corresponds to a logRatio of 0.7/−0.7). We applied the following selection procedure to our expression data: (1) signals intensities of less than twofold above local background level were excluded and (2), only expression logRatios higher than 0.7 (lower than −0.7) values with Coefficient of Variance (CV)-values (calculated from the average and standard deviation as follows: 100 × SD/average.) below 60 were regarded as significant. For statistical analysis according Cleveland (1981) the Acuity 4.0 software suite was used (Molecular Devices).
Realtime quantitative PCR of Arabidopsis response regulators (ARR’s)
Plant material was harvested 4 h, 1 and 4 days after C6-HSL treatment or from untreated plants. Target RNA from C6-HSL treated plants or controls were extracted as described above. For cDNA synthesis 5 μg RNA was mixed with 1 μl Poly-d(T)12–18 primer (0.5 μg/μl), adjusted to a final volume of 12 μl and incubated for 10 min at 70°C and 1 min on ice. Afterward, 4 μl 5× buffer, 1 μl 10 mM dNTPs, 2 μl 0,1 M DTT, 1 μl SuperScript II (200U/μl) (Invitrogen, Carlsbad, CA, USA) and 0.5 μl RNaseOUT (40U/μl) (Invitrogen) were added to the reaction and stepwise incubated for 60 min at 42°C, 15 min at 70°C and finally on ice. For RT qPCR received cDNA was diluted 20 times in DEPC treated water.
For RT qPCR of Arabidopsis Response Regulators (ARR’s) the following specific primers were used: ARR3-fwd: tggaatcttcggactctacggtgg, ARR3-rev: cgaatccacaagcgaagttgcagac ARR4-fwd: cgatgaagatgatgacgtgttgacg; ARR4-rev: cacggcatcccagaatagttccact; ARR5-fwd: gcagctaaaacgcgcaaagatct; ARR5-rev: ccgaaagaatcaggacatgcatg; ARR6-fwd: caaacgcgcaaagatctgagct; ARR6-rev: gcgagaatcatcagtgtaggctcaa; ARR7-fwd: gctgaggaatgcaaaatcttaagcc; ARR7-rev: agaagtatcatcatgacttg; ARR15-fwd: aggtggtgaagctgaagaaggaaaa; ARR15-rev: ttgatgatgatgatggagatgaatc.
PCR amplification was done in a total volume of 25 μl containing 10.5 μl diluted cDNA, 1 μl of each primer (10 μM) and 12.5 μl QPCR SYBR low Rox Mix (ABgene, Epsom, UK). For relative quantification of gene expression the comparative CT Method (Livak and Schmittgen 2001) with the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA) was used. The following qPCR thermal cycling program was applied: 1 time 2 min at 50°C, 1 time 15 min at 95°C, 40 times cycling for 15 s at 95°C and 1 min at 60°C. The amount of target was normalized to the endogenous reference gene 18S rRNA. Each data point represents the average of three independent experiments. For technical control each RT qPCR experiment was repeated four times on the same 96-well plate.
Cytokinin and IAA determination
To determine cytokinin and auxin (indole-3-acetic acid, IAA) concentrations the hydroponic system described in plant growth conditions was used. C6-HSL was added in concentration of 10 μM. Root and leaf materials were harvested 1 and 4 days after C6-HSL treatment, homogenized in liquid nitrogen and stored at −80°C. Auxin and cytokinins (CKs) were determined according to Dobrev et al. (2005) and Dobrev and Kaminek (2002). IAA and CKs were extracted overnight at −20°C using modified Bieleski solvent (methanol:water:formic acid, 14:5:1, by vol; Dobrev and Kaminek 2002). [5-3H]IAA (740 GBq/mmol, ARC Inc., St.Louis, MO, USA; 1,000 Bq per sample) and deuterium-labeled CKs ([2H5]Z, [2H5]ZR, [2H5]Z-7G, [2H5]Z-9G, [2H5]Z-OG, [2H5]ZR-OG, [2H3]DZ, [2H3]DZR, [2H6]iP, [2H6]iPR, [2H6]iP-7G, [2H6]iP-9G; Apex Organics, Honiton, UK; 50 pmol per sample each) were added as recovery markers and internal standards. After centrifugation (20,000g, 4°C, 20 min) the extracts were purified using Sep-Pak C18 cartridges (Waters Corporation, Milford, MA, USA) and evaporated to water phase. Hormones were trapped on an Oasis MCX mixed mode, cat ion exchange, reverse-phase column (150 mg, Waters). After a wash with 1 M HCOOH, IAA was eluted with 100% MeOH and evaporated to dryness. Further, CK phosphates (CK nucleotides) were eluted with 0.35 M NH4OH in water and CK bases, ribosides, and glucosides were eluted with 0.35 M NH4OH in 60% (v/v) MeOH. The latter eluate was evaporated to dryness. Prior to analysis, CK nucleotides were transformed to their corresponding ribosides as follows. NH4OH was evaporated from the eluted fraction with CK nucleotides. Samples were alkalinized (0.1 M ammonium acetate, pH 9.6) and incubated with alkaline phosphatase (90 min at 37°C). After neutralization (acetic acid), the solution was passed through a C18 Sep-Pak cartridge. CK ribosides were eluted with 80% (v/v) methanol and evaporated to dryness. Evaporated IAA and CK samples were stored at −20°C until further analysis. IAA was quantified by 2D-HPLC according to Dobrev et al. (2005).
Purified CK samples were analyzed by LC-MS system consisting of HTS PAL autosampler (CTC Analytics, Zwingen, Switzerland), Rheos 2000 quaternary pump (FLUX, Geneva, Switzerland) with Csi 6200 Series HPLC Oven (Cambridge Scientific Instruments, Ely, UK) and LCQ Ion Trap mass spectrometer (Finnigan Corp, San Jose, CA, USA) equipped with an electrospray. 10 μl of sample was injected into a C18 column (AQUA, 250 × 2 mm, 5 µm, Phenomenex, Torrance, CA, USA) and eluted with 0.0005% acetic acid (A) and acetonitrile (B). The HPLC gradient profile was as following: 5 min 10% B (and 90% of A), then increasing to 17% B within 10 min, and to 46% within further 10 min at a flow rate of 0.2 ml min−1. The column temperature was kept at 30°C. The effluent was introduced in mass spectrometer being operated in the positive ion, full-scan MS/MS mode. Quantification was performed using a multilevel calibration graph with deuterated CKs as internal standards. As standards of cis-zeatin-glucosides and cis-zeatin-9-riboside-O-glucoside were not available, the amounts of these compounds were estimated only from the calibration graphs of the corresponding trans-isomers. Presented results are median values of three independent experiments. Statistical significant differences between treatments were calculated with student t-test and only P values below 0.01 were accepted as significant.
Bacterial inoculations
For application of Serratia liquefaciens to plant roots we used the hydroponic system described in plant growth conditions. Bacteria dissolved in 10 mM MgSO4 were added to the growth medium until an OD435 of 0.1 was reached. An adequate amount of 10 mM MgSO4 was also added to control trays.
For application of AHLs to the rhizosphere we also used the hydroponic system. AHLs were added in concentrationsof 10 μM. Bacterial pathogen inoculation was carried out 4 days after the treatment with the AHL in ethanol or an appropriate amount of only ethanol as control. Bacterial growth assay was performed according to Katagiri et al. (2002). The bacterial strain used in this study was Pseudomonas syringae pv. tomato DC3000 (Pst DC3000). Overnight culture of Pst DC3000 was washed and resuspended in 10 mM MgSO4. Plants were removed from hydroponic system and leaves were dipped in a bacterial suspension of Pst DC3000 containing 108 cfu/ml. For this purpose plant roots were held with tweezers and leaves were dipped headfirst into the bacterial solution for about 3 s. Following inoculation the plants were placed in Vitro Vent boxes containing 0.5% agar and located in growth chambers for 4 days. Leaves were harvested, extracts from three different surface sterilized leaf discs were prepared and serial dilutions were performed to determine bacterial population densities within leaf tissues. Presented results are mean values of three independent experiments.
Determination of root elongation and shoot diameter
To determine root elongation and shoot diameter after application of AHLs, we used the hydroponic system described in plant growth conditions. Plants were grown on ½ MS medium, incl. vitamins, 1% sucrose and 0.25% Gelrite for 11 days. Seedlings were transplanted in a floating hydroponic system (Battke et al. 2003) in Vitro Vent boxes containing liquid ½ MS medium incl. vitamins and 1% sucrose. AHLs were added in concentrations of 10 μM. Six different types of AHLs were tested: N-butanoyl-dl-HSL (C4-HSL), N-hexanoyl-dl-HSL (C6-HSL), N-octanoyl-dl-homoserine lactone (OHL), N-decanoyl-dl-homoserine lactone (C10-HSL), N-dodecanoyl-dl-homoserine lactone (dC10-HSL) and N-tetradecanoyl-dl-homoserine lactone (tC10-HSL).
Measurement of root elongation and shoot diameter was performed 14 days after the treatment with the AHL in ethanol or an appropriate amount of only ethanol as control. All results are indicated as means of at least three independent experiments, each experiment consisting of one Vitro Vent box containing nine plants. Experiments were repeated several times leading to similar results. Statistically significant differences between treatments were calculated with student t-test and only P values below 0.01 were accepted as significant.
Detection of C6-HSL and C10-HSL in plant tissue by FTICR mass spectrometry
For the detection of C6-HSL and C10-HSL in leaf and root tissues, the hydroponic system described in plant growth conditions was used. AHLs were added as racemate in concentrations of 10 μM to the root system. Plant material was harvested 4 and 14 days after AHL treatment and root tissues were washed several times in H2O to remove adhering AHLs. Tissues from three independent experiments were pooled, homogenized in liquid nitrogen and stored at −80°C. 10 ml of water with acetonitrile content of 10% (v/v) was added onto the material and ultrasonicated for 15 min. The liquid phase was then separated by centrifugation at 6,500g at room temperature for 5 min and cleaned with a SPE procedure presented earlier (Li et al. 2006). Briefly, 9 ml of the sample was added onto MegaBond Elute SPE cartridge (Varian, Palo Alto, CA, USA) after it was conditioned with 2 ml of methanol and water, respectively. The loaded cartridge was then washed with 4 ml methanol/water mixture, dried under vacuum and the solutes eluted with a 2-propanol/hexane mixture (85/15, v/v). The eluate was dried under a nitrogen stream and resolved with water containing 10% acetonitrile and filtered through PTFE discs (Merck, Darmstadt, Germany) before analysis with electrospray Fourier transformation ion cyclotron mass spectrometry (FTICR/MS).
Positive electrospray spectra were acquired on a Bruker Daltonics (Bremen, Germany) Apex Qe 12 T system equipped with Apollo II and microelectrospray source. The spectra were acquired in broadband mode (m/z 150–1,000) and were calibrated externally on clusters of arginine (ca. 10 mg/l in 50% of methanol with 0.1% of formic acid) in the required mass range (m/z 175.11895, m/z 349.23062, m/z 523.34230 and m/z 697.45398). The calibration was verified internally using low mass diester impurities (m/z 207.15909, m/z 207.15909, m/z 229.14103, m/z 279.15909, m/z 315.25299). Mass accuracy was always better than 0.1 ppm (0.00001%).
Results
Gene expression in A. thaliana after C6-HSL treatment
We used the Arabidopsis 3 Oligo Microarray (Agilent Technologies) to determine expression of differentially regulated genes in leaf and root tissue after application of 10 μM C6-HSL to the Arabidopsis root system. The concentration of 10 μM C6-HSL was chosen because of previous experiences with tomato (Schuhegger et al. 2006). We are aware that this concentration might not reflect the available AHL concentration throughout the rhizosphere and higher AHL concentrations might prevent/reduce transcriptional responses. However, as reviewed by Hense and colleagues, biofilms show a strong spatial heterogeneity regarding bacteria as well as quorum sensing signals, allowing for AHL concentrations even higher than the one applied (Hense et al. 2007). Such hot spots are certainly not possible in hydroponic conditions. Gene expression was assayed for three different time points (4 h, 1 and 4 days). The presented results are mean values of three independent biological experiments, where up- or down-regulation of genes is defined as a minimum 1.6-fold change in its transcript level (equivalent to 0.7 for induced and −0.7 for repressed genes in indicated logarithmic data). Taking together, the data from all time-points, we found transcriptional changes for 721 and 1095 genes in leave and root tissue, respectively. Leaf tissue showed upregulation of about 230 genes and downregulation between 65 (4 h) and 118 (1 day) genes (Suppl. Fig. 1). For root tissue the total number of expressed genes differed much more depending on the time point. 4 h after C6-HSL treatment 744 genes—a threefold higher number than tested for all other time points—showed a significant upregulation in gene expression. In contrast, 1 and 4 days after inoculation only few genes in roots were affected (Suppl. Fig. 1). It should be noted, however, that our 1.5 threshold of 1.6/−1.6 induction/repression is rather low. Setting this threshold to 2.0 (another common threshold in transcriptomics) would reduce the number of regulated genes by almost 80%.
Differentially transcribed genes were grouped into 14 functional categories (Fig. 1), based on the TAIR A. thaliana database (http://www.arabidopsis.org). Up to 39% of these genes could not be grouped, as there is no annotated function in the data bases yet. These genes are designated as expressed or unknown proteins. About 15% could not be grouped in any of the 14 defined functional categories and fall into the “others” cluster. Tables of all differentially expressed genes can be found in the Suppl. Tables 3, 4.
Functional classification of differentially transcribed genes after C6-HSL treatment. Genes were grouped based on the TAIR Arabidopsis thaliana database. Three different time points in leaf and root tissues were tested using microarray analysis. Note that some genes may belong to more than one functional category
Most genes expressed in root and leaf tissues could be assigned to the categories energy (3–11%), metabolism (6–11%), transcription/translation (5–12%) and lipid-, protein-, nucleic acid-binding (4–8%) (Fig. 1). Notably interesting were the results we obtained for 1-day C6-HSL treatment. In leaf tissue 8% of all differentially expressed genes could be classified into a group of cell wall and cell growth related genes (Fig. 1). Among them many expansin and extensin family proteins were found mostly up-regulated. Expansins are known mainly to play roles in cell wall organization and biogenesis (Cosgrove et al. 2002). In comparison, only 1% of the regulated genes in root tissue are classified into this group after 1 day, but getting up to 4% after 4 days. However, unlike in leaf tissue, 11 different arabinogalactan-proteins were particularly activated in root tissue mainly 4 h after C6-HSL treatment. Arabinogalactan-proteins (AGPs) are the family of hydroxyproline-rich glycoproteins implicated in various aspects of plant growth and development (Schultz et al. 2000; Showalter 2001). In contrast to that only four arabinogalactan-proteins in leaf tissue showed changes in gene expression (for gene expression levels see Suppl. Tables 1, 2a, b).
Strikingly, we found up to 6% of differentially regulated genes in leaf tissue and up to 3% in root tissue involved in plant hormone response. All genes implicated in plant hormone response are presented in Suppl. Table 1. Especially, two types of plant hormones, auxins and cytokinins play critical roles in control of plant growth and many developmental processes (Ferreira and Kieber 2005; Woodward and Bartel 2005). In leaf tissue, particularly 12 auxin responsive genes and 5 two-component response regulator (ARR) genes (for locus details see Suppl. Table 1), involved in cytokinin signaling (D’Agostino et al. 2000), could be classified into the group of plant-hormone-response genes.
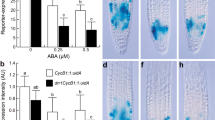
The type-A ARRs act as negative regulators for cytokinin signaling and their transcription is rapidly induced in response to exogenous cytokinin (D’Agostino et al. 2000). To verify the results obtained from microarray analysis qRT PCR experiments were performed (Fig. 2b). The strongest repression levels resulted from 1-day C6-HSL treatment for ARR5, ARR6, ARR7 and ARR15. Results of array and qRT PCR experiments exhibited in part high standard deviation values, caused by extremely low RNA content of one of the biological replicates, but the PCR data convincingly confirm our array analyses. Only in one case (ARR4; 4 h and 4 days) the PCR and array analyses differed from each other.
Expression of Arabidopsis Type-A response regulators (ARRs) in leaf tissue after C6-HSL treatment to root system. a ARRs comprise 22 genes divided into two main groups (type-A, type-B).The encircled type-A ARRs were found repressed in leaf tissue. The diagram was modified according to To et al. (2004). b Expression levels of 6 type-A ARRs in leaf tissue obtained from RT qPCR and microarray analysis 4 h, 1 and 4 days after application of C6-HSL. Mean values ± SD (n = 3, in case of PCR n = 4). The high standard deviation (resulting in a statistical non-significance for many samples) of the PCR data is the result from one sample with extremely low RNA content
In root tissue 15 auxin associated genes were differentially regulated. Most of them were up-regulated 4 h after C6-HSL treatment. Down-regulation was observed for three different IAA-amino acid hydrolases in leaf tissue and the acetaldehyde oxidase protein AAO1 gene in root tissue. Beside auxin and cytokinin responsive proteins also ethylene-responsive proteins, gibberellin family proteins and ABA-responsive element-binding proteins were differentially regulated in both, leaf and root tissues. Beside the above-mentioned arabinogalactan-proteins, auxin responsive proteins and expansin/extensin proteins, other gene families appeared frequently, including disease resistance proteins, glycosyl/glycoside hydrolase and transferase proteins, LTP protease inhibitor/seed storage/lipid transfer proteins, proteins belonging to the photosystem, ribosomal proteins and zinc finger. Twenty-eight different ribosomal proteins were mainly activated at the 4-h time point in root tissue, indicating high protein biosynthesis rates shortly after C6-HSL treatment.
Unlike many other bacterial compounds, C6-HSL did not induce a typical defense response pattern. Most of the disease resistance proteins affected by C6-HSL belong to the TIR-NBS-LRR class and were specifically down regulated in leaf tissue 1 day after C6-HSL treatment. None of the typical markers-genes of systemic acquired resistance (pathogenesis-related-proteins, e.g., PR1), nor markers for jasmonic acid depended resistance (e.g., defensine PDF1.2, thinons (THI2.1), hevine-like proteins (HEL), vegetative storagem protein (VSP)) were found differentially regulated by application of C6-HSL at the tested timepoints.
Infection of A. thaliana with Pseudomonas syringae
Previously, macroarray experiments after infection of tomato plants with the fungal leaf pathogen Alternaria alternata indicated that AHL-molecules systemically induce SA- and ethylene-dependent defense genes (Schuhegger et al. 2006). Hence we tested whether C6-HSL could likewise elicit induced systemic resistance against the plant pathogen Pseudomonas syringae pv. tomato DC3000 in A. thaliana. Bacterial colony-forming units (cfu) were evaluated 0, 1, 2, and 3 days after inoculation. We did not find a significant reduction of bacterial growth at any of these measured timepoints (Fig. 3c). In addition, visual evaluation of leave damage 5 days after bacterial inoculation showed no difference (Fig. 3a, b). As a next step, we checked whether root inoculation with living Serratia liquefaciens bacteria (a producer of C4-HSL and C6-HSL in the rhizosphere) had an influence on the resistance of A. thaliana against Pst DC3000. Successful root colonization of A. thaliana by Serratia liquefaciens MG1::gfp and its AHL-negative mutant Serratia liquefaciens MG44::gfp was established. A distinct colonization could be measured at various time points) reaching values of 108 colony forming units (cfu) per 100 mg root fresh weight after 7 days (data not shown). Growth of the wild-type MG1 and the AHL-negative mutant MG44 in the rhizosphere was analogous during 7 days post inoculation. We found a difference between control plants (no inoculation) and the Serratia (either wt or mutant) pretreated plants: root colonization by the living bacteria significantly reduced the pathogen spread. This effect was most pronounced 2 days after pathogen inoculation but less pronounced after 3 days (see Fig. 4). However, we found no significant difference in induction of resistance between AHL-producing and AHL-non-producing strains. Thus, in contrast to tomato the positive influence of root colonization of Serratia liquefaciens on pathogen resistance of A. thaliana is not dependent on AHL-production, but on colonization by Serratia spp. per se.
Bacterial pathogen inoculation with Pst DC3000 4 days after inoculation of A. thaliana roots with C6-HSL. a Disease symptoms in control plants 5 days after infection. b Disease symptoms in C6-HSL treated plants 5 days after infection. c Number of Pst DC3000 bacteria extracted from leaf tissue 0, 1, 2, and 3 days after infection, respectively. No enhanced disease resistance after C6-HSL treatment was observed for all tested time points compared to control plants. Mean values ± SE (n = 3)
Bacterial pathogen inoculation with Pst DC3000 4 days after inoculation of A. thaliana roots with S. liquefaciens MG1 and MG44. Number of Pst DC3000 bacteria extracted from leaf tissue 0, 1, 2, and 3 days after infection, respectively. Disease susceptibility was reduced after bacterial root inoculation with both Serratia strains. Mean values ± SE (n = 3)
Plant growth-promoting effects in A. thaliana roots by different types of AHLs
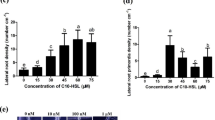
Six different types of AHLs were tested for their ability to promote Arabidopsis plant growth. Short chain N-hexanoyl-dl-HSL (C6-HSL) (ttest, P = 0.0005) and N-butanoyl-dl-HSL (C4-HSL) (P = 0.0000006) significantly promoted root length in relation to control up to 1.2-fold 14 days after inoculation, whereas the long chain homoserinelactones N-octanoyl-dl-HSL (OHL), N-decanoyl-HSL (C10-HSL), N-dodecanoyl-HSL (dC10-HSL) and N-tetradecanoyl-HSL (tC10-HSL) did not (Fig. 5a). Inoculation with C10-HSL even led to a significantly decreased root growth (P = 0.0006). Application of different concentrations of C4-HSL to Arabidopsis roots resulted in significant effects (P < 0.005) down to concentrations of 1nM (data not shown). In case of C6-HSL a significant root elongation could only be measured beyond 10 μM. Concentrations of 1 μM or lower induced no significant increase in root growth (data not shown). Figure 4b and c shows C6-HSL treated and untreated control plants with visible differences in root length. We obtained similar results for treatments with C4-HSL. On the other hand, no growth promoting effects on leaf tissue by any of the tested AHLs could be detected: Neither the measurement of rosette diameter (Fig. 5a), nor determination of shoot weight (data not shown) resulted in any significant changes after AHL treatment.
Plant growth promoting effects in A. thaliana by different types of AHLs. a Measurement of root elongation and shoot diameter 14d after inoculation of different AHLs (10 μM). Asterisk roots significantly longer than in control plants (t-test P < 0.005) plus sign root or shoot significantly smaller than in control plants (t-test P < 0.005). Black columns indicate rosette diameter, gray columns indicate root length. C6-HSL and C4-HSL increase growth of roots, but not leaf rosette, C10-HSL decreases growth of root and leaf rosette. b A. thaliana 14 days after C6-HSL treatment. c 14-day-old control plants, mock inoculated. Mean values ± SE (n = 3)
Cytokinin and IAA concentrations after C6-HSL treatment
The induction of several hormone-related genes by C6-HSL treatment in conjunction with growth-promoting effects found for roots made us look for changes in plant growth hormone concentrations in root and leaf tissues. Here we focused on cytokinins and IAA because of their critical roles in plant growth and development. Hormone concentrations were determined 1 and 4 days after C6-HSL and control treatments. Concentrations of active cytokinins (i.e., free cytokinin bases and their ribosides, Laloue et al. 1981) declined in leaf tissue compared to control plants both 4 and 1 day after C6-HSL treatment (Table 1). 4 days after inoculation 5.1 pmol/g FW active cytokinins was measured in untreated leaf tissues, whereas C6-HSL-treated plants contained only 3.2 pmol/g FW. Unfortunately, cytokinin concentrations in root tissues exhibited values with high standard deviations; therefore, only cautious conclusions could be made. As observed for leaf tissue, 4 days after C6-HSL treatment the concentration of active cytokinins declined compared to untreated control plants. Other, biologically almost inactive cytokinin metabolites (riboside-monophosphates, glucosides, dihydrozeatin- and cis-zeatin-type cytokinins) did not show significant changes after C6-HSL treatment (data not shown).
Determination of free IAA resulted in contrary results compared to cytokinin concentrations. Particularly, the 4-day time point showed higher IAA concentrations in C6-HSL treated leaf and root tissues compared to untreated control plants. Notably interesting was the big difference we received for root tissue 4 days after C6-HSL inoculation. The IAA concentrations were more than twofold higher in treated roots than obtained for control plants (6.31:2.97 pmol/g FW). 4 days after C6-HSL treatment the IAA to cytokinin ratio exhibited a marked difference between treated and control plants (Fig. 6). Generally, C6-HSL treatment resulted in an obvious and statistically significant, shift of auxin:cytokinin ratio in both leaf (P = 0.0005) and root (P = 0.005) tissues toward higher auxin levels.
Ratio free IAA:active cytokinin. Means of auxin and cytokinin concentrations (Table 1) were correlated for leaf and root tissues after 1 and 4 days in C6-HSL-treated and control plants. Changes of ratio in C6-HSL treated leaf and root tissues after 4 days toward higher IAA levels compared to control plants. Bars marked with an asterisk have a statistical significant (t-test: P < 0.005) higher IAA/CK value than controls of the same timepoint and tissue. Mean values ± SE (n = 3)
C6-HSL and C10-HSL accumulation in plant tissue
Having realized the changes in gene expression and hormone concentration levels in leaf tissue as well as root growth effects we asked for uptake and transport of short-chain C6-HSL and long-chain C10-HSL. Detection of AHLs by FTICR mass spectrometry revealed the time dependent appearance of C6-HSL in plant tissue. Relative intensities increased from 1 to 4 days in leaf tissue (Fig 6a) and from 4 to 14 days post inoculation in leaf and root tissues. In the mass spectra of the treated plant extracts both [M−H]+ and [M+Na]+ ions were detected with mass error less than 0.3 ppm. The match of the isotopic pattern of the proposed chemical composition to the spectra allowed the confirmation of their identification. The intensities were higher in leaves than in roots and increased from day 4 to day 14 confirming the transport hypothesis of C6-HSL into the leaves. Detection of C10-HSL by FTICR mass spectrometry revealed totally different results compared to C6-HSL treated plants. C10-HSL intensities were much higher in root tissue compared to leaf tissue and increased from day 4 to day 14 (Fig. 7b, c). Accumulation of C10-HSL in roots and negligible transport to shoots might be due to higher hydrophobicity compared to C6-HSL. In addition, there might be the possibility of selective C14-HSL destruction in shoots (see Bauer and Mathesius 2004).
Measurement of C6-HSL and C10-HSL accumulation in leaves and roots by FTICR-MS after inoculation of A. thaliana roots with 10 μM AHLs. a FTICR-MS spectra of leaf tissue 1 and 4 days after inoculation of A. thaliana roots with 10 μM C6-HSL. C6-HSL peaks are marked with arrows. Relative intensities increased from 1 to 4 days post inoculation. b Control plants were mock inoculated with EtOH. In leaf tissue intensities increased from 4 to 14 days post inoculation for C6-HSL treated plants and decreased for C10-HSL treated plants. c In root tissue intensities for C6-HSL and C10-HSL treated plants increased from 4 to 14 days
Discussion
The interaction of plants with microorganisms plays a key role in ecosystems, and determines growth, development and death of both partners. The conditions of the (micro) environment differ considerably between the highly variable aerial plant part and the more stable root system. Coexistence of plants and bacteria for millions of years has led to the evolution of complex networks consisting of different signaling molecules. A growing body of evidence suggests that the mechanisms of plant resistance to pathogens or a plants’ responsiveness to beneficial microbes share common principles. The AHLs, together with pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides, are believed to be important in mediating the interactions between plants and pathogenic, symbiotic, and saprophytic bacteria, respectively. Quorum sensing signals are not only used by the bacteria, but also by plants: Both plants and algae have been shown to produce and secrete compounds that mimic quorum sensing components and influence bacterial gene expression (Gao et al. 2003; Degrassi et al. 2007). In addition, plants seem to be able to detect various AHLs at quite low concentrations (Mathesius et al. 2003). For tomato, Schuhegger et al. (2006) reported that C6-HSL-producing Serratia liquefaciens MG1 were able to induce resistance to the fungal leaf pathogen, Alternaria alternata while the AHL-negative Serratia liquefaciens MG44 did not (Schuhegger et al. 2006). These data suggested that AHL play an important role in plant bacterial communication and a possible role in pathogen defense, and we decided to analyze the effect of AHLs on A. thaliana with the primary focus on the transcriptional level. It should be noted that in a recent report You and colleagues did not find a significant induction of genes after spraying leaves with 3-oxo-octanoyl-l-homoserine lactone (OOHL) (You et al. 2006). On the other hand, their experimental system differed from the one we and others (Mathesius et al. 2003; Schuhegger et al. 2006) used especially regarding AHL application (uptake through roots vs. spraying on leaf surface). Furthermore, the cut-off You et al. (2006) applied to their array data was log ± 1, while we worked with log ± 0.7. Nevertheless, while our treatments of Arabidopsis with C6-HSL induced clear changes in transcript levels, only a small number of the induced genes were related to or associated with defense. Only between 3 and 6% of the genes regulated by C6-HSL are annotated as defense genes. Corresponding values for typical bacterial elicitors such as harpin or LPS are between 20 and 30% (Krause and Durner 2004; Zeidler et al. 2004). None of the classical markers for systemic acquired resistance (e.g., PR1, PR2 or PR5), or for jasmonic acid depended resistance (e.g., PDF1.2, HEL, THI2.1) were differentially regulated after C6-HSL-treatment and most of the disease resistance proteins belonging to the TIR-NBS-LRR class were down regulated after treatment with C6-HSL. Since the classical marker genes for systemic resistance are usually induced 8 h after treatment and stay upregulated for more than 24 h to provide a prolonged defense against pathogens (Zhou et al. 1998; Moran and Thompson 2001), they should have been visible in our microarrays. Thus we conclude that C6-HSL does not induce a classical defense response in Arabidopsis. It might be important for PGPRs to overcome the plants’ direct defense responses to form a successful mutualistic interaction (Khan 2005; Zhuang et al. 2007). On the other hand, induction of defense and/or resistance seems to depend on the specific plant-microbe interaction, since tomato showed a strong defense signature in response to Serratia spp. or AHLs (Schuhegger et al. 2006; see below).
As shown in Fig. 3, C6-HSL did not induce a priming effect either since plants showed no increased resistance against the biotrophic leave pathogen Pseudomonas syringae DC3000. Furthermore, while root colonization by living Serratia liquefaciens led to reduced infection by Pseudomonas syringae, resistance was not influenced by the ability of the rhizobacterium to synthesize AHL (S. liquefaciens MG1 the AHL-negative mutant S. liquefaciens MG44) (Fig. 4). In sum, our findings are in contrast to the results of Schuhegger et al. (2006), who found that C6-HSL-producing Serratia liquefaciens MG1 were able to induce resistance to the fungal leaf pathogen. Currently we do not have a satisfying answer for this discrepancy. Bacterial-plant communication is certainly highly complex and other factors may influence the response of a plant to AHLs. In a natural system, AHLs may act in combination with PAMPs or other bacterial effectors. It is also possible that C6-HSL-treated Arabidopsis would have shown resistance against a different biotrophic or necrotrophic pathogen. Most likely, a successful interaction depends on the combination of the interacting organisms.
Interestingly, while we could not demonstrate a direct influence of C6-HSL on defense we found an effect on root growth of A. thaliana. Two short-chain AHLs (C4-HSL and C6-HSL) increased root length in Arabidopsis (Fig. 5). Plant hormones most directly involved in the regulation of many developmental processes in plants including root and shoot growth are auxins and cytokinins. Auxin mediates a negative control of cytokinin biosynthesis, indicating that both hormones could interact and thereby control plant development (Nordstrom et al. 2004). In our hands, interaction of A. thaliana roots with the bacterial quorum sensing signal C6-HSL, led to changes of transcriptional levels of plant hormone related genes and alteration of hormone concentrations toward lower cytokinin and higher auxin concentrations (Table 1; Fig. 6). Auxin has been shown to be important for many aspects of root development, including emergence and initiation of lateral roots and root elongation (Benkova et al. 2003; Ljung et al. 2005). Furthermore, study of cytokinin receptor loss-of-function mutants revealed a negative regulatory role for cytokinin in root growth regulation (Riefler et al. 2006). The observed root growth promoting effects of short chain AHLs (Fig. 5) may be due to changes in the auxin to cytokinin ratio. However, while the changes in auxin levels alone cannot explain the increased root growth, we have to take into account the fact that several (unknown) factors might have been influenced even more than the measured hormones auxin and cytokinin. Another point is that some rhizosphere bacteria have been shown to utilize auxin as a carbon source for growth. Pseudomonas pudita strain 1290 was even able to grow with auxin as the sole carbon source (Leveau and Lindow 2005). In this way root colonizing bacteria gain on several levels and may use AHL-production to tune plant growth to accommodate the microbial composition of the rhizosphere. Previously, proteome analysis of Medicago truncatula roots after treatment of 3-day-old seedlings with long-chain AHLs revealed 5% of proteins with changed accumulation levels assigned to plant hormone responses or synthesis (Mathesius et al. 2003). Similar to our results several auxin-induced proteins were differentially accumulated. To test whether AHLs generate changes in gene expression Mathesius et al. (2003) used an auxin-inducible GH3 promoter. Upregulation of GUS reporter fused was seen in all cell types in 3-oxo-C12-HSL treated roots (Mathesius et al. 2003). While alterations in auxin responses or synthesis after C6-HSL treatment appear not to be species-specific, the structure of AHLs seems to influence the reaction of plants to some degree. Mathesius et al. (2003) tested two different long chain AHLs, 3-oxo-C12-HSL and 3-oxo-C16:1-HL, which were found to have similar effects on the accumulation of about two-thirds of proteins.
While C4-HSL promoted root growth significantly at concentrations of 1nM, C6-HSL did not show an effect at concentrations below 10 μM. At a first glance these concentrations may appear very high. However, Charlton et al. (2000) found natural AHL concentrations of up to 630 μM in biofilms of Pseudomonas aeroginosa. While such high concentrations will certainly not occur in the rhizosphere, there might be significant differences in spatial distributions that could lead to areas of high AHL concentrations (Hense et al. 2007).
AHLs with longer side-chains did not have any significant root growth promoting effect. This could be due to increasing hydrophobicity of the AHLs. Uptake studies of C6-HSL, N-octanoyl-dl-homoserine-lactone (OHL) and C10-HSL (Gotz et al. 2007) revealed that only C6-HSL was transported to the leaves of yam beans and only C6-HSL and OHL were transported to barley leaves, which is in accordance with our findings: As shown by FTICR mass spectrometry short-chain C6-HSL can diffuse from roots into leaf tissue in A. thaliana and thereby directly elicit responses in all plant tissues, whereas long chain C10-HSL, possibly due to higher hydrophobicity, were not transported into leaf tissue and thereby accumulated in root tissue (Fig. 7). Accumulation of long-chain C10-HSL in root tissue seems to exert toxic effect in roots and thus possibly resulted in the reduction of root length.
In sum, our study reports on previously unknown responses of A. thaliana to bacterial quorum sensing signals of AHL type. Changes in gene expression patterns or root growth may well be part of the growth-promoting action of rhizosphere bacteria. Further genetic and functional studies should concentrate on the fate of AHLs in plants and downstream signaling after contact with AHL.
Abbreviations
- AHL:
-
N-acyl-l-homoserine lactones
- ARR:
-
Arabidopsis response regulators
- C4-HSL:
-
N-butyryl-dl-HSL
- C6-HSL:
-
N-hexanoyl-dl-HSL
- C10-HSL:
-
N-decanoyl-dl-homoserine lactone
- IAA:
-
Indole-3-acetic acid
- 3-Oxo-C12-HSL:
-
N-3-oxo-dodecanoyl homoserine lactone
- PGPR:
-
Plant growth promoting rhizobacteria
- SA:
-
Salicylic acid
References
Battke F, Schramel P, Ernst D (2003) A novel method for in vitro culture of plants: cultivation of barley in a floating hydroponic system. Plant Mol Biol Rep 21:405–409
Bauer WD, Mathesius U (2004) Plant responses to bacterial quorum sensing signals. Curr Opin Plant Biol 7:429–433
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S (2000) A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2:530–541
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Cleveland WS (1981) Lowess—a program for smoothing scatterplots by robust locally weighted regression. Am Stat 35:54–62
Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D (2002) The growing world of expansins. Plant Cell Physiol 43:1436–1444
D’Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124:1706–1717
Degrassi G, Devescovi G, Solis R, Steindler L, Venturi V (2007) Oryza sativa rice plants contain molecules that activate different quorum-sensing N-acyl homoserine lactone biosensors and are sensitive to the specific AiiA lactonase. FEMS Microbiol Lett 269:213–220
Dobrev PI, Havlicek L, Vagner M, Malbeck J, Kaminek M (2005) Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J Chromatogr A 1075:159–166
Dobrev PI, Kaminek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950:21–29
Ferreira FJ, Kieber JJ (2005) Cytokinin signaling. Curr Opin Plant Biol 8:518–525
Fuqua C, Parsek MR, Greenberg EP (2001) Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35:439–468
Gao M, Teplitski M, Robinson JB, Bauer WD (2003) Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol Plant Microbe Interact 16:827–834
Gotz C, Fekete A, Gebefuegi I, Forczek ST, Fuksova K, Li X, Englmann M, Gryndler M, Hartmann A, Matucha M, Schmitt-Kopplin P, Schroder P (2007) Uptake, degradation and chiral discrimination of N-acyl-d:/l: -homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal Bioanal Chem 389:1447–1457
Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft JU (2007) Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol 5:230–239
Joseph C, Phillips D (2003) Metabolites from soil bacteria affect plant water relations. Plant Physiol Biochem 41:189–192
Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana–Pseudomonas syringae interaction. The Arabidopsis book. American Society of Plant Biologists, Rockville. doi:10.1199/tab.0111, http://www.aspb.org/publications/arabidopsis/
Khan AG (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18:355–364
Krause M, Durner J (2004) Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant Microbe Interact 17:131–139
Laloue M, Pethe-Terrine C, Guern J (1981) Uptake and metabolism of cytokinins in tobacco cells: Studies in relation to the expression of their biological activities. Springer, Berlin
Leveau JH, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371
Li X, Fekete A, Englmann M, Gotz C, Rothballer M, Frommberger M, Buddrus K, Fekete J, Cai C, Schroder P, Hartmann A, Chen G, Schmitt-Kopplin P (2006) Development and application of a method for the analysis of N-acylhomoserine lactones by solid-phase extraction and ultra high pressure liquid chromatography. J Chromatogr A 1134:186–193
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17:1090–1104
Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA 100:1444–1449
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199
Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125:1074–1085
Newton JA, Fray RG (2004) Integration of environmental and host-derived signals with quorum sensing during plant-microbe interactions. Cell Microbiol 6:213–224
Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101:8039–8044
Quinones B, Dulla G, Lindow SE (2005) Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant Microbe Interact 18:682–693
Riefler M, Novak O, Strnad M, Schmulling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, van Breusegem F, Eberl L, Hartmann A, Langebartels C (2006) Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918
Schultz CJ, Johnson KL, Currie G, Bacic A (2000) The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 12:1751–1768
Showalter AM (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58:1399–1417
Smith RS, Iglewski BH (2003) P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 6:56–60
To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16:658–671
Wagner VE, Frelinger JG, Barth RK, Iglewski BH (2006) Quorum sensing: dynamic response of Pseudomonas aeruginosa to external signals. Trends Microbiol 14:55–58
Wei HL, Zhang LQ (2006) Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89:267–280
Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25:365–404
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95:707–735
You YS, Marella H, Zentella R, Zhou Y, Ulmasov T, Ho TH, Quatrano RS (2006) Use of bacterial quorum-sensing components to regulate gene expression in plants. Plant Physiol 140:1205–1212
Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA 101:15811–15816
Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10:1021–1030
Zhuang X, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33:406–413
Acknowledgments
This work was supported by Deutsche Forschungsgeeinschaft (IK, UvR, JD) and by the Ministry of Education of the Czech Republic, project LC06034 (PID, JK, EZ). The authors thank Elke Mattes for excellent technical assistance and J. Malbeck for accomplishment of MS analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
U. von Rad and I. Klein contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
von Rad, U., Klein, I., Dobrev, P.I. et al. Response of Arabidopsis thaliana to N-hexanoyl-dl-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229, 73–85 (2008). https://doi.org/10.1007/s00425-008-0811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0811-4