Abstract
The volume-regulated anion channel (VRAC), also known as the volume-sensitive outwardly rectifying (VSOR) anion channel or the volume-sensitive organic osmolyte/anion channel (VSOAC), is essential for cell volume regulation after swelling in most vertebrate cell types studied to date. In addition to its role in cell volume homeostasis, VRAC has been implicated in numerous other physiological and pathophysiological processes, including cancer, ischemic brain edema, cell motility, proliferation, angiogenesis, programmed cell death, and excitotoxic glutamate release. Although VRAC has been extensively biophysically, pharmacologically, and functionally characterized, its molecular identity was highly controversial until the recent identification of the leucine-rich repeats containing 8A (LRRC8A) protein as essential for the VRAC current in multiple cell types and a likely pore-forming subunit of VRAC. Members of this distantly pannexin-1-related protein family form heteromers, and in addition to LRRC8A, at least another LRRC8 family member is required for the formation of a functional VRAC. This review summarizes the biophysical and pharmacological properties of VRAC, highlights its main physiological functions and pathophysiological implications, and outlines the search for its molecular identity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms controlling cell volume are essential for normal cell function. Their importance extends far beyond cell volume homeostasis, as regulated cell volume changes are involved in numerous physiological processes, and conversely, cell volume perturbation is associated with a range of pathological states. After cell swelling, volume is recovered in the process of regulatory volume decrease (RVD), a major component of which is activation of an anion current. Historically, the channel mediating this current is known as the volume-regulated anion channel (VRAC), the volume-sensitive outwardly rectifying (VSOR) anion channel, or the volume-sensitive organic osmolyte/anion channel (VSOAC). For the sake of consistency with the recently published papers on LRRC8, we refer in the following to the channel as VRAC. The recent identification of leucine-rich repeats containing 8A (LRRC8A) as essential for VRAC currents and likely part of the channel pore (see review by Jentsch and coworkers elsewhere in this volume) opens up for novel understanding of the regulation and roles of this previously so elusive channel. It is therefore timely–and the purpose of this review–to recapitulate briefly the discovery of VRAC, its biophysical properties, pharmacology and regulation, and its physiological and pathophysiological roles.
The discovery of the VRAC current as an essential part of the RVD response
The discovery that osmotic swelling increases cellular anion (and cation) permeability was first made in Ehrlich ascites tumor cells [1–3] and human lymphocytes [4–6], followed by electrophysiological recording of an outwardly rectifying Cl− current after hypotonic swelling of human T lymphocytes and intestine 407 human epithelial cells [7, 8]. Shortly thereafter, the first comprehensive biophysical analysis of VRAC was published [9], as further detailed below and in Fig. 1. Moreover, this current was shown to be necessary for the RVD response [7, 8].
Biophysical properties of VRAC. a Time course of the activation and deactivation of currents through VRAC in a mouse aorta endothelial cell (measured at +80 and −80 mV). The solid bar marks the application of a 25 % hypotonic solution (upper panel). Note the slow activation of VRAC. Current–voltage (I–V) curves (lower panel) obtained at the time points indicated by a and b in the upper panel. b Time dependent current–voltage relationship. The upper panel shows current traces under isotonic conditions taken at the time indicated by I in panel (a). The lower panel shows current traces during a hypotonic challenge (indicated by II in panel (a)). Traces are from a step-voltage protocol (holding potential 0 mV, 2 s steps from −80 to +100 mV, spaced 20 mV). Note the inactivation at positive potentials. c Permeation properties of VRAC: permeability ratios calculated from the shifts in E rev by substitution of Cl− (asp aspartate, glu glutamate, gluc gluconate, lact lactate, taur taurine, gly glycine, HCO 3 − bicarbonate, SCN − thiocyanate). Note the permeation of large anion such as taurine, aspartate, and glutamate. d Stoke’s diameters of the anions used for Cl− substitution plotted against their permeability relative to Cl−. The diameter of the open VRAC pore was estimated from the excluded volume model (solid line). e Single channel properties of VRAC. Ensemble-averaged membrane currents in a BC3H1 myoblast cell after a 40 % decrease in extracellular osmolarity, recorded after voltage steps from −80 to + 120 mV (upper panel, left). Bottom left: single VRAC currents from an outside-out patch obtained from the same cell and using the same voltage protocol. Note the clustering of openings at the beginning of the step indicating a decrease in open probability during the step. Top right: current-trace (ensemble-averaged current) for a step from +80 to 100 mV. Note the slow increase in current, reflecting recovery from inactivation at the positive holding potential. Bottom right: outside-out patch, single channel recordings from the same cell as above. Note the delay in the appearance of channel openings, which parallels the slow increase in the macroscopic inward current. f Amplitude histograms from single channel openings after cell swelling (outside-out patch pulled from a swollen cell held at −80 mV and stepped to +120 mV). The histogram was fitted by four Gaussian functions. Single channel amplitude was 5.0 pA. Plotted below is the entire single channel current–voltage relationship from these experiments (BC3H1 cells). Note that the outward rectification is due to the different slope conductance at positive and negative potentials. Panels a–f are modified, with permission from Figures 13–15 in [10]
The search for the molecular identity of VRAC
As discussed elsewhere, several properties of VRAC contributed to its long resistance to discovery at the molecular level [11, 12]. The colorful history of the search for VRAC involves numerous now-discarded molecular candidates (reviewed in [11–18]). The first proposed candidates were P-glycoprotein (P-gp, aka multidrug resistance protein-1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1)) [19], pICln [20], and ClC-3 [21], which were all later shown to exhibit biophysical properties incompatible with those of VRAC and/or to be unnecessary for the VRAC current [10, 16, 22]. Thus, depletion or inhibition of P-gp had no effect on VRAC in intestine 407 cells [23] and VRAC current and P-gp expression do not correlate across cell types [24]; ClC-3 knockout mice exhibit normal VRAC currents [25–27]; and pICln is a protein involved in spliceosomal snRNP biogenesis [28, 29]. Furthermore, pICln, when expressed in lipid bilayers, has been shown to form highly cation-selective channels [30], although ion selectivity was later proposed to reflect the specific lipid composition of the bilayer [31]. Additional proteins proposed as VRAC include band 3, aka the AE1 Cl−/HCO3 − exchanger [32]; the FXYD family protein phospholemman [33–35]; the voltage-dependent mitochondrial anion channel VDAC [36]; and the intracellular Cl− channel CLIC1 [37, 38]. Evidence against all of these as molecular candidates for VRAC has later been brought forward (see [10, 13, 15, 16, 22]). In addition to LRRC8A, two other candidates have been brought forward in recent years. Firstly, TMEM16A [39] was proposed as a VRAC candidate; however, its Ca2+ sensitivity clearly distinguishes it from VRAC. Also knockdown of several other TMEM16 family members (TMEM16F, −H, and −J) was found to reduce VRAC [39]. Later studies showed that TMEM16F does not mediate VRAC, yet it likely contributes to RVD under conditions with increased free intracellular Ca2+ concentration ([Ca2+] i ) [40, 41] (for a discussion of the variable role of Ca2+ in RVD, see [14]). Notably, TMEM16F is an ATP-independent phospholipid scramblase, and recent work has proposed that the ionic current is a nonspecific leak current resulting from the scramblase activity [42]. On the other hand, recent evidence suggests that members of the TMEM16 family, at least, under some conditions induce VRAC currents and, moreover, may engage in a functional relationship with LRRC8A [43, 44].
Finally, bestrophin has been described as the molecular entity underlying VRAC in Drosophila melanogaster, first by Chien and Hartzell [45] and later independently verified in a genome-wide siRNA screen [46]. This suggests, interestingly, a divergence of volume-sensitive Cl− channels between invertebrates and vertebrates in the course of evolution. The possible role of bestrophin in mediating VRAC currents in vertebrates is controversial. VRAC is unaffected in macrophages from mBest1/2 double knockout mice [47], and the biophysical properties of VRAC in mammalian cells are clearly at variance with those of the Drosophila volume-sensitive Cl− current [46]. On the other hand, a recent report found the mammalian bestrophin 1 (BEST1) protein to be essential for RVD in human retinal pigment epithelial cells and to mediate currents in these cells with biophysical properties comparable to those of VRAC [48]. It may however be noted that the ohmic current–voltage relationship and essentially absent voltage-dependent inactivation are at variance with those of VRAC (compare Fig. 4 in [48] with Fig. 1 of this review). Notably, it was shown in this study that LRRC8A was dispensable for both VRAC and RVD [48].
A major breakthrough came, as noted above, simultaneously from two groups in 2014: the Jentsch group [59] and the Patapoutian group [60], who both identified the LRRC8 family as essential for VRAC. This work will be covered elsewhere in this volume, and only the major conclusions are briefly recapitulated here. Functional VRAC currents appear to require LRRC8A and at least one other LRRC8 isoform. Further, although the structure-function characterization of the assumed LRRC8 hexamer (or other multimers) is still very incomplete and some contradictory evidence is obtained (see [56]), it was proposed based on mutagenesis studies that LRRC8A may form part of the pore or be located very close to it [59, 60]. Specifically, it was suggested that residue T44 of human LRRC8A, which is predicted to localize in the external part of TM1, forms part of the channel pore [60]. The recent report that the subunit composition of the LRRC8 heteromer determines its permeability properties is also most consistent with the LRRC8 proteins contributing directly to the pore [52, 61]. On the other hand, the fact that mutations of charged amino acids in predicted transmembrane domains have little effect on the current [60] is surprising if these comprise part of the pore [56]. Also consistent with its role as VRAC, LRRC8A is very broadly expressed, localizes to the plasma membrane, is, like VRAC (see below, and [62–64]), isovolumetrically activated by reduced intracellular ionic strength [60], and, finally, its knockdown inhibits RVD [59, 60]. Finally, an important open question, which can now begin to be addressed, is whether LRRC8A may in fact have roles beyond its function as an ion channel or whether the phenotype of the LRRC8A knockout mouse [65] and that of a patient with a truncated LRRC8A variant [66] reflects novel VRAC functions.
VRAC biophysical and permeation properties
Current characteristics
The biophysical characteristics of VRAC have been described in detail in a wide range of cell types (e.g. [9, 16, 17, 22, 56, 67]; for reviews, see e.g. [10, 11, 17, 22, 49, 56, 68]). The VRAC current activates slowly when cells are exposed to a hypotonic challenge. It exhibits a modest outward rectification which is known to reflect voltage-dependent enhancement of the single channel conductance [16, 68, 69] (Fig. 1a, b). The single channel conductance is in the intermediate conductance range, approximately 50–80 pS at positive, and 10–20 pS at negative membrane potentials [69–74] (Fig. 1e, f). It may be noted that the single channel current was initially greatly underestimated due to incompatibility of the current activation properties with the assumptions of stationary noise analysis [70, 71]. Specifically, Jackson and Strange demonstrated that activation of the current by swelling may involve a sudden switching of single channels from a closed state, where channel open probability is zero, to a state, and where open probability is near unity [70, 71]. The current generally exhibits a characteristic, but variable, voltage-dependent inactivation at positive membrane potentials, the time course of which is sensitive to extracellular concentrations of H+, Mg2+, and Cl−, as well as on the current magnitude [9, 16, 22, 56, 67, 75] (Fig. 1b). Similar voltage-dependent inactivation is observed at the whole cell and single channel level [69, 75] (Fig. 1b, e).
Permeability profile
The VRAC permeability sequence has been characterized in detail and is generally reported as SCN− > I− > NO3 − > Br− > Cl− > HCO3 − > glycine > F− > taurine > lactate > gluconate > glutamate > aspartate [9, 10, 16, 22, 76–78] (Fig. 1c). A fit of the relative permeabilities of these ions to their Stoke’s diameter predicts a pore diameter of about 11 Å (Fig. 1d). The pore geometry was also more precisely estimated by use of 4 sulfonic-calix(n)arene anions as permeation reporters indicating that calix(4)arene permeates but calix(6)arene blocks the VRAC pore, indicating an 11 by 17 Å pore [79, 80]. Non-electrolyte partition studies pointed to a cut-off diameter of the VRAC pore of 12.6 Å [81].
The permeability of VRAC to organic anions, leading to its naming also as VSOAC (volume-sensitive osmolyte anion channel), has been widely studied and whether inorganic and organic anions permeated the same or different channels has been subject to major controversy (that may in fact reflect the involvement of partially different LRRC8 heteromers, see below); for the earliest evidence of organic anion transport via VRAC, see [82]; for discussions of this topic, see [14, 83, 84].VRAC has also been proposed to be permeable to the ATP anion [85–87] yet is inhibited by ATP via open-channel block under physiological voltage conditions [17, 88]. Furthermore, VRAC was recently found to potentiate the uptake of the protein synthesis inhibitor blasticidin S [89] and, as further described below, the chemotherapeutic platinum drugs cisplatin and carboplatin (but not oxaliplatin) [52] into mammalian cells. The discovery that LRRC8 channel subunit composition determines its permeability profile [52] suggests that the permeability to both anions and large organic molecules of different charge may reflect the expression of VRAC channels of varying stoichiometry. Thus, the LRRC8A/D heteromer favors permeation of organic osmolyte over anions and also allows uncharged compounds such as platinum drugs to pass the channel. In contrast, the LRRC8A + B/C/E heteromers favor anion permeation above taurine permeation (for a discussion, see [52]).
VRAC pharmacology
The pharmacology of VRAC has been extensively described (see [15, 16, 56]). Although no fully selective VRAC inhibitors are yet available, numerous compounds strongly or partially inhibit the VRAC current. Such compounds that are widely used include 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS), 5-nitro-2-(3-phenylpropyl-amino) benzoic acid (NPPB), and tamoxifen [15, 16, 56]. The acidic di-aryl-urea NS3728 inhibits VRAC in HEK-293 cells and Ehrlich Lettré (ELA) cells with an IC50 value of around 0.4 μM [90, 91] and has gained relatively wide use in recent years. This compound, however, also inhibits Ca2+-activated Cl− currents [91]. Another apparently specific VRAC inhibitor is 4-(2-butyl-6,7-dichlor-2-cyclopentyl-indan-1-on-5-yl)-oxybutyric acid (DCPIB) shown to specifically inhibit VRAC in the heart and CNS [92–94]. Also serotonin reuptake inhibitors (fluoxetin, i.e., Prozac) [95, 96], anti-malarials (mefloquin) [97], anti-estrogen (clomiphene, nafoxidine) [98], and the “T-type Ca2+ channel blocker” mibefradil [99] exhibit relatively strong inhibitory effects on VRAC currents. Finally, numerous other compounds, albeit not specific to VRAC, have been shown to inhibit the VRAC current, including pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) [100], suramin [100], phloretin [101], and carbenoxolone [102]. Obviously, the high chemical variability of the VRAC-modulating compounds has impeded the evaluation of specific functional properties of VRAC.
VRAC regulation
The regulation of VRAC has been extensively studied, with a particular focus on understanding the signal of activation upon cell swelling and how this information is transmitted to the channel. A complete picture of the mechanisms regulating VRAC and their possible interrelationship is still missing, and some of the findings may have been compromised by the lack of a molecular candidate and specific pharmacological tools for VRAC. Needless to say, the identification of LRRC8A opens for the investigation of this using state-of-the art tools and will undoubtedly soon lead to leaps in the understanding of VRAC regulation. However, in this context, it is useful to know the major evidence on pathways of VRAC activation and modulation identified so far, and these are therefore summarized in the following.
VRAC activation
As its name implies, VRAC is regulated by cell swelling. However, as recently discussed in detail [11], VRAC is likely activated not by the change in cell volume per se – an extensive thermodynamic parameter–but by the accompanying decrease in intracellular ionic strength–an intensive thermodynamic parameter. Indeed, a reduction of ionic strength under isosmotic conditions does not cause a change in cell volume but results in activation of VRAC and vice versa; an increased intracellular ionic strength, in the form of a hypertonic intracellular solution, causes a volume increase but inhibits VRAC activation [62, 64]. It has been proposed, in part, based on pioneering experiments in erythrocytes [103], that the relative activities of channels/transporters mediating the swelling-activated efflux of organic and inorganic osmolytes may be determined by the extent of ionic strength change during cell swelling [104, 105]. It is emphasized that ionic strength is clearly not the only mechanism capable of activating VRAC. In addition to its activation by reduced intracellular ionic strength [62–64], VRAC is isovolumetrically activated by intracellular GTPγS [106, 107]. VRAC was also shown to be activated isovolumetrically by purinergic signaling [54, 108], at least in part involving Ca2+-signaling and protein phosphorylation events [109] and via activation of bradykinin (BK) receptors and metabotropic glutamate receptors (mGluR), in a manner involving reactive oxygen species (ROS) and again Ca2+ signaling [54–56, 58]. ROS were shown to directly induce VRAC activation in a manner independent of cell swelling [53, 110, 111] and to be involved in activation of VRAC by epidermal growth factor (EGF) signaling [111] and by inducers of apoptosis [53]. Swelling-induced VRAC activation was furthermore shown to involve a swelling-induced interaction between α-actinin-4 (ACTN4) and a cytosolic ABC transporter family member, ABCF2, which prevented the inhibitory action of ABCF2 on VRAC [112]. The inflammatory mediator Sphingosine-1-phosphate (S1P) acting via its G-protein-coupled receptor S1PR1 was also recently reported to activate VRAC isovolumetrically. S1P is generated by sphingosine kinase, which is activated by multiple stimuli, including bacterial lipopolysaccharide (LPS), platelet-derived growth factor (PDGF), tumor necrosis factor alpha (TNFα), thrombin, IgE-bound antigen, and ATP [86, 113].
Intracellular signaling pathways in VRAC activation and modulation
The activation of VRAC by intracellular GTPγS suggests that GTP is part of the activation pathway, possibly via small GTP-binding proteins [107]. This is consistent with the finding that Rho and Rho kinase (ROCK) are required for VRAC activation but cannot itself activate the channel (hence denoted permissive pathways) in several cell types [114–117]. Other signaling pathways implicated in VRAC activation or modulation have been reviewed elsewhere [14, 22, 49, 56] and will only briefly be outlined here. They include bona fide signaling molecules such as phosphatidyl-inositol-3-kinase (PI3K) [118] and tyrosine kinases [107, 119–122], membrane lipids including cholesterol, various lipid-derived signaling molecules, the actin cytoskeleton [114, 123, 124], and other structural proteins including annexin-II [125] and caveolin-1 [126, 127].
VRAC physiology and pathophysiology
By far the most widely described function of VRAC is its essential role in RVD and hence cell volume homeostasis, in most cell types studied [14, 128, 129]. In addition, however, VRAC has been assigned a wide range of other important physiological functions. These include, but are not limited to, roles in electrogenesis, cell proliferation, angiogenesis, cell motility, and apoptosis (summarized in Fig. 2). These functions have been widely reviewed, and only a brief overview of pertinent aspects will be given below. It should be kept in mind that because of the hitherto elusive molecular identity of VRAC, conclusions on its function and dysfunction have drawn heavily on pharmacology, and it will be important to validate the proposed roles using specific molecular tools.
Roles of VRAC in electrogenesis
Studies in vascular endothelial cells have demonstrated that VRAC plays a central role in electrogenesis and hence is important for offsetting and regulating the driving forces for other ion channels and transporters [22]. While mainly studied in endothelial cells, obviously, VRAC inhibition will in general hyperpolarize cells with a depolarized membrane potential (V m), and its activation will depolarize cells with a more negative membrane potential. As discussed in [22], the resting V m of at least some endothelial cell types has been shown to exhibit a bimodal distribution, with one population with a resting V m of −70 to −60 mV which is dominated by a K+ conductance, and another in which the Cl− conductance is dominating, and which consequently exhibits a resting V m of −40 to −10 mV. In macrovascular bovine pulmonary aortic endothelial cells (BAEC), resting membrane currents are dominated by a combination of an inwardly rectifying K+ (IRK) current and a VRAC Cl− current (Fig. 3a–c), and inhibition of VRAC by mibefradil elicits hyperpolarization because the IRK current is now dominating (Fig. 3d; for details see [10, 22, 72]). Similarly, in calf pulmonary artery endothelial (CPAE) cells, mibefradil induces rapid hyperpolarization [99]. In general, however, the electrogenic effects of VRAC are understudied given their likely physiological importance and should be addressed in further detail and in a wider range of cell types.
Roles of VRAC in endothelial cell electrogenesis. The figure illustrates aspects of electrogenesis in non-stimulated bovine aortic pulmonary endothelial cells (BPAEC). a Current–voltage relationships from linear voltage ramps (from −150 to +100 mV), (1) under control conditions, (2) in the presence of 1 mM Ba2+ to inhibit the inwardly rectifying potassium (IRK) current, and after osmotic shrinkage (100 mM mannitol) to inhibit VRAC, still in the presence of Ba2+ (3). The remaining current is a nonselective cation (NSC) current. b The difference current between conditions 1 and 2 is IRK, mediated through Kir2.1 [130, 131]. c The VRAC current is the difference current between conditions 2 and 3. d The resting V m reflects the respective contributions of the three conductances. Upon inhibition of VRAC by Mibefradil (10 μM), the K+ conductance becomes dominant and the membrane hyperpolarizes. The figure is modified from [22], with permission, and is based on data from [132] (panels a-c) and [72] (panel d)
Roles of VRAC in apoptotic cell death and chemotherapy resistance
A role for VRAC in apoptotic cell death has been demonstrated in multiple studies in a variety of cell types. Apoptosis following activation of the intrinsic apoptotic pathway (e.g., by staurosporine, STS) or of the extrinsic pathway [by death receptor ligands, e.g., Fas ligand (FasL) or TNFα (Fig. 4 upper scheme)] is causatively associated with persistent cell shrinkage [133]. This normotonic shrinkage has been denoted apoptotic volume decrease (AVD) [51, 134]. Thus, VRAC is activated by apoptotic stimuli, although intracellular ionic strength is increasing during apoptotic stress. One signal that can lead to VRAC activation under these conditions is ROS [53]. The AVD process is accompanied by efflux of KCl and osmotically obliged water [134] and involves VRAC activation [53, 135]. Additionally, pharmacological inhibition of VRAC inhibits apoptosis induced by STS [51, 136], FasL [51], TNFα [51], cisplatin [50], ROS [53, 137], and ischemia-reperfusion [138]. Conversely, isovolumetric cell shrinkage is sufficient to induce apoptosis [134, 139, 140]. It is therefore interesting to note that VRAC is downregulated in several drug-resistant cancer cell types, resulting in a decreased propensity for apoptosis [141–143]. Notably, in human ovarian cancer cells, cisplatin resistance was found to correlate with reduced swelling-activated taurine efflux and reduced expression of LRRC8A [143]. Recent findings from the Jentsch laboratory based on The Cancer Genome Atlas (TCGA) data suggested that while LRRC8A expression levels had no effect on survival of ovarian cancer patients treated with platinum drugs, reduced expression of LRRC8D correlated with reduced survival, consistent with the important role of this subunit in cellular cisplatin uptake [52, 61]. Collectively, the findings above show that VRAC is important for the cellular response to apoptotic stimuli. The recent demonstration that VRAC heteromers containing LRRC8D represent an important uptake mechanism for cisplatin and carboplatin shows that this at least in part reflects that VRAC is important for the cellular uptake of these compounds. On the other hand, the VRAC dependence of cell death in response to STS- or FasL treatment, ROS, or isotonic Cl− efflux [51, 139, 144, 145] confirms the role of VRAC in the cell death process per se, as these stimuli are unlikely to be dependent on VRAC-mediated drug uptake (Fig. 4 upper scheme). While the broader relevance of VRAC in cancer clearly needs to be defined, it is interesting to note that a recent study of genes essential to net growth of human CML and Burkitt’s lymphoma cell lines identified LRRC8A, C, D, and E as non-essential for this process ([146], Suppl. Tables 1–2). This is in congruence with the possibility that there may be other molecular candidates for VRAC than the LRRC8 family. However, it is equally consistent with the known roles of other types of Cl− currents than VRAC (e.g., Ca2+-activated Cl− currents, see above) in RVD as well as in growth in some cell types and with the contributions of other transporters, e.g., KCl cotransporters, to these processes (see [14]).
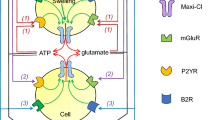
Roles of VRAC in AVD and NVI. Upper scheme: roles of VRAC activity in the AVD in response to STS, FasL/TNFα, ischemia-reperfusion, and cisplatin. Under these conditions, VRAC is normotonically activated, resulting in a cell shrinkage that is necessary for the ensuing apoptotic cell death. In addition, VRAC serves as an uptake pathway for cisplatin. Lower scheme: roles of VRAC activity in NVI in response to glutamate-induced excitotoxicity and in glial glutamate release in response to glutamate-, BK-, and ATP-induced activation of mGluR, BKR, and P2YR, respectively. VRAC-mediated glutamate release further enhances, in a positive feedback manner, the release of glutamate via activation of mGluR in glial cells. Under these conditions, cell swelling induced by NaCl uptake further enhances neuronal VRAC activity but elicits Cl− inflow rather than efflux due to the extensive depolarization resulting from iGluR activation. One of important characteristics of necrosis is the release of harmful or inflammatory factors to surrounding cells and tissues from the dying cells due to cell rupture. It must however be noted that numerous cell signaling mechanisms are involved in the processes between NVI, cell rupture and necrosis, as well as between AVD and cell fragmentation. AVD apoptotic volume decrease, BK bradykinin, BKR BK receptor, FasL Fas ligand, iGluR ionotropic glutamate receptor cation channel, mGluR metabotropic glutamate receptor, Na v voltage-gated Na+ channel, NVI necrotic volume increase, P2YR purinergic type 2Y receptor, ROS reactive oxygen species, STS staurosporine, TNFα tumor necrosis factor; VRAC(d) LRRC8 heteromer containing LRRC8D. The figure is modified from [49], with permission, and is based on data from [50–53] (upper scheme) and [54–58] (lower scheme)
Roles of VRAC in CNS function and necrotic cell death under excitotoxicity
As discussed in further detail by Mongin and coworkers elsewhere in this volume, VRAC is functionally expressed in brain neurons, astrocytes, and microglia (see [56]). In neurons, activation of voltage-gated Na+ channels and ionotropic glutamate receptor cation channels (iGluR) leads to cell swelling, thereby activating VRAC (Fig. 4 lower scheme). Whereas the role of VRAC after cell swelling associated with physiological neuronal firing activity remains incompletely understood, its role in neuronal RVD has been widely studied (see [56]). As noted above, in addition to Cl−, VRAC carries amino acids such as taurine, glutamate, and aspartate [92, 147–151]. This has important pathophysiological consequences because VRAC activation during, e.g., ischemic insults such as stroke causes swelling-induced neurotransmitter release from glia cells, contributing to excitotoxic damage [92, 148–154] (Fig. 4 lower scheme).
Necrotic cell death occurs in parallel with a marked normotonic cell swelling which has been denoted necrotic volume increase (NVI) [134, 155]. Neurotoxicity caused by prolonged exposure to excessive glutamate released from glial cells is termed excitotoxicity [156] and is associated with stroke, cerebral ischemia, brain trauma, and some neurodegenerative disorders, including epilepsy and Alzheimer’s, Huntington’s, and Parkinson’s diseases [157]. Under excitotoxic conditions, neuronal swelling is induced by water inflow driven by Na+ influx via iGluR and Cl− influx via GABAA receptor anion channels, in turn, leading to activation of VRAC as described above (Fig. 4 lower scheme). Glutamate also activates mGluR, which enhance VRAC activity in a G-protein-dependent manner [56] (Fig. 4 lower scheme). In addition, ATP (acting as a neuro- and gliotransmitter) and BK (acting as an inflammatory mediator) also activate VRAC via G-protein-coupled receptors in a manner independent of cell swelling [54, 55, 58]. Once activated under these conditions, VRAC serves as a pathway not for volume-regulatory Cl− efflux but for swelling-exacerbating Cl− inflow because of the prominent V m depolarization produced by iGluR activation (Fig. 4 lower scheme). This “reverse-mode” operation of VRAC leads to NVI and necrotic cell death in neurons [57].
VRAC in cell cycle progression and proliferation
VRAC currents are differentially regulated through the cell cycle, and inhibition of VRAC has been shown to inhibit proliferation in a wide range of cell types [111, 158–161]. Specifically, in SiHa human cervical cancer cells, VRAC inhibition resulted in G0/G1 arrest and delayed G1-S transition [160]. The precise cell cycle phase affected may differ between species: In nasopharyngeal carcinoma cells, VRAC activity was found to be downregulated in S phase compared to G1 and M [162]. In contrast, in SiHa cells, VRAC activity was reported to be increased in S compared to G0 and G1 [160], and a similar pattern was found in ELA cells [91]; for a review, see [163]. Also in congruence with a role of VRAC in control of proliferation status, downregulation of the VRAC current has been shown to be required for muscle cell differentiation [164]. While these studies need to be revisited at the molecular level given the unspecific nature of VRAC inhibitors, these data point to the importance of VRAC for cell cycle progression. The precise mechanism(s) remain to be elucidated, but several can be envisaged: The importance of V m changes in proliferation have long been known, and the role of VRAC is paralleled by an important role of K+ channels in cell proliferation [165]. Additionally, specific cell volume changes have been assigned important roles in cell cycle progression [166–170]. Interestingly, it was recently suggested that G1/S progression may involve a size-discriminatory process in G1, such that cells exit G1 with similar sizes [166].
VRAC in cell migration and angiogenesis
Another important physiological role of VRAC is the regulation of cell migration, which in several cell types is inhibited by VRAC inhibitors [171–174], presumably at least in part reflecting the involvement of local cell volume changes in cell motility (see [175]). Notably, a wide array of VRAC inhibitors attenuate the formation of new blood vessel in several model systems, e.g., matrigel-tube formation assay (in vitro), fibrin gel assay, and chorioallantoic membrane (CAM) assay (in ovo) [176, 177]. While inhibition of migration and/or proliferation could contribute to the observed effects of VRAC in angiogenesis, this may render VRAC inhibitors interesting in the context of angiogenesis inhibition in, e.g., cancer.
Concluding remarks
This review has provided an overview of the current knowledge on VRAC biophysical properties, pharmacology, regulation, physiology, and pathophysiology–the great majority obtained before the recent breakthrough in the understanding of the molecular identity of VRAC. These studies can now be used as an important starting point for novel discoveries and structure-function understanding, based on the identification of LRRC8A as an essential component of VRAC. Centrally open questions include the precise identity of the VRAC pore; whether other molecular entities than the LRRC8 proteins contribute to VRAC, perhaps via interactions with the LRRC8 proteins; the mechanisms through which VRAC is activated by cell volume perturbations and other stimuli; and, of course, the molecular validation of the multiple roles of VRAC regulation and dysregulation in human physiology and pathophysiology that have so far relied on pharmacological tools. The coming years will surely see a surge of new replace “important” with “pivotal” (to avoid two times “important” in the same sentence) discoveries about the functions of this ubiquitously important channel.
References
Hoffmann EK (1978) Regulation of cell volume by selective changes in the leak permeabilities of Ehrlich ascites tumor cells. Alfred Benzon Symp XI:397–417
Hoffmann EK, Simonsen LO, Lambert IH (1984) Volume-induced increase of K+ and Cl− permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol 78:211–222
Hoffmann EK, Simonsen LO, Sjoholm C (1979) Membrane potential, chloride exchange, and chloride conductance in Ehrlich mouse ascites tumour cells. J Physiol 296:61–84
Grinstein S, Clarke CA, Dupre A, Rothstein A (1982) Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol 80:801–823
Grinstein S, Clarke CA, Rothstein A (1982) Increased anion permeability during volume regulation in human lymphocytes. Philos Trans R Soc Lond B Biol Sci 299:509–518
Sarkadi B, Attisano L, Grinstein S, Buchwald M, Rothstein A (1984) Volume regulation of Chinese hamster ovary cells in anisoosmotic media. Biochim Biophys Acta 774:159–168
Cahalan MD, Lewis RS (1988) Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43:281–301
Hazama A, Okada Y (1988) Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 402:687–702
Nilius B, Oike M, Zahradnik I, Droogmans G (1994) Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J Gen Physiol 103:787–805
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Pedersen SF, Klausen TK, Nilius B (2015) The identification of a volume-regulated anion channel: an amazing Odyssey. Acta Physiol (Oxf) 213:868–881
Stauber T (2015) The volume-regulated anion channel is formed by LRRC8 heteromers–molecular identification and roles in membrane transport and physiology. Biol Chem 396:975–990
Clapham DE (1998) The list of potential volume-sensitive chloride currents continues to swell (and shrink). J Gen Physiol 111:623–624
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Strange K (1998) Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln. J Gen Physiol 111:617–622
Valverde MA, Diaz M, Sepulveda FV, Gill DR, Hyde SC, Higgins CF (1992) Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature 355:830–833
Paulmichl M, Li Y, Wickman K, Ackerman M, Peralta E, Clapham D (1992) New mammalian chloride channel identified by expression cloning. Nature 356:238–241
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421
Nilius B, Droogmans G (2001) Ion channels and their functional role in vascular endothelium. Physiol Rev 81:1415–1459
Tominaga M, Tominaga T, Miwa A, Okada Y (1995) Volume-sensitive chloride channel activity does not depend on endogenous P-glycoprotein. J Biol Chem 270:27887–27893
De GC, Sehrer J, Viana F, van AK, Eggermont J, Mertens L, Raeymaekers L, Droogmans G, Nilius B (1995) Volume-activated chloride currents are not correlated with P-glycoprotein expression. Biochem J 307(Pt 3):713–718
Arreola J, Begenisich T, Nehrke K, Nguyen HV, Park K, Richardson L, Yang B, Schutte BC, Lamb FS, Melvin JE (2002) Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl- channel gene. J Physiol 545:207–216
Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, Uchida S, Sasaki S, Okada Y (2004) ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol Biochem 14:213–224
Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Pu WT, Krapivinsky GB, Krapivinsky L, Clapham DE (1999) pICln inhibits snRNP biogenesis by binding core spliceosomal proteins. Mol Cell Biol 19:4113–4120
Pu WT, Wickman K, Clapham DE (2000) ICln is essential for cellular and early embryonic viability. J Biol Chem 275:12363–12366
Li C, Breton S, Morrison R, Cannon CL, Emma F, Sanchez-Olea R, Bear C, Strange K (1998) Recombinant pICln forms highly cation-selective channels when reconstituted into artificial and biological membranes. J Gen Physiol 112:727–736
Garavaglia ML, Rodighiero S, Bertocchi C, Manfredi R, Furst J, Gschwentner M, Ritter M, Bazzini C, Botta G, Jakab M, Meyer G, Paulmichl M (2002) ICln channels reconstituted in heart-lipid bilayer are selective to chloride. Pflugers Arch 443:748–753
Haynes JK, Goldstein L (1993) Volume-regulatory amino acid transport in erythrocytes of the little skate, Raja erinacea. Am J Physiol 265:R173–R179
Davis CE, Patel MK, Miller JR, John JE III, Jones LR, Tucker AL, Mounsey JP, Moorman JR (2004) Effects of phospholemman expression on swelling-activated ion currents and volume regulation in embryonic kidney cells. Neurochem Res 29:177–187
Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'Brian JJ, Szabo G, Jones LR (1995) Unitary anion currents through phospholemman channel molecules. Nature 377:737–740
Moorman JR, Jones LR (1998) Phospholemman: a cardiac taurine channel involved in regulation of cell volume. Adv Exp Med Biol 442:219–228
Dermietzel R, Hwang TK, Buettner R et al (1994) Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci U S A 91:499–503
Landry D, Sullivan S, Nicolaides M, Redhead C, Edelman A, Field M, al-Awqati Q, Edwards J (1993) Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J Biol Chem 268:14948–14955
Redhead CR, Edelman AE, Brown D, Landry DW, al-Awqati Q (1992) A ubiquitous 64-kDa protein is a component of a chloride channel of plasma and intracellular membranes. Proc Natl Acad Sci U S A 89:3716–3720
Almaca J, Tian Y, Aldehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca2+. Pflugers Arch 466:1899–1910
Shimizu T, Iehara T, Sato K, Fujii T, Sakai H, Okada Y (2013) TMEM16F is a component of a Ca2+-activated Cl− channel but not a volume-sensitive outwardly rectifying Cl- channel. Am J Physiol Cell Physiol 304:C748–C759
Yu K, Whitlock JM, Lee K, Ortlund EA, Cui YY, Hartzell HC (2015) Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife 4:e06901
Hammer C, Wanitchakool P, Sirianant L, Papiol S, Monnheimer M, Faria D, Ousingsawat J, Schramek N, Schmitt C, Margos G, Michel A, Kraiczy P, Pawlita M, Schreiber R, Schulz TF, Fingerle V, Tumani H, Ehrenreich H, Kunzelmann K (2015) A coding variant of ANO10, affecting volume regulation of macrophages, is associated with Borrelia seropositivity. Mol Med 21:26–37
Kunzelmann K (2015) TMEM16, LRRC8A, bestrophin: chloride channels controlled by Ca2+ and cell volume. Trends Biochem Sci 40(535–543):2015
Chien LT, Hartzell HC (2007) Drosophila bestrophins are dually regulated by calcium and cell volume. J Gen Physiol 130:21A–22A
Stotz SC, Clapham DE (2012) Anion-sensitive fluorophore identifies the Drosophila swell-activated chloride channel in a genome-wide RNA interference screen. PLoS ONE 7:e46865
Chien LT, Hartzell HC (2008) Rescue of volume-regulated anion current by bestrophin mutants with altered charge selectivity. J Gen Physiol 132:537–546
Milenkovic A, Brandl C, Milenkovic VM, Jendryke T, Sirianant L, Wanitchakool P, Zimmermann S, Reiff CM, Horling F, Schrewe H, Schreiber R, Kunzelmann K, Wetzel CH, Weber BH (2015) Bestrophin 1 is indispensable for volume regulation in human retinal pigment epithelium cells. Proc Natl Acad Sci U S A 112:E2630–E2639
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587:2141–2149
Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y (2005) Roles of volume-sensitive Cl- channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol 205:139–145
Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci U S A 97:9487–9492
Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, Jentsch TJ (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J 34:2993–3008
Shimizu T, Numata T, Okada Y (2004) A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci U S A 101:6770–6773
Akita T, Fedorovich SV, Okada Y (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Akita T, Okada Y (2014) Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 275:211–231
Inoue H, Okada Y (2007) Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J Neurosci 27:1445–1455
Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y (2009) Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol 587:2197–2209
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Voets T, Nilius B, Vennekens R (2015) VRACs swallow platinum drugs. EMBO J 34:2985–2987
Nilius B, Prenen J, Voets T, Eggermont J, Droogmans G (1998) Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J Physiol 506(Pt 2):353–361
Sabirov RZ, Prenen J, Tomita T, Droogmans G, Nilius B (2000) Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch 439:315–320
Voets T, Droogmans G, Raskin G, Eggermont J, Nilius B (1999) Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc Natl Acad Sci U S A 96:5298–5303
Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, Geha RS (2014) Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med 211:929–942
Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, Ozono K, Hara J (2003) A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest 112:1707–1713
Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B (1998) Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J Membr Biol 163:97–110
Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730
Okada Y, Petersen CC, Kubo M, Morishima S, Tominaga M (1994) Osmotic swelling activates intermediate-conductance Cl− channels in human intestinal epithelial cells. Jpn J Physiol 44:403–409
Jackson PS, Strange K (1995) Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J Gen Physiol 105:643–660
Jackson PS, Strange K (1996) Single channel properties of a volume sensitive anion channel: lessons from noise analysis. Kidney Int 49:1695–1699
Nilius B, Voets T, Eggermont J, Droogmans G (1999) VRAC: a multifunctional volume-regulated anion channel in vascular endothelium. In: Chloride channels. Oxford: Isis Medical Media Ltd
Solc CK, Wine JJ (1991) Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol 261:C658–C674
Worrell RT, Butt AG, Cliff WH, Frizzell RA (1989) A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol 256:C1111–C1119
Voets T, Droogmans G, Nilius B (1997) Modulation of voltage-dependent properties of a swelling-activated Cl− current. J Gen Physiol 110:313–325
Hagiwara N, Masuda H, Shoda M, Irisawa H (1992) Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol 456:285–302
Kubo M, Okada Y (1992) Volume-regulatory Cl− channel currents in cultured human epithelial cells. J Physiol 456:351–371
Rasola A, Galietta LJ, Gruenert DC, Romeo G (1992) Ionic selectivity of volume-sensitive currents in human epithelial cells. Biochim Biophys Acta 1139:319–323
Droogmans G, Maertens C, Prenen J, Nilius B (1999) Sulphonic acid derivatives as probes of pore properties of volume-regulated anion channels in endothelial cells. Br J Pharmacol 128:35–40
Droogmans G, Prenen J, Eggermont J, Voets T, Nilius B (1998) Voltage-dependent block of endothelial volume-regulated anion channels by calix[4]arenes. Am J Physiol 275:C646–C652
Ternovsky VI, Okada Y, Sabirov RZ (2004) Sizing the pore of the volume-sensitive anion channel by differential polymer partitioning. FEBS Lett 576:433–436
Kirk K, Ellory JC, Young JD (1992) Transport of organic substrates via a volume-activated channel. J Biol Chem 267:23475–23478
Kirk K (1997) Swelling-activated organic osmolyte channels. J Membr Biol 158:1–16
Shennan DB (2008) Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem 21:15–28
Blum AE, Walsh BC, Dubyak GR (2010) Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells. Am J Physiol Cell Physiol 298:C386–C396
Burow P, Markwardt F (2014) When S1P meets ATP. Channels (Austin) 8:385–386
Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M (2002) Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 119:511–520
Tsumura T, Oiki S, Ueda S, Okuma M, Okada Y (1996) Sensitivity of volume-sensitive Cl- conductance in human epithelial cells to extracellular nucleotides. Am J Physiol 271:C1872–C1878
Lee CC, Freinkman E, Sabatini DM, Ploegh HL (2014) The protein synthesis inhibitor blasticidin s enters mammalian cells via leucine-rich repeat-containing protein 8D. J Biol Chem 289:17124–17131
Helix N, Strobaek D, Dahl BH, Christophersen P (2003) Inhibition of the endogenous volume-regulated anion channel (VRAC) in HEK293 cells by acidic di-aryl-ureas. J Membr Biol 196:83–94
Klausen TK, Bergdahl A, Hougaard C, Christophersen P, Pedersen SF, Hoffmann EK (2007) Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich Lettre ascites cells. J Cell Physiol 210:831–842
Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA (2006) Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol 572:677–689
Decher T, Lang TJ, Nilius B, Bruggemann A, Busch TE, Steinmeyer K (2001) DCPIB is a novel selective blocker of I-Cl, I-swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol 134:1467–1479
Harrigan TJ, Abdullaev IF, Jourd'heuil D, Mongin AA (2008) Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem 106:2449–2462
Maertens C, Droogmans G, Verbesselt R, Nilius B (2002) Block of volume-regulated anion channels by selective serotonin reuptake inhibitors. Naunyn Schmiedebergs Arch Pharmacol 366:158–165
Maertens C, Wei L, Voets T, Droogmans G, Nilius B (1999) Block by fluoxetine of volume-regulated anion channels. Br J Pharmacol 126:508–514
Maertens C, Wei L, Droogmans G, Nilius B (2000) Inhibition of volume-regulated and calcium-activated chloride channels by the antimalarial mefloquine. J Pharmacol Exp Ther 295:29–36
Maertens C, Droogmans G, Chakraborty P, Nilius B (2001) Inhibition of volume-regulated anion channels in cultured endothelial cells by the anti-oestrogens clomiphene and nafoxidine. Br J Pharmacol 132:135–142
Nilius B, Prenen J, Kamouchi M, Viana F, Voets T, Droogmans G (1997) Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl− channels in macrovascular endothelial cells. Br J Pharmacol 121:547–555
Poletto Chaves LA, Varanda WA (2008) Volume-activated chloride channels in mice Leydig cells. Pflugers Arch 457:493–504
Fan HT, Morishima S, Kida H, Okada Y (2001) Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol 133:1096–1106
Ye ZC, Oberheim N, Kettenmann H, Ransom BR (2009) Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57:258–269
Motais R, Guizouarn H, Garcia-Romeu F (1991) Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochim Biophys Acta 1075:169–180
Emma F, McManus M, Strange K (1997) Intracellular electrolytes regulate the volume set point of the organic osmolyte/anion channel VSOAC. Am J Physiol 272:C1766–C1775
Strange K (1994) Are all cell volume changes the same? News Physiol Sci 9:223–228
Doroshenko P, Neher E (1992) Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol 449:197–218
Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B (1998) Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol 506(Pt 2):341–352
Wang Y, Roman R, Lidofsky SD, Fitz JG (1996) Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A 93:12020–12025
Mongin AA, Kimelberg HK (2002) ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol 283:C569–C578
Browe DM, Baumgarten CM (2004) Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl− current elicited by beta1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol 124:273–287
Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A (2004) NAD(P)H oxidase-derived H(2)O(2) signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem 279:13301–13304
Ando-Akatsuka Y, Shimizu T, Numata T, Okada Y (2012) Involvements of the ABC protein ABCF2 and alpha-actinin-4 in regulation of cell volume and anion channels in human epithelial cells. J Cell Physiol 227:3498–3510
Burow P, Klapperstuck M, Markwardt F (2015) Activation of ATP secretion via volume-regulated anion channels by sphingosine-1-phosphate in RAW macrophages. Pflugers Arch 467:1215–1226
Klausen TK, Hougaard C, Hoffmann EK, Pedersen SF (2006) Cholesterol modulates the volume-regulated anion current in Ehrlich-Lettre ascites cells via effects on Rho and F-actin. Am J Physiol Cell Physiol 291:C757–C771
Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J (1999) Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J Physiol 516(Pt 1):67–74
Pedersen SF, Beisner KH, Hougaard C, Willumsen BM, Lambert IH, Hoffmann EK (1992) Rho family GTP binding proteins are involved in the regulatory volume decrease process in NIH3T3 mouse fibroblasts. J Physiol 541:779–796
Tilly BC, Edixhoven MJ, Tertoolen LG, Morii N, Saitoh Y, Narumiya S, de Jonge HR (1996) Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol Biol Cell 7:1419–1427
Feranchak AP, Roman RM, Schwiebert EM, Fitz JG (1998) Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. J Biol Chem 273:14906–14911
Carton I, Trouet D, Hermans D, Barth H, Aktories K, Droogmans G, Jorgensen NK, Hoffmann EK, Nilius B, Eggermont J (2002) RhoA exerts a permissive effect on volume-regulated anion channels in vascular endothelial cells. Am J Physiol Cell Physiol 283:C115–C125
Du XL, Gao Z, Lau CP, Chiu SW, Tse HF, Baumgarten CM, Li GR (2004) Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J Gen Physiol 123:427–439
Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F (1998) The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. J Cell Biol 141:281–286
Tilly BC, den Van BN, Tertoolen LG, Edixhoven MJ, de Jonge HR (1993) Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem 268:19919–19922
Levitan I, Christian AE, Tulenko TN, Rothblat GH (2000) Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol 115:405–416
Romanenko VG, Rothblat GH, Levitan I (2004) Sensitivity of volume-regulated anion current to cholesterol structural analogues. J Gen Physiol 123:77–87
Nilius B, Gerke V, Prenen J, Szucs G, Heinke S, Weber K, Droogmans G (1996) Annexin II modulates volume-activated chloride currents in vascular endothelial cells. J Biol Chem 271:30631–30636
Trouet D, Hermans D, Droogmans G, Nilius B, Eggermont J (2001) Inhibition of volume-regulated anion channels by dominant-negative caveolin-1. Biochem Biophys Res Commun 284:461–465
Trouet D, Nilius B, Jacobs A, Remacle C, Droogmans G, Eggermont J (1999) Caveolin-1 modulates the activity of the volume-regulated chloride channel. J Physiol 520(Pt 1):113–119
Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RKH (2003) Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148:1–80
Forsyth SE, Hoger A, Hoger JH (1997) Molecular cloning and expression of a bovine endothelial inward rectifier potassium channel. FEBS Lett 409:277–282
Kamouchi M, Trouet D, De GC, Droogmans G, Eggermont J, Nilius B (1997) Functional effects of expression of hslo Ca2+ activated K+ channels in cultured macrovascular endothelial cells. Cell Calcium 22:497–506
Voets T, Droogmans G, Nilius B (1996) Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J Physiol 497(Pt 1):95–107
Shimizu T, Maeno E, Okada Y (2007) Prerequisite role of persistent cell shrinkage in apoptosis of human epithelial cells. Sheng Li Xue Bao 59:512–516
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol-Lond 532:3–16
Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N (2006) Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol 209:21–29
Tanabe S, Wang X, Takahashi N, Uramoto H, Okada Y (2005) HCO3 −-independent rescue from apoptosis by stilbene derivatives in rat cardiomyocytes. FEBS Lett 579:517–522
Wang X, Takahashi N, Uramoto H, Okada Y (2005) Chloride channel inhibition prevents ROS-dependent apoptosis induced by ischemia-reperfusion in mouse cardiomyocytes. Cell Physiol Biochem 16:147–154
Inoue H, Ohtaki H, Nakamachi T, Shioda S, Okada Y (2007) Anion channel blockers attenuate delayed neuronal cell death induced by transient forebrain ischemia. J Neurosci Res 85:1427–1435
Maeno E, Shimizu T, Okada Y (2006) Normotonic cell shrinkage induces apoptosis under extracellular low Cl conditions in human lymphoid and epithelial cells. Acta Physiol (Oxf) 187:217–222
Nukui M, Shimizu T, Okada Y (2006) Normotonic cell shrinkage induced by Na+ deprivation results in apoptotic cell death in human epithelial HeLa cells. J Physiol Sci 56:335–339
Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y (2007) Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J Cell Physiol 211:513–521
Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK (2010) Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol 298:C14–C25
Sorensen BH, Thorsteinsdottir UA, Lambert IH (2014) Acquired cisplatin resistance in human ovarian A2780 cancer cells correlates with shift in taurine homeostasis and ability to volume regulate. Am J Physiol Cell Physiol 307:C1071–C1080
Dezaki K, Maeno E, Sato K, Akita T, Okada Y (2012) Early-phase occurrence of K+ and Cl- efflux in addition to Ca2+ mobilization is a prerequisite to apoptosis in HeLa cells. Apoptosis 17:821–831
Szabo I, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F (1998) Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc Natl Acad Sci U S A 95:6169–6174
Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM (2015) Identification and characterization of essential genes in the human genome. Science 350:1096–1101
Banderali U, Roy G (1992) Anion channels for amino acids in MDCK cells. Am J Physiol 263:C1200–C1207
Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA (2014) LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol 592:4855–4862
Jackson PS, Strange K (1993) Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol 265:C1489–C1500
Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10:1583–1591
Mulligan SJ, MacVicar BA (2006) VRACs CARVe a path for novel mechanisms of communication in the CNS. Sci STKE 2006:e42
Kimelberg HK (2005) Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 50:389–397
Kimelberg HK, MacVicar BA, Sontheimer H (2006) Anion channels in astrocytes: biophysics, pharmacology, and function. Glia 54:747–757
Mongin AA (2007) Disruption of ionic and cell volume homeostasis in cerebral ischemia: the perfect storm. Pathophysiology 14:183–193
Barros LF, Hermosilla T, Castro J (2001) Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol A Mol Integr Physiol 130:401–409
Olney JW (1990) Excitotoxicity: an overview. Can Dis Wkly Rep 16 Suppl 1E: 47–57
Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP (1998) Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp Neurol 154:241–258
Doroshenko P, Sabanov V, Doroshenko N (2001) Cell cycle-related changes in regulatory volume decrease and volume-sensitive chloride conductance in mouse fibroblasts. J Cell Physiol 187:65–72
Klausen TK, Preisler S, Pedersen SF, Hoffmann EK (2010) Monovalent ions control proliferation of Ehrlich Lettre ascites cells. Am J Physiol Cell Physiol 299:C714–C725
Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B (2000) Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol 529(Pt 2):385–394
Voets T, Szucs G, Droogmans G, Nilius B (1995) Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflugers Arch 431:132–134
Chen L, Wang L, Zhu L, Nie S, Zhang J, Zhong P, Cai B, Luo H, Jacob TJ (2002) Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol 283:C1313–C1323
Nilius B (2001) Chloride channels go cell cycling. J Physiol 532:581
Voets T, Wei L, De SP, van DW, Eggermont J, Droogmans G, Nilius B (1997) Downregulation of volume-activated Cl− currents during muscle differentiation. Am J Physiol 272:C667–C674
Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA (2014) Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci 369:20130094
Ginzberg MB, Kafri R, Kirschner M (2015) Cell biology. On being the right (cell) size. Science 348:1245075
Habela CW, Sontheimer H (2007) Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle 6:1613–1620
Pendergrass WR, Angello JC, Kirschner MD, Norwood TH (1991) The relationship between the rate of entry into S phase, concentration of DNA polymerase alpha, and cell volume in human diploid fibroblast-like monokaryon cells. Exp Cell Res 192:418–425
Rouzaire-Dubois B, Malo M, Milandri JB, Dubois JM (2004) Cell size-proliferation relationship in rat glioma cells. Glia 45:249–257
Rouzaire-Dubois B, Milandri JB, Bostel S, Dubois JM (2000) Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflugers Arch 440:881–888
Mao J, Wang L, Fan A, Wang J, Xu B, Jacob TJ, Chen L (2007) Blockage of volume-activated chloride channels inhibits migration of nasopharyngeal carcinoma cells. Cell Physiol Biochem 19:249–258
Ransom CB, O'Neal JT, Sontheimer H (2001) Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci 21:7674–7683
Schneider L, Klausen TK, Stock C, Mally S, Christensen ST, Pedersen SF, Hoffmann EK, Schwab A (2008) H-ras transformation sensitizes volume-activated anion channels and increases migratory activity of NIH3T3 fibroblasts. Pflugers Arch 455:1055–1062
Soroceanu L, Manning TJ Jr, Sontheimer H (1999) Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J Neurosci 19:5942–5954
Schwab A, Fabian A, Hanley PJ, Stock C (2012) Role of ion channels and transporters in cell migration. Physiol Rev 92:1865–1913
Manolopoulos VG, Liekens S, Koolwijk P, Voets T, Peters E, Droogmans G, Lelkes PI, De CE, Nilius B (2000) Inhibition of angiogenesis by blockers of volume-regulated anion channels. Gen Pharmacol 34:107–116
Ziegelhoeffer T, Scholz D, Friedrich C, Helisch A, Wagner S, Fernandez B, Schaper W (2003) Inhibition of collateral artery growth by mibefradil: possible role of volume-regulated chloride channels. Endothelium 10:237–246
Acknowledgments
The work in the author’s laboratories is supported by grants from the Danish Council for Independent Research and the Kirsten and Freddy Johansen Foundation (SFP) as well as by JSPS KAKENHI grants (YO).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on: “Molecular physiology of anion channels: dual function proteins and new structural motifs”
Rights and permissions
About this article
Cite this article
Pedersen, S.F., Okada, Y. & Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflugers Arch - Eur J Physiol 468, 371–383 (2016). https://doi.org/10.1007/s00424-015-1781-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1781-6