Abstract
N-acyl-L-homoserine lactones (AHLs) are involved in cell-to-cell communication in Gram-negative bacteria through a process termed quorum-sensing (QS). In this report, we evaluated the response of Arabidopsis thaliana primary roots to abscisic acid (ABA) in wild-type (WT) and decanamide resistant root 1 (drr1) mutant, previously reported to be resistant to N-decanoyl-L-homoserine lactone (C10-HL). When compared to WT seedlings, drr1 mutants were hypersensitive to ABA and had primary roots shorter, which correlated with lower cell division in meristems, a higher concentration of endogenous ABA, and a greater expression of ABI5 gene; and this shortened primary root phenotype was reversed in drr1abi5 double mutants. An analysis of expression of ABSCISIC ACID INSENSITIVE 4 (ABI4) showed an ABA-inducible pattern in primary root tips, which was further increased in drr1 mutant seedlings. Comparison of seed germination in WT, drr1, abi5, and drr1abi5 lines showed higher germination percentages in the following order under control condition: abi5 > drr1abi5 > WT > drr1, while abi5 and drr1abi5 germinated faster and drr1 germinated slower with respect to WT under ABA condition. Taken together, our results suggest that DRR1 is a negative regulator of ABA signaling probably acting upstream of the transcription factors ABI4 and ABI5, which influence ABA responsiveness in primary roots and seed germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants and microorganisms communicate through chemical signaling. Plants biosynthesize a wide range of organic compounds including sugars, organic acids, and vitamins, which can be released into the rhizosphere and are sensed as nutritional or regulatory cues by fungi and bacteria. On the other hand, microorganisms release phytohormones, small molecules, or volatile compounds that directly or indirectly influence plant immunity or regulate plant growth and morphogenesis (Ortiz-Castro et al. 2009). Examples of bioactive molecules produced by bacteria that are perceived by roots are the N-acyl-L-homoserine lactones (AHLs). These compounds act as quorum-sensing (QS) signals in Gram-negative bacteria and mediate pathogenic and symbiotic interactions with their host plants (Schikora et al. 2016; Ortiz-Castro and López-Bucio 2019).
AHLs contain a conserved L-homoserine lactone (HL) ring and an amide (N)-linked acyl side chain ranging from 4 to 18 carbons (Cs), saturated or unsaturated, and with or without a C-3 substituent (Waters and Bassler 2005; Camilli and Bassler 2006). These chemical signals are produced by AHL synthase enzymes, and are detected by a wide variety of transcription factors called “R-proteins”, such as LuxR or LasR, and by a small family of sensor kinases related to LuxN (Duerkop et al. 2007). The binding of the AHL by most of the characterized R-proteins initiates their interaction with the promoters of genes to induce or repress transcription (Pearson et al. 1994; Parsek et al. 1999; Churchill and Chen 2011). The specific activity of the different AHLs can be determined by the HL ring, the amide group, and the acyl chain length (Churchill and Chen 2011; Lintz et al. 2011).
AHLs are neutral compounds that diffuse freely through cell membranes and accumulate in the surrounding medium as the bacterial population increases (Lee and Zhang 2015). In the rhizosphere, AHLs accumulate and act as bioactive plant signals. For instance, medium- and long-chain AHLs repress Arabidopsis thaliana primary root growth, while improving lateral root and root hair formation in a dose-dependent manner, giving rise to more branched root systems (Ortiz-Castro et al. 2008). Two major classes of secondary metabolites have been identified in plants that share structural similarity with AHLs, namely, alkamides and N-acyl ethanolamines (NAEs) (Blancaflor et al. 2003; Ramírez-Chávez et al. 2004). A recessive alkamide resistant mutant of Arabidopsis thaliana termed decanamide resistant root 1 (drr1) was identified by Morquecho-Contreras et al. (2010), due to its continued primary root growth in media supplied with either the plant compound N-isobutyl decanamide or the bacterial QS molecule N-decanoyl-L-homoserine lactone. Characterization of wild-type (WT) and drr1 plants along the entire life cycle suggested that QS signaling influences plant developmental programs, plant phase transitions, and senescence, while detailed analysis in root system showed that DRR1 regulates root system architecture by an abscisic acid (ABA)-dependent mechanism in the primary root, and another jasmonic acid-dependent mechanism in the lateral roots; however, the identity of this gene is currently unknown (Morquecho-Contreras et al. 2010).
An analysis of mutant and overexpressing lines for an Arabidopsis thaliana fatty acid amide hydrolase (AtFAAH) gene which encodes an enzyme that hydrolyzes NAEs indicates that plants have the enzymatic machinery to metabolize a broad range of signaling molecules, including the AHLs (Wang et al. 2006; Ortiz-Castro et al. 2008). Since AtFAAH was found to regulate NAE signaling and seedling establishment, and an intact ABA signaling pathway was required for NAE action (Teaster et al. 2007), it is possible that some ABA components could be shared during the root response to bacterial QS signals. This scenario is further supported by recent structural studies and identification of more FAAH enzymes in angiosperms with versatile substrate-binding pocket that help explain FAAH's ability to recognize and utilize various N-acyl amides from plant or bacterial origin as substrates (Aziz et al. 2019; Aziz and Chapman 2020).
ABA regulates important aspects of plant growth and development, such as embryo and seed development, seed desiccation tolerance and dormancy, germination, seedling establishment, vegetative development, and reproduction (Cutler et al. 2010). The ABA signaling pathway starts when ABA promotes the interaction of PYR/PYL/RCARs receptors and PP2Cs phosphatases, resulting in PP2C inactivation (Ma et al. 2009; Park et al. 2009). PP2Cs inactivate SnRK2s kinases by direct de-phosphorylation, and PP2C inactivation by the receptors allows kinases to phosphorylate downstream proteins (Fujii et al. 2007; Umezawa et al. 2009, 2010; Vlad et al. 2009). In the nucleus, key targets are the basic leucine zipper transcription factor ABSCISIC ACID INSENSITIVE 5 (ABI5) and related ABRE-BINDING FACTORS (ABFs). Phosphorylated ABI5 and ABFs bind as dimers to the ABA-responsive cis-element and in concert with other transcriptional regulators control the ABA-responsive transcription (Nakamura et al. 2001; Furihata et al. 2006; Raghavendra et al. 2010). Among the transcriptional regulators different of ABI5 and ABFs is ABSCISIC ACID INSENSITIVE 4 (ABI4), an AP2-type transcription factor that binds directly to the promoter of ABA-responsive genes and activates their expression (Finkelstein et al. 1998; Bossi et al. 2009; Cutler et al. 2010; Reeves et al. 2011).
The fact that AtFAAH can hydrolyze NAEs and AHLs, and since NAE signaling interacts with ABA signaling, allows us to hypothesize that AHLs also interact with ABA signaling. To test this hypothesis, we decided to use drr1 mutant, which is affected in a gene necessary for the AHL perception in root system and regulates primary root growth in an ABA-dependent manner; therefore, here we performed plant growth, chemical, genetic, and gene expression analysis in Arabidopsis thaliana WT seedlings and drr1 mutants, which demonstrates a C10-HL/ABA signaling crosstalk in regulating primary root growth and seed germination.
Materials and Methods
Plant Material and Growth Conditions
Arabidopsis thaliana drr1 [Ws ecotype] (Morquecho-Contreras et al. 2010), abi1 [Ler ecotype] (Koornneef et al. 1984), abi2 [Ler ecotype] (Koornneef et al. 1984), abi3 [Ler ecotype] (Koornneef et al. 1984), abi4 [Col-0 ecotype] (Finkelstein 1994), and abi5 [Ws ecotype] (Finkelstein 1994) mutants, as well as CycB1;1:uidA [Col-0 ecotype] (Colón-Carmona et al. 1999) and ABI4:GUS [Col-0 ecotype] (Söderman et al. 2000) transgenic lines were used for the experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 4 min and 10% (v/v) bleach for 4 min. After five washes with sterile distilled water, seeds were germinated and grown on agar plates containing 0.2 × MS medium (Murashige and Skoog 1962). MS medium (MS basal salts mixture) was purchased from Sigma. The suggested formulation to medium for tobacco tissue cultures is 4.3 g L−1 salts for a 1 × concentration; we used 0.9 g L−1, which we consider and refer to as 0.2 × MS. This medium lacks amino acids and vitamins. Phytagar (micropropagation grade) was purchased from Phytotechnology. Plates were placed in a plant grown chamber (Percival Scientific AR-95L) with a photoperiod of 16 h of light and 8 h of darkness, light intensity of 100 µmol m−2 s−1, and temperature of 22 °C.
Pharmacological Treatments
The 0.2 × MS nutrient medium was supplemented with kanamycin (Km), abscisic acid (ABA), or N-decanoyl-L-homoserine lactone (C10-HL). Dissolved compounds (sterile deionized water for Km, ethanol for C10-HL and dimethyl sulfoxide for ABA) were added to cooled (50 °C) molten medium and poured onto plates. Control plates were supplied with the greatest concentration of solvent used in the treatments. Chemicals were purchased from Sigma.
Abscisic Acid Quantification in Seedlings
Abscisic acid (ABA) was quantified as previously described by Contreras-Cornejo et al. (2014). Briefly, ABA in seedlings was extracted and subsequently derivatized in ABA methyl ester (ABA-ME, volatile compound) for its detection by gas chromatography-mass spectrometry (GC–MS). For the extraction, approximately, 300 mg of plant tissue was frozen and ground in liquid nitrogen, and subsequently 500 µL of a mixture containing isopropanol, distilled water, and concentrated HCl (2:1:0.002; v/v/v) was added. Sample was shaken at 1400 rpm for 30 s and centrifuged at 11,500 rpm for 3 min. Supernatant was collected and mixed with 200 µL of dichloromethane. Then, the dichloromethane phase was separated and dried with gaseous nitrogen. For the derivatization, dry sediment was resuspended with a mixture of acetyl chloride in methanol (20% v/v), sonicated for 15 min, and finally heated for 1 h at 75 °C. For the detection, the derivatized sample was evaporated with gaseous nitrogen and resuspended in 20 µL of ethyl acetate for GC–MS analysis. Retention time for ABA-ME was established at 15.76 min and its fragment ions (m/z) were 134, 190, and 278. ABA in a defined concentration was processed like the plant tissue to construct a standard curve and determine the amount of compound in the samples.
Genetic Analysis
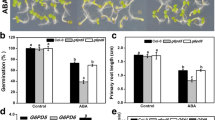
The ABI4:GUS (or CycB1;1:uidA) reporter were transferred into drr1 mutants via manual pollination using pollen from Arabidopsis thaliana ABI4:GUS (or CycB1;1:uidA) transgenic line to fertilize the gynoecium of drr1 flowers. The siliques developed from the crosses were labeled and subsequently, the seeds (F1) were collected, disinfected, and screened to identify heterozygous seedlings with normal root growth responses to C10-HL. F1 seedlings were selected, transferred to soil by allowing to self-fertilize to obtain the F2 generation, and seeds from these plants were disinfected, germinated, and allowed to grow over the surface of Petri plates containing agar-solidified 0.2 × MS medium supplemented with C10-HL. As expected, homozygous drr1 seedlings, whose roots are resistant to grow in medium supplied with 30 µM C10-HL, segregated in a 3:1 WT/drr1 Mendelian proportion. drr1 mutants harboring the AB14:GUS (or CycB1;1:uidA) reporter were selected by staining the primary root tip with a GUS staining kit, and positive seedlings were then transferred to soil to grow and reproduce. Seeds from individual drr1ABI4:GUS (or drr1CycB1;1:uidA) were harvested separately and each lot was analyzed again for GUS activity. drr1 mutants with GUS activity were propagated once again to ensure that the progeny individuals were truly homozygous for the mutation and harbor the ABI4:GUS (or CycB1;1:uidA) construction. To generate the drr1abi5 double mutant, a similar genetic strategy was employed. In this case, pollen from an abi5 plant was used to fertilize the gynoecium of drr1 flowers. The heterozygous F1 progeny was recovered by allowing to self-fertilize. Seedlings from F2 populations were screened via a double selection procedure supplying 3 µM ABA (this concentration was used by Finkelstein in 1994 to select abi5 mutants) and seedlings resistant to the ABA repressor effect were subsequently transferred to 100 µM kanamycin (Km). The seedlings that survived this double selection were propagated again to ensure that the progeny was truly homozygous (Fig. S1). This selection scheme was possible because the mutation in DRR1 is caused by a T-DNA insertion, which contains a Km resistance cassette (NPTII gene), by allowing to grow seedlings in the medium supplemented with kanamycin (Krysan et al. 1999; Morquecho-Contreras et al. 2010). Finally, a RT-PCR reaction was employed to confirm that the drr1abi5 mutant expresses NPTII but does not ABI5, which occurs because NPTII is contained in the T-DNA that causes DRR1 mutation and ABI5 is not expressed in abi5 mutant (Fig. 4a, b). drr1ABI4:GUS and drr1CycB1;1:uidA were not checked by the NPTII expression because ABI4:GUS and CycB1;1:uidA constructs carry NPTII gene to facilitate their selection with antibiotics (Colón-Carmona et al. 1999; Söderman et al. 2000).
Expression Analysis
Plant RNA was isolated using RNA isolation kit (ZR Plant RNA MiniPrep™ Kit of Zymo Research) from frozen and ground tissue with liquid nitrogen. Then, cDNA was synthesized using a reaction mixture containing 3 µg of DNase-treated RNA, 2 µL [0.5 µg/µL] of oligo (dT)23, 6 µL [5×] of Reaction Buffer, 4 µL [2.5 mM] of dNTP Mix, 3 µL [0.1 mM] of DTT, 1 µL [40 U/µL] of RiboLock RNase Inhibitor, and 1 µL [200 U/µL] of RevertAid Reverse Transcriptase (DNase/RNase-Free Distilled Water was used to achieve a final volume of 30 µL), which was incubated for 1 h at 42 °C; and subsequently, the enzyme in this sample was inactivated at 70 °C during 15 min. For end-point RT-PCR, 1.5 µL of cDNA were used as template to carry out a PCR reaction with 3 µL [10× with (NH4)2SO4] of Taq Buffer, 2 µL [5 µM] of mixtured-oligos (a pair of oligonucleotides used as primers), 2 µL [2.5 mM] of dNTP Mix, 3 µL [25 mM] of MgCl2, and 1 µL [1 U/µL] of Taq DNA polymerase (recombinant) (DNase/RNase-Free Distilled Water was used to achieve a final volume of 30 µL), which was performed for 36 cycles of denaturation during 30 s at 95 °C, alignment during 30 s at 60 °C, and amplification during 60 s at 72 °C, in addition to a final extension of 5 min at 72 °C. PCR products were loaded in 1% agarose gels (with [1×] TAE Buffer and [0.5 µg/mL] propidium iodide) and resolved by electrophoresis. For qRT-PCR, 1 µL of cDNA was used as template to carry out a qPCR reaction with 5 µL [2×] of Maxima SYBR Green/ROX qPCR Master Mix and 1 µL [5 µM] of mixtured-oligos (a pair of oligonucleotides used as primers) (DNase/RNase-Free Distilled Water was used to achieve a final volume of 10 µL), which was performed for 40 cycles of denaturation during 30 s at 95 °C, alignment during 30 s at 60 °C, and amplification during 60 s at 72 °C, in addition to a final extension of 5 min at 72 °C. The Cq value (quantitation cycle) from qPCR was used to calculate the relative expression with ACT7 gene as internal control. Oligonucleotides were acquired in “Unidad de Síntesis y Secuenciación de ADN” of “Instituto de Biotecnología UNAM”, and the other compounds and enzymes were purchased from Thermo Scientific™. T-100™ Thermal Cycler (BIO-RAD), C-1000™ Thermal Cycler (BIO-RAD), EP-2000 Run One™ electrophoresis chamber (EmbiTec®), and Gel Doc™ EZ Imager (BIO-RAD) were used to perform the expression analysis. The specific set of primers for each PCR reaction was as follows: 5′-CTTCCGCCGTAAAAGC-3′ (forward) and 5′GTCCCAGCACCACAGG-3′ (reverse) for UBI6 (At2g47110) (López-Bucio et al. 2014); 5′-CGGTGCCCTGAATGAAC-3′ (forward) and 5′-GCCAACGCTATGTCCTG-3′ (reverse) for NPTII (AF458479 [GenBank U00004]); 5′-CAGTGTCTGGATCGGAGGAT-3′ (forward) and 5′-TGAACAATCGATGGACCTGA-3′ (reverse) for ACT7 (At5g09810); 5′-TGGTGGTGAGAATCATCCGTTTA-3′ (forward) and 5′-CCAAAGTTCTTGCCGTTCTCACA-3′ (reverse) for ABI5 (At2g36270).
Analysis of Plant Growth and Seed Germination
The growth of primary root was registered using a ruler and 15 individuals of each condition were evaluated for each treatment. The root fresh weight from 30 seedlings grown on the same plate was measured on an analytical scale (Ohaus) immediately after plant harvest and 3 plates of each condition were evaluated. Lateral roots of each seedling were quantified with a stereoscopic microscope (Leica MZ6) and lateral root density was calculated from lateral root number/primary root length. The percentage of germination was obtained from a population of 50 seeds per triplicate in each line evaluated (WT, drr1, abi5 and drr1abi5). Plates with 0.2 × MS medium (without or with 1 µM ABA) and the seeds were placed in a plant growth chamber with the conditions described above, but in the absence of light. Germination was registered with a stereoscopic microscope (Leica MZ6) at the time when radicle was completely emerged.
Histochemical Analysis of GUS Activity
Transgenic seedlings expressing the GUS reporter gene (Jefferson et al. 1987) were stained in phosphate buffer (NaH2PO4 and Na2HPO4, 0.1 M, pH 7) with 0.1% 5-bromo-4-chlorium-3-indolyl-β-D-glucuronide (X-Gluc), 2 mM potassium ferrocyanide, and 2 mM potassium ferricyanide overnight at 37 °C. Seedlings were cleared and fixed as described previously by Malamy and Benfey (1997). The processed roots were included in glass slips, covered with coverslips and sealed with commercial nail varnish. Subsequently, seedlings were photographed using Nomarski optics on a Leica DM5000-B microscope. For each treatment, at least 10 transgenic seedlings were analyzed. Measurements related to GUS activity was quantified by determining the blue pixels present in an area (quantified in square micrometers) or in cells (quantified in cell number) from primary root tips, using the ImageJ software (http://rsbweb.nih.gov/ij/). We invert the image and split color channels, the red channel (formed by blue pixels before inversion) was used to obtain an value, which was considered 1 in wild-type individuals grown in control condition, and those for mutants and treatments were adjusted relative to these (AU = arbitrary unit). Then, the quantification of AU is referred in the figures as GUS expression intensity.
Statistical Analysis
For all experiments, the overall data were statistically analyzed using STATISTICA 10.0 Software (Dell StatSoft, Austin, Texas, USA). Univariate and multivariate analyses with a Tukey’s post hoc test were used for testing differences in the analyzed parameters. In the graphs, the different letters are used to indicate means that differ significantly (ρ < 0.05), and the asterisks indicate a significant difference to the control (ρ < 0.05).
Results
The Arabidopsis thaliana Decanamide Resistant Root 1 Mutant has a Diminished CycB1 Expression in Response to ABA
We previously reported that decanamide resistant root 1 (drr1) mutants are more sensitive to the primary root growth inhibitory effect of abscisic acid than wild-type (WT) seedlings (Morquecho-Contreras et al. 2010), and along with this, Wang et al. (2011) showed that ABA treatments reduces CycB1 expression in root apical meristem (RAM); thus the CycB1;1:uidA construct was introduced into drr1 mutants by outcrossing, and the reporter expression was compared in seedlings 7 days after germination (dag) grown in 0.2 × MS-agar media supplemented with 0 µM, 0.25 µM, and 0.5 µM ABA. In WT seedlings, ABA decreased the amount of reporter-expressing cells (Fig. 1a, c, e, g) and inhibited GUS expression intensity (Fig. 1b, c, e, g) in a concentration-dependent manner. On the other hand, drr1 mutants showed a greater response than WT seedlings to the repressor effect of ABA (Fig. 1a, b, d, f, h).
Abscisic acid [ABA] effects on CycB1;1:uidA and drr1CycB1;1:uidA roots. a reporter-expressing cells and b GUS expression intensity were recorded from photographs of root meristems in c, e, g CycB1;1:uidA and d, f, h drr1CycB1;1:uidA seedlings, which were germinated (2 days) and grown (7 days) on 0.2 × MS-agar media supplemented with c, d 0 µM, e, f 0.25 µM, and g, h 0.5 µM of ABA (Scale bar = 50 µm). Values shown represent the mean ± standard deviation (n = 10 seedlings) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. AU arbitrary units; µM micromolar, µm micrometers. ≈ ≥
DRR1 Negatively Regulates ABA Biosynthesis and Signaling
To find out why ABA decreased CycB1 expression more efficiently in the drr1 meristems, we decided to evaluate if the mutation affects hormone biosynthesis and/or signaling. First, ABA concentration was determined in wild-type (WT) and drr1 seedlings, which showed that the mutant presents 1.6 times more ABA than WT (Fig. 2). Subsequently, we used a genetic and molecular strategy to clarify whether signaling was involved. The ABA signaling pathway involves several genetic components initially identified on the basis of ABA-resistant germination (Finkelstein 1994; Finkelstein and Lynch 2000; Lopez-Molina et al. 2001). To genetically test the specific role of ABA on primary root growth, ABA sensitivity of WT (Col-0, Ler and Ws) and abi1, abi2, abi3, abi4, and abi5 mutants were assessed on 0.2 × MS-agar media supplemented with increasing ABA concentrations. From this analysis, only the abi5 mutants were able to sustain primary root growth in the ABA treatments performed (Fig. 3a–f). The above results motivated us to check whether ABI5 could be in the same signaling pathway of DRR1 since the corresponding mutants show opposite phenotypes to ABA in roots. Therefore, we genetically obtained homozygous drr1abi5 double mutants, which were molecularly characterized by evaluating the presence of NPTII expression as in drr1 (Fig. 4a) and the absence of ABI5 expression as in abi5 (Fig. 4b). Interestingly, a comparative analysis of ABI5 expression in Ws and drr1 responding to 0.5 µM ABA indicated that exogenously applied phytohormone increased ABI5 expression by almost four times in Ws (Fig. 4c), while the increment in drr1 was approximately 14-fold (Fig. 4d). The root response to ABA was evaluated in WT, single, and double mutants and a new intermediate response between drr1 and abi5 was observed in drr1abi5 mutants for both primary root growth (Fig. 5a) and root biomass (Fig. 5b), which was different from the WT response in 0.5 µM and 1 µM ABA. These results indicate that ABI5 mediates root growth responses to ABA and that the mutation in DRR1 influences ABA responses likely acting upstream of ABI5.
ABI5 Participation in the Root Response to C10-HL
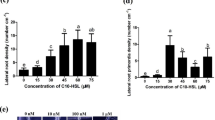
The drr1 mutant was isolated because it shows resistance to primary root growth repression by C10-HL (Morquecho-Contreras et al. 2010). Next, Ws wild-type (WT) and drr1, abi5, and drr1abi5 mutants were grown on 0.2 × MS-agar media supplemented with 0 µM, 10 µM, 20 µM, 30 µM, and 40 µM C10-HL. In these assays, WT and abi5 seedlings shortened their primary roots in a concentration-dependent manner during the response to quorum-sensing (QS) signal (Fig. 6a), and no significant difference was observed between these two genotypes. However, when compared to the WT, drr1 and drr1abi5 seedlings were able to sustain primary root growth in media supplied with 30 µM and 40 µM C10-HL, where drr1abi5 primary roots were slightly shorter than drr1 primary roots in the last C10-HL concentration (Fig. 6a). Lateral root formation increased in treatments with C10-HL in WT and abi5 seedlings, and this response was similarly reduced in drr1 and drr1abi5 seedlings (Fig. 6b). These results show that only a slight differential effect of primary root growth was apparent in drr1abi5 seedlings with respect to drr1 seedlings during the response to the bacterial QS signal.
ABI4 Expression is Modulated by DRR1 in Response to Abscisic Acid
Söderman et al. (2000) reported that ABI4 and ABI5 transcription factors function in a combinatorial network, rather than a regulatory hierarchy, controlling ABA response, and they made ABI4:GUS marker, which is expressed in primary root of seedlings up to 3 days old. To determine the possible influence of DRR1 in modulating this combinatorial network at the level of transcription factors in ABA signaling pathway during the first days of development, the ABI4:GUS marker was transferred into drr1 seedlings by outcrossing. Then, we compared the ABI4:GUS expression pattern in 3-day-old seedlings grown in 0.2 × MS-agar media supplemented with 0 µM, 0.25 µM, and 0.5 µM ABA and found that in the wild-type (WT), this reporter gene was mainly expressed in the root tip and increased its expression depending upon the ABA concentration (Fig. 7a, b, c, e, g), while ABI4:GUS expression was further increased in drr1 mutants (Fig. 7a, b, d, f, h). An analysis to evaluate changes in ABI4:GUS expression in MS 0.2 × media supplemented with 0 µM, 20 µM, and 40 µM C10-HL showed that this quorum-sensing signal did not significantly alter the expression pattern of the reporter in 2-day-old WT or drr1 seedlings when compared to the control condition (Fig. S2).
DRR1 Influences Seed Germination Acting in an ABI5 Pathway
Arabidopsis thaliana mutants deficient in ABA biosynthesis or signaling show increased germination rates (León-Kloosterziel et al. 1996). To determine if the ABA oversensitive root responses of drr1 mutants could be related to seed dormancy and to establish possible epistatic relations of AHL signaling-related DRR1 with ABI5, we compared germination frequencies between wild-type (WT), drr1, abi5, and drr1abi5 seeds at 0, 8, 16, 24, 32, 40, 48, 56, 64, and 72 h (h) in 0.2 × MS-agar media supplemented with 0 and 1 µM of ABA in darkness. WT seeds started germination 24 h after release of stratification and reached 100% around 56 h in the control condition (Fig. 8a). The earlier germination occurred as follows: abi5 > drr1abi5 > WT > drr1, and this later attained 100% around 64 h, which indicated that in control condition, drr1 has a slower germination than WT, which could be because this mutant accumulates ABA, while abi5 and drr1abi5 have a faster germination than WT. In ABA treatment (1 µM) at 48 h, only 15% and 7% germination were obtained in WT and drr1, respectively, but 55% germination could be achieved in abi5 and drr1abi5 (Fig. 8b); and at 72 h, WT, drr1, abi5, and drr1abi5 had 67%, 44%, 100%, and 100% germination, respectively (Fig. 8c). These results indicate that the ABI5 mutation normalizes germination in drr1 mutants and the ABA response, thus positioning DRR1 and ABI5 in the same signaling pathway.
Discussion
Accumulating information reveals a symbiotic relationship between plants and bacteria via N-acyl-L-homoserine lactones (AHLs) and structurally related metabolites from plant origin, which regulate root system architecture and influence plant fitness. Ortiz-Castro et al. (2008) investigated the root architectural changes of Arabidopsis thaliana following application of commercially available AHLs ranging from 4 to 14 carbons in length. Developmental changes elicited by C10-HL, the most active compound included repression of primary root growth and inhibition of cell division in root meristems. The isolation of drr1 Arabidopsis thaliana mutants resistant to C10-HL suggested that this quorum-sensing (QS) signal accelerates developmental programs and senescence, since the mutants showed delayed flowering and increased their vegetative growth period (Morquecho-Contreras et al. 2010).
The in vivo role of the QS signal for plant performance remains to be characterized, however, recent data about the interaction between the Gram-negative plant growth promoting rhizobacteria (PGPR) Burkholderia phytofirmans PsJN and Arabidopsis thaliana throughout the entire plant life cycle, demonstrated that the bacteria instead accelerates flowering and shortened the vegetative growth period; these modifications correlated with the early upregulation of flowering control genes (Poupin et al. 2013). In addition, when Arabidopsis thaliana seedlings were inoculated with Gluconacetobacter diazotrophicus, a root endophyte, growth promotion was consistently observed for up to 50 days, which correlated with higher canopy photosynthesis, lower plant transpiration, and increased water-use efficiency, aspects related to ABA signaling (Rangel de Souza et al. 2016). Thus, inoculations with bacteria that produce and release AHLs could affect the whole life cycle of a plant, accelerating its growth rate and improving photosynthesis and water-use efficiency, effects highly relevant for most crops. Our data indicate that at least in part, the influence of AHLs on plant functional processes may be related to its regulation of ABA signaling.
Palmer et al. (2014) reported that AHL amidolysis by a plant-derived fatty acid amide hydrolase (FAAH) yield L-homoserine, and accumulation of the latter appears to encourage plant growth at low concentrations by stimulating transpiration, while higher concentrations inhibit growth by stimulating ethylene production. Such research has interesting experimental strategies and findings, but their experimental results do not fully support that L-homoserine is a product derivate from AHL amidolysis. The authors did not prove that the FAAH over-expressor line (OE7A, Wang et al. 2006) emphasizes the AHL effects; also, they did not demonstrate that L-homoserine lactone (the intermediary to produce L-homoserine via AHLs) causes the AHL effects on Arabidopsis thaliana, furthermore, the research did not explain how the L-homoserine lactone ring opens to originate L-homoserine, which would likely require an additional enzymatic step. Therefore, the aforementioned prevent us from supporting the model where AHLs are degraded to L-homoserine to cause alterations in plant growth and development, instead, we support the hypothesis of Ortiz-Castro et al. (2008), which proposes that AHLs can mimic endogenous plant molecules and cause changes in plant growth and development.
AHLs share structural chemical similarity with alkamides and N-acyl-ethanolamines (NAEs), compounds naturally produced by plants with interesting biological properties. NAEs and alkamides do not have the L-homoserine lactone ring in their chemical structures, but the three types of compounds need the functioning of some genes in common, as in the case of drr1 mutant, which diminishes alkamide and AHL effects in plants. Although the molecular identity of the DRR1 is at present unknown, its loss of function in Arabidopsis thaliana caused increased sensitivity to the repressor effects of ABA in root growth. This indicates that DRR1 is involved in ABA signaling, influencing plant size and longevity according to the reported phenotype of drr1 mutants grown in soil (Morquecho-Contreras et al. 2010). In particular, the Arabidopsis thaliana primary root shortens in response to ABA, because the latter suppresses cell proliferation in root apical meristem (RAM) (Wang et al. 2011), which is upregulated by DRR1 at the CycB1 level, because DRR1 likely blocks ABA biosynthesis, being that the DRR1 loss-in-function mutation caused an arrest of CycB1;1:uidA expression in RAM and ABA accumulation in seedlings (Figs. 1 and 2). We genetically defined that among ABA-related genes, the ABI5 gene encoding ABI5 transcription factor mediates the ABA-repressing effect on primary root growth since the corresponding abi5 mutant renders Arabidopsis thaliana roots resistant to pharmacological ABA application (Fig. 3), and we showed that DRR1 is a negative regulator of ABI5 expression by affecting ABA signaling pathway, because the mutation in the gene encoding DRR1, caused an overexpression of ABI5 with or without ABA (Fig. 4). drr1abi5 double mutants further revealed that DRR1 may act upstream of ABI5 in the ABA signaling pathway, since root ABA hypersensitivity of drr1 mutants is reversed in the drr1abi5 double mutant, the result is a phenotype similar to the wild-type (WT) rather than a phenotype like abi5 (Fig. 5), which possibly occurs because ABF genes encoding transcription factors with redundant function to ABI5 in ABA signaling pathway are functional in drr1abi5 mutant (Raghavendra et al. 2010).
Content of abscisic acid [ABA] in Ws and drr1 seedlings. Arabidopsis thaliana seedlings (Ws and drr1) were germinated (2 days) and grown (7 days) on 0.2 × MS-agar; then, the ABA concentration was analyzed in samples of complete seedlings (approximately 300 mg of plant tissue). Values shown represent the mean ± standard deviation (n = 3 samples) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. Pg picograms, mg milligrams
The root response to abscisic acid of wild-type (Col-0, Ler and Ws) and ABA mutants (abi1, abi2, abi3, abi4 and abi5). Arabidopsis thaliana seedlings were germinated (2 days) and grown (7 days) on 0.2 × MS-agar media supplemented with 0 µM, 0.25 µM, 0.5 µM, and 1 µM of ABA; and primary root lengths from a wild types and b abi1, c abi2, d abi3, e abi4, and f abi5 mutants were recorded. Values shown represent the mean ± standard deviation (n = 15 seedlings) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. µM micromolar, cm centimeters
Expression analysis of NPTII and ABI5 genes in Ws (wild-type), drr1, abi5, and drr1abi5. Arabidopsis thaliana seedlings were germinated (2 days) and grown (7 days) on 0.2 × MS-agar media supplemented with 0 µM and 0.5 µM of ABA, and RNA was extracted to carry out a reverse transcription (RT) reaction obtaining cDNA; a an end-point RT-PCR was performed from cDNA to the NPTII gene using UBI6 as a control, while a qRT-PCR was performed from cDNA to the ABI5 gene using ACT7 as a control. Then, b ABI5 expression was compared in Ws, drr1, abi5, and drr1abi5 grown in control condition and also evaluated in c Ws and d drr1 grown in 0 µM and 0.5 µM ABA. Values shown represent the mean ± standard deviation (n = 2 samples) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. UD undetected within 40 cycles
Root response of Ws (wild-type), drr1, abi5, and drr1abi5 to abscisic acid. Arabidopsis thaliana seedlings were germinated (2 days) and grown (7 days) on 0.2 × MS-agar media supplemented with 0 µM, 0.25 µM, 0.5 µM, and 1 µM of ABA; then, a the primary root length and b the root fresh weight were recorded. Panels “c” and “d” included representative photographs from all seedlings (Ws and drr1, abi5 and drr1abi5) grown on control condition (0 µM ABA) and an ABA treatment (0.5 µM ABA), respectively (Scale bar = 1 cm). Values shown represent the mean ± standard deviation (n = 15 seedlings for “a”; n = 3 plates with 6 seedlings for “b”) and the different letters indicate significant differences at P ≤ 0.05. The experiment was replicated three times with similar results. µM micromolar, cm centimeters, mg milligrams
It is worth mentioning that variations in the sensitivity to a bioactive molecule between Arabidopsis thaliana ecotypes have been previously reported. Gómez-Gómez et al. (1999) reported that Ws ecotype was more tolerant to the growth-suppressing effect of flagellin, when was compared to Col-0 and Ler ecotypes. This phenomenon also occurred in the ABA response, where Col-0 was more tolerant to the growth-suppressing effect of the phytohormone, with respect to Ws and Ler (Fig. 3a). This can be explained because the ecotypes have adapted to specific environmental conditions in their geographical areas of origin; therefore, a less active ABA signaling pathway can be required in the region where Col-0 was isolated, by causing decreased sensitivity to the exogenously supplemented compound. In this way, we did not discard ABI4 participation due to the pharmacogenetic screening because abi4 mutant is a Col-0 background, and the tolerance of Col-0 to ABA could mask resistance of abi4 to ABA in the concentration range used in this study.
Medium chained AHLs strongly induce lateral root formation. Since drr1 seedlings exhibit greater response to ABA, which negatively regulates lateral root formation (De Smet et al. 2003), it is possible that the reported phenotype of few lateral roots in drr1 seedlings would depend on an increased ABA responsiveness. However, our data show that in contrast to primary root growth, the formation of lateral roots does not necessarily involve ABI5 since the WT and abi5 mutants behaved similarly, forming increasing numbers of lateral roots in response to C10-HL treatments and even, the ABI5 loss-in-function mutation did not show alterations in lateral root formation in response to C10-HL in drr1abi5 double mutants (Fig. 6). For the case of primary root, the subtle difference in length between drr1 and drr1abi5 mutants during the response to C10-HL in each replicate of the experiment in Fig. 6 prevents us from saying that ABI5 does not participate during the primary root response to C10-HL, but it allows us to hypothesize that said participation is indirect or easily substitutable with some protein of redundant function, then, we did not go deep into this part.
Root response of Ws (wild-type), drr1, abi5, and drr1abi5 to N-decanoyl-L-homoserine lactone [C10-HL]. Arabidopsis thaliana seedlings were germinated (2 days) and grown (10 days) on 0.2 × MS-agar media supplemented with 0 µM, 10 µM, 20 µM, 30 µM, and 40 µM of C10-HL; then, the a primary root length and the b lateral root density were recorded. In the panels, "c" and “d” are included representative photographs from all seedlings (Ws, drr1, abi5 and drr1abi5) grown on the control condition (0 µM C10-HL) and a C10-HL treatment (40 µM C10-HL) respectively (Scale bar = 1 cm). Values shown represent the mean ± standard deviation (n = 15 seedlings) and the different letters indicate a significant difference at P ≤ 0.05. The experiment was replicated three times with similar results. µM micromolar, cm centimeters
ABA signaling pathway influences several transcription factors that activate gene expression in a developmental and tissue specific context. Particularly, ABI4 has been reported to regulate root system architecture by inhibiting lateral root formation (Shkolnik-Inbar and Bar-Zvi 2010). Our data are consistent with these previous findings since ABI4:GUS expression in roots increase following ABA treatment, and drr1 mutants show an exacerbated ABI4:GUS expression when compared to WT seedlings, therefore, ABA hypersensitivity of drr1 mutants correlates with a greater expression of the ABA-inducible transcription factor ABI4. Bossi et al. (2009) reported that ABI4 is an essential activator of its own expression during development, acting in ABA signaling and in sugar responses besides inducing ABI5 expression, and the latter was also upregulated in drr1 mutants. Therefore, a higher ABI4:GUS expression would indicate that more ABI4 and ABI5 transcription factors are recruited to the ABA signaling pathway and this would be influenced when DRR1 does not work in drr1 mutant during the ABA response, but ABI4 and ABI5 would not be recruited to take part in the ABA signaling pathway when ABI4:GUS expression did not change, as occurred in WT and drr1 seedlings by carrying ABI4:GUS construction in response to C10-HL (Fig. 7).
Abscisic acid effects on ABI4:GUS and drr1ABI4:GUS roots. a GUS expression area and b GUS expression intensity were recorded from photographs of root meristems in c, e, g ABI4:GUS and d, f, h drr1ABI4:GUS seedlings, which were germinated (2 days) and grown (3 days) on 0.2 × MS-agar media supplemented with c, d 0 µM, e, f 0.25 µM, and g, h 0.5 µM of ABA (Scale bar = 50 µm). Values shown represent the mean ± standard deviation (n = 10 seedlings) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. µm2 square micrometers, AU arbitrary units, µM micromolar, µm micrometers
Arabidopsis thaliana exhibits seed dormancy, allowing the seeds in the natural situation to survive the dry summer period and germinate when the environmental conditions are appropriate for growth and development. The degree of seed dormancy is reflected in the germination percentage and mutants deficient in ABA signaling are among the most non-dormant, or instead, early germinating mutants. Consistently, we found that the rate of germination of the abi5 mutants were higher than that of the WT, conversely, the drr1 mutants showed delayed germination, which could be because this mutants accumulates ABA (Fig. 2). Interestingly, the drr1abi5 double mutants had a seed germination phenotype similar to the wild-type but different from the abi5 or drr1 single mutants in control condition, which did not happen during the ABA treatment, where the abi5 and drr1abi5 germination was similar. This also confirms the hypothesis that DRR1 and ABI5 act in the same signaling pathway, and suggests that DRR1 regulates ABA-mediated germination primarily via ABI5 (Fig. 8).
Seed germination of Ws (wild-type), drr1, abi5, and drr1abi5 in response to abscisic acid [ABA]. Arabidopsis thaliana seeds were sown on 0.2Χ MS-agar media supplemented with 0 µM and 1 µM ABA; then, the germination percentage was recorded for 72 h (h). a 72 h kinetics of germination in control condition. The germination percentage with 0 µM and 1 µM of ABA at b 48 h and c 72 h. Values shown represent the mean ± standard deviation (n = 3 plates with 50 seeds) and the asterisks in “a” indicate significant difference to the control, as well as the different letters in “b” and “c” indicate a significant difference at P ≤ 0.05. The experiment was replicated three times with similar results. µM micromolar, % percentage
In natural ecosystems, seeds germinate in the presence of a wide range of microorganisms and the soil microbiome may be crucial to germination and early plant growth. However, to the best of our knowledge, no studies have investigated the effects of AHL-producing bacteria on germinating seeds. This report shows that the AHL-related drr1 mutant may be part of a signaling network determining not only seed dormancy and primary root growth, but also other possible functional aspects related to ABA such as stress and senescence, the ecological roles of such molecular interactions remain to be clarified.
Change history
15 February 2021
The correct Handling Editor is Alexander Christmann
References
Aziz M, Chapman KD (2020) Fatty acid amide hydrolases: an expanded capacity for chemical communication? Trends Plant Sci 25:236–249
Aziz M, Wang X, Tripathi A, Bankaitis VA, Chapman KD (2019) Structural analysis of a plant fatty acid amide hydrolase provides insights into the evolutionary diversity of bioactive acylethanolamides. J Biol Chem 294:7419–7432
Blancaflor EB, Hou G, Chapman KD (2003) Elevated levels of N-lauroylethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta 217:206–217
Bossi F, Cordoba E, Dupré P, Santos Mendoza M, San Román C, León P (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59:359–374
Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311:1113–1116
Churchill MEA, Chen L (2011) Structural basis of acyl-homoserine lactone-dependent signaling. Chem Rev 111:68–85
Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20:503–508
Contreras-Cornejo HA, Macías-Rodríguez L, Alfaro-Cuevas R, López-Bucio J (2014) Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol Plant Microbe Interact 27:503–514
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang HM (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33:543–555
Duerkop BA, Ulrich RL, Greenberg EP (2007) Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J Bacteriol 189:5034–5040
Finkelstein RR (1994) Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5:765–771
Finkelstein RR, Lynch TJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12:599–609
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Fujii H, Verslues PE, Zhu JK (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19:485–494
Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103:1988–1993
Gómez-Gómez L, Felix G, Boller T (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18:277–284
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Eur Mol Biol Organization J 6:3901–3907
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61:377–383
Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 12:2283–2290
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41
Léon-Kloosterziel KM, van de Bunt GA, Zeevaart JA, Koornneef M (1996) Arabidopsis mutants with a reduced seed dormancy. Plant Physiol 110:233–240
Lintz MJ, Oinuma K-I, Wysoczynski CL, Greenberg EP, Churchill MEA (2011) Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc Natl Acad Sci USA 108:15763–15768
López-Bucio JS, Dubrovsky JG, Raya-González J, Ugartechea-Chirino Y, López-Bucio J, de Luna-Valdez LA, Ramos-Vega M, León P, Guevara-García AA (2014) Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J Exp Bot 65:169–183
Lopez-Molina L, Mongrand S, Chua N-H (2001) A post-germination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98:4782–4787
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064–1068
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Morquecho-Contreras A, Méndez-Bravo A, Pelagio-Flores R, Raya-González J, Ortiz-Castro R, López-Bucio J (2010) Characterization of drr1, an alkamide-resistant mutant of Arabidopsis, reveals an important role for small lipid amides in lateral root development and plant senescence. Plant Physiol 152:1659–1673
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakamura S, Lynch TJ, Finkelstein RR (2001) Physical interactions between ABA response loci of Arabidopsis. Plant J 26:627–635
Ortiz-Castro R, López-Bucio J (2019) Phytostimulation and root architectural responses to quorum-sensing signals and related molecules from rhizobacteria. Plant Sci 284:135–142
Ortiz-Castro R, Martínez-Trujillo M, López-Bucio J (2008) N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31:1497–1509
Ortiz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4:701–712
Palmer AG, Senechal AC, Mukherjee A, Ané J-M, Blackwell HE (2014) Plant responses to bacterial N-acyl L-homoserine lactones are dependent on enzymatic degradation to L-homoserine. ACS Chem Biol 9:1834–1845
Park S, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SM, Bonetta D, Finkelstein RR, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu J, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Parsek MR, Val DL, Hanzelka BL, Cronan JE Jr, Greenberg EP (1999) Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci USA 96:4360–4365
Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91:197–201
Poupin MJ, Timmermann T, Vega A, Zuñiga A, González B (2013) Effects of the plant growth promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 8:1–15
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signaling. Trends Plant Sci 15:395–401
Ramírez-Chávez E, López-Bucio J, Herrera-Estrella L, Molina-Torres J (2004) Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiol 134:1058–1068
Rangel de Souza ALS, De Souza SA, De Oliveira MVV, Ferraz TM, Figueiredo FAMMA, Da Silva ND, Rangel PL, Panisset CRS, Olivares FL, Campostrini E, De Souza Filho GA (2016) Endophytic colonization of Arabidopsis thaliana by Gluconacetobacter diazotrophicus and its effect on plant growth promotion, plant physiology, and activation of plant defense. Plant Soil 399:257–270
Reeves WM, Lynch TJ, Mobin R, Finkelstein RR (2011) Direct targets of the transcription factors ABA-Insensitive (ABI) 4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 75:347–363
Schikora A, Schenk S, Hartmann A (2016) Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol Biol 90:605–612
Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22:3560–3573
Söderman EM, Brocard IM, Lynch TJ, Finkelstein RR (2000) Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol 124:1752–1765
Teaster ND, Motes CM, Tang Y, Wiant WC, Cotter MQ, Wang Y-S, Kilaru A, Venables BJ, Hasenstein KH, Gonzalez G, Blancaflor EB, Chapman KD (2007) N-acylethanolamine metabolism interacts with abscisic acid signaling in Arabidopsis thaliana seedlings. Plant Cell 19:2454–2469
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106:17588–17593
Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51:1821–1839
Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21:3170–3184
Waters CM, Bassler BL (2005) Quorum Sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346
Wang YS, Shrestha R, Kilaru A, Wiant W, Venables BJ, Chapman KD, Blancaflor EB (2006) Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines. Proc Natl Acad Sci USA 103:12197–12202
Wang L, Hua D, He J, Duan Y, Chen Z, Hong X, Gong Z (2011) Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet 7:e1002172
Acknowledgements
This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT, México, grants no. A1-S-34768 and CB2015-251848), the Consejo de la Investigación Científica (UMSNH, México, grant no. CIC 2.26) and Universidad Nacional Autónoma de México (UNAM, México; Academic support program DGAPA-PAPIIT through grants IN210917 and IN209420). We thank Dr. Javier Raya-González for the technical support to perform the quantification in the qRT-PCR assays.
Author information
Authors and Affiliations
Contributions
SBO performed experiments and revised the manuscript; CMLG performed experiments and about cyclin B1 and revised the manuscript; ROC supervised experiments and revised the manuscript. AAGG contributed with funds, revised, and edited the manuscript. JLB led the research, provided funds, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling Editor: Alexander Christmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
344_2021_10302_MOESM1_ESM.jpg
Supplementary file1 Figure S1. Kanamycin [Km] resistance of drr1 and drr1abi5 mutants. Arabidopsis thaliana seedlings (Ws, drr1, abi5 and drr1abi5) were germinated (2 days) and grown (10 days) on 0.2x MS-agar media supplemented with 100 µM of Km; photographs show two representative individuals from at least 15 seedlings grown on kanamycin media (Scale bar = 1 millimeter). The experiment was replicated three times with similar results. (JPG 296 kb)
344_2021_10302_MOESM2_ESM.jpg
Supplementary file 2 Figure S2. N-decanoyl-L-homoserine lactone [C10-HL] effects on ABI4:GUS and drr1 ABI4:GUS roots. (a) GUS expression area and (b) GUS expression intensity were recorded from photographs of root meristems in (c,e,g) ABI4:GUS and (d,f,h) drr1ABI4:GUS seedlings, which were germinated (2 days) and grown (2 days) on 0.2x MS-agar media supplemented with (c,d) 0 µM, (e,f) 20 µM and (g,h) 40 µM of C10-HL (Scale bar = 50 µm). Values shown represent the mean ± standard deviation (n = 10 seedlings) and the different letters indicate a significant difference at P ≤ 0.05. The experiments were replicated three times with similar results. µm2 = square micrometers; AU = arbitrary units; µM = micromolar; µm = micrometers. (JPG 958 kb)
Rights and permissions
About this article
Cite this article
Barrera-Ortiz, S., López-García, C.M., Ortiz-Castro, R. et al. Bacterial Quorum-Sensing Signaling-Related drr1 Mutant Influences Abscisic Acid Responsiveness in Arabidopsis thaliana L.. J Plant Growth Regul 41, 376–390 (2022). https://doi.org/10.1007/s00344-021-10302-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10302-9