Abstract
Background
Postoperative adhesions are frequent and significant complications that typically arise following abdominal surgery. Currently, the existing evidence for predicting the risk of adhesive small bowel obstruction (ASBO) after emergency gastrointestinal surgery (EGS) remains inadequate. A reliable perioperative model that quantifies the risk of ASBO after EGS serves as a practical tool for guiding individually tailored surveillance.
Methods
A consecutive series of 1296 patients who underwent EGS for radiologically confirmed bowel/visceral inflammation or perforation between 2012 and 2022 at a tertiary academic medical center were included in this study to establish a best-fit nomogram. The nomogram was externally validated by assessing discrimination and calibration using an independent cohort from a separate medical center.
Results
A total of 116 patients (8.9%) developed at least one episode of ASBO after EGS during a median follow-up duration of 26 months. The results of multivariable logistic analysis indicated that male sex (P = 0.043), preoperative albumin level (P = 0.002), history of pelvic radiotherapy (P = 0.038), laparotomy (P = 0.044), and intensive care unit stay ≥ 72 h (P = 0.047) were identified as independent risk factors for developing ASBO. By incorporating these predictors, the developed nomogram exhibited good accuracy in risk estimation, as evidenced by a guide-corrected C-index score of 0.852 (95% CI 0.667–0.920) in the external validation cohort. Decision curve analysis and clinical impact curve demonstrated a clinically effective predictive model.
Conclusion
By incorporating the nomogram as a supplemental tool in perioperative management, it becomes possible to accurately assess the individual's likelihood of developing ASBOs. This quantification enables surgeons to implement appropriate preventive measures, ultimately leading to improved outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emergency gastrointestinal surgery (EGS) refers to a range of surgical interventions conducted in the abdominal cavity to address acute gastrointestinal conditions. Postoperative mortality is much higher in EGS than in elective surgery (10–30% vs. 1–5%), and morbidity after EGS is twice as high as that of elective surgery [1,2,3]. Intra-abdominal adhesions are almost inevitable after EGS and remain a significant cause of long-term adhesion-related complications. During an autopsy study conducted in the early 1970s, Weibel and Majnov [4] found that adhesions were present in 51 percent, 72 percent, and 93 percent, respectively, of subjects who had undergone one minor, one major, or multiple surgeries. While the majority of intra-abdominal adhesions are asymptomatic, a subset of patients may experience significant symptoms such as small bowel obstruction, female infertility, and chronic pain [5].
Postoperative adhesions account for 40% of all cases of bowel obstruction, with involvement of the small bowel in approximately 65–75% of these cases. [6, 7]. Previous studies have documented a range of 1.3% to 9.5% for the occurrence of adhesive small bowel obstruction (ASBO) among patients undergoing elective surgery [8]. Additionally, the majority of cases tend to manifest within the first year of postoperative follow-up [8]. Furthermore, ASBO is frequently linked with persistent co-occurring medical conditions, protracted periods of hospitalization, and elevated healthcare expenditures. Notably, the complexity of disease diagnosis, coupled with significant heterogeneity among the populations studied and the failure to perform reliable follow-up, adds to the challenge of evaluating the true risk of ASBO. Estimating the precise prevalence of SABO following EGS poses a significant challenge. Moreover, the prevention of intraabdominal adhesions has garnered significant attention from researchers, leading to the evaluation of various products. Nevertheless, there is a dearth of data, and a consensus regarding the effectiveness of this intervention has yet to be reached.
Promising diagnostic tools hold the potential to identify patients with a high risk of developing ASBO, demonstrating high specificity and sensitivity. Previous studies have primarily focused on identifying various risk factors for ASBO occurrence following elective surgery, including the duration of surgery, the surgical approach, intraoperative blood loss, previous abdominal surgery, and the presence of an ileostomy [9,10,11]. However, as per our understanding, no studies have been published to date that specifically investigate the aforementioned factors in relation to EGS.
This retrospective study attempted to develop a nomogram for predicting the risk of ASBO after EGS and comprehensively assessed the available evidence identifying risk factors predisposing to this condition. This information can assist physicians in accurately identifying patients at high risk of developing ASBO, enabling them to implement promising interventions in which change surgical approaches or optimize medical management to favorably impact the prognosis of high-risk patients.
Material and methods
Ethics approval
Ethics approvals were obtained from the Institutional Review Committee of all participating centers, and patient informed consent was waived due to the retrospective nature of the study. This study was performed in accordance with the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statements [12].
Data collection and study population
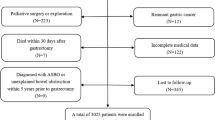
Patients who underwent at least one EGS for radiologically verified inflammation or perforation of the intestine or viscera during the period from January 2012 to December 2022 were considered for inclusion in our study. Patients who underwent EGS for acute gastrointestinal injury due to malignancy or trauma were also part of the study collective. Patients with a former abdominal operation prior to the index emergency operation, those diagnosed with unexplained bowel obstruction or ASBO within a five-year period prior to the index emergency operation, those with incomplete medical records regarding critical variables, and those who were lost during follow-up were excluded from the study. In addition, many patients, particularly those with mild disease, declined surgery. Nevertheless, in order to avoid compromising the clinical applicability of the nomogram, we excluded patients with less severe disease or who had undergone less invasive procedures, such as simple appendicitis and irreducible hernias with normal bowel viability. According to a prespecified protocol, the patient information was retrospectively extracted from a prospectively updated registry of the clinical database. A flow diagram for patient selection is shown in Fig. 1.
Definition of ASBO
ASBO was strictly defined as a combination of clinical manifestations (nausea, vomiting, abdominal pain, abdominal distention, and cessation of defecation), physical examinations (abdominal tenderness, accentuation of bowel sounds, and tympanic sounds on percussion), and abdominal CT findings (adhesion structures, beak sign, intestinal wall thickening, and dilated bowel with air-fluid planes). In addition, we summarize the clinical experience of the imaging findings for adhesion structure. Comparing the signs of CT with the intraoperative exploration findings, it can be found that CT findings of the site of SBO due to adhesions depend on the degree of the angulation/kinking and torsion. Angulation/kinking is a sharp turn of the long axis of the bowel. Angulation/kinking can lead to an acute-angled, U- or J- shaped configuration consisting of a proximal dilated and distal collapsed intestine that is visible on CT. The presence of obstructive symptoms as well as the above-mentioned imaging findings require the surgeon to consider the presence of adhesions. Furthermore, the following caveat is worth mentioning, early postoperative bowel paralysis (within 30 days postoperatively) was not registered as ASBO but rather as a surgical complication.
Follow-up
All eligible patients who survived for at least 30 days after surgery were considered eligible for follow-up. All surviving patients who underwent EGS for bowel/visceral inflammation or perforation were systematically followed up by the surgeon through outpatient interviews or telephone calls to collect data on postoperative ASBO and health status every 3–6 months for the first 2 years after surgery and then annually thereafter. The deadline was March 31, 2023.
Predictor variables
Preoperative predictor variables were as follows: age, sex, ASA, diabetes, preoperative albumin levels, history of abdominal surgery, and history of pelvic radiotherapy. Preoperative evaluation of surgical risk was completed by the attending anesthesiologist according to the American Society of Anesthesiologists physical status classification [13]. Patients’ ASA scores were obtained directly from preoperative anesthesiology screening records.
Intraoperative predictor variables included surgical approach (laparotomy or laparoscopic), the duration of surgery, blood loss, blood transfusions, intestinal status (viable and non-viable), the use of an indwelling drainage tube, and sites of surgery: mid or hindgut (abdominal wall, small intestine, appendix, rectum, colon), and foregut (stomach, gallbladder, pancreas). Intestines with strangulation, torsion, or internal herniation that the surgeon decided to remove were classified as "non-viable." It should be noted that none of the patients in the present study was administered intraoperative intraperitoneal anti-adhesion preparations.
Postoperative predictor variables included surgical complications (incisional or intraabdominal), post-operative immobilization [length of intensive care unit (ICU) stay], and the duration of in-hospital stay.
Statistical analysis
All statistical analyses were performed using R software (version 4.1.0, http://www.r-project.org/). Continuous variables are presented as mean ± standard deviation (SD) or median with first quartile and third quartile, and were compared using either the independent-sample t-test or the Mann–Whitney U-test. Categorical variables were presented as frequency and percentage, and analyzed using either the Chi-square test or Fisher's exact test. Univariate and multivariate logistic regression analyses were employed to identify potential risk factors for ASBO. Statistical significance was defined as a two-sided P value of < 0.05.
Nomogram construction and evaluation
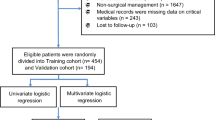
A total of 1296 patients were enrolled from the Affiliated Hospital of Qingdao University and randomly divided into a training cohort and an internal validation cohort at a ratio of 7:3 by setting the randomization seed “859929351” in the “caTools” package of R. A predictive nomogram was constructed based on selected independent predictive factors identified using multivariate logistic regression analysis of the training cohort. In addition, a dataset of 196 patients from Qilu Hospital of Shandong University was harnessed as an external validation cohort.
The discriminative ability of the nomogram was determined by Harrell’s concordance index (C-index) and area under the receiver operating characteristic curve (AUC). Calibration curves were plotted via bootstrapping with 1000 resamples to assess the calibration of the nomogram, accompanied by the Hosmer–Lemeshow goodness-of-fit test. Decision-curve analysis (DCA) and clinical impact curve (CIC) were performed to quantify the net benefits at different threshold probabilities to evaluate the clinical usefulness of the nomogram. A P-value < 0.05 was considered a statistically significant difference.
Results
Baseline characteristics
A total of 1812 consecutive patients who underwent EGS were retrospectively extracted from a prospectively maintained database dedicated to emergency cases. 1296 patients were included in the study based on the predefined inclusion and exclusion criteria. Among the included cases, 908 were allocated to the training cohort, while 388 were assigned to the internal validation cohort, following a random allocation process. The patients’ median age was 51 years (IQR 37.5–60.5), with a male predominance. The median follow-up duration was 26 (IQR 7–48) months. Detailed information on the baseline characteristics is presented in Table 1.
Overall, 116 patients (8.9%) experienced at least one episode of ASBO. The median time since the first postoperative seizure of ASBO was 24 months (IQR 14–36 months). Among these 116 patients, 83 (71.6%) required conservative treatment, while 33 (28.4%) required surgery to relieve the obstruction. Among the surgically treated patients, 12 underwent intestinal adhesions release and 21 underwent bowel resection and anastomosis. In addition, during the follow-up of ASBO patients, 28 (24.1%) experienced recurrence.
Risk factors for ASBO after EGS
Univariate and multivariate analyses of risk factors for developing ASBO are shown in Table 2. Multivariable backward stepwise logistic regression analysis revealed several independent risk predictors strongly associated with ASBO. These predictors included male sex (OR = 24.263, P = 0.043), a history of pelvic radiotherapy (OR = 26.103, P = 0.038), undergoing laparotomy (OR = 22.855, P = 0.044), preoperative albumin levels (OR = 0.644, P = 0.002), and ICU stay of 72 h or longer (OR = 23.337, P = 0.047).
Development and validation of an ASBO-predicting nomogram
Based on the results of the multivariate logistic regression analysis, five independent predictors were integrated to establish a nomogram for predicting ASBO (Fig. 2A). Detailed information on the nomogram is as follows: male sex (no [0] or yes [1]), history of pelvic radiotherapy (no [0] or yes [1]), surgical approach (laparoscopy [0] or laparotomy [1]), preoperative albumin levels, and ICU stay (< 72 h [0] or ≥ 72 h [1]). A higher total score, based on the sum of the assigned number of points for each factor in the nomogram, was associated with a higher risk of ASBO.
The favorable discrimination of the nomogram was confirmed using the training [C-index (95% CI), 0.976 (0.957–0.995)] and validation [0.910 (0.824–0.995)] sets. Moreover, the nomogram yielded a favorable AUC, indicating better capability for ASBO prediction (Fig. 3). Calibration plots vividly indicated good concordance between the risk probabilities of the model obtained from the training cohort and the actual probabilities in the training and internal validation cohorts (Fig. 2B and C).
To validate the stability of the nomogram, we performed external validation using data from 196 patients at Qilu Hospital of Shandong University. The detailed characteristics of the external validation cohort are presented in Table 3. The C-index was 0.852 (95% CI 0.667–0.920). The calibration plot of the external validation was shown in Fig. 4.
Clinical usefulness
The DCA curve of the nomogram is shown in Fig. 5A. It was demonstrated that when the threshold probability was greater than 0.1, using the nomogram to predict ASBO after EGS added more net benefit than all-or-none treatment strategies. We further constructed a CIC curve by digitizing the predicted results for 1000 patients, and it was observed that the predicted results were close to the actual results (Fig. 5B). The DCA and CIC analyses demonstrated a clinically effective predictive model.
DCA and CIC analysis of the nomogram. A When the threshold probability was greater than 0.1, using the nomogram to predict ASBO after EAS added more net benefit than all-or-none treatment strategies; B Depicted the prediction of risk stratification of 1000 patients by using resample bootstrap method. EGS, Emergency gastrointestinal surgery
Discussion
ASBO constituted a significant public health concern, contributing to considerable morbidity, increased reoperation rates, and elevated costs. Previous studies have reported the incidences of ASBO ranging from 1.3% to 9.5% following elective surgeries [8]. Our study similarly observed a comparable occurrence, with 116 out of 1296 patients developing ASBO. Accurate identification of patients at a heightened risk of ASBO is paramount for the implementation of effective preventive strategies. However, in spite of extensive research, definitive risk factors predisposing patients to adhesion-related morbidity, specifically ASBO, remain elusive [9,10,11].
To the best of our knowledge, the present study is the first to comprehensively investigate the occurrence of ASBO after EGS through a large-scale retrospective cohort analysis of patient data. With multivariate analysis, we have identified several risk factors for ASBO that have not been previously demonstrated. More importantly, we developed a visual nomogram that incorporates the identified independent predictors to generate a probability of ASBO that is unique to an individual. Our assessments of discriminatory capacity and calibration ability indicated that the model is robust and possesses strong predictive capabilities for ASBO.
Before commencing this study, we formulated a hypothesis suggesting that immobilization would impact the postoperative recovery of both mesenteric blood flow and intestinal dynamics. Thus, we investigated the factors affecting the duration of immobilization, such as age, severity of illness, and ICU stay. Our study revealed that patients with a prolonged ICU stay (> 72 h) faced an elevated risk of developing ASBO. Surprisingly, no studies have reported any association between shorter immobilization times and reduced risk of ASBO or shorter duration of episodes until then. So far, the evidence supporting the notion that early mobilization improves the recovery of bowel function is insufficient. Further confirmation of this mechanism via more in-depth studies is still required.
A strong association was found between preoperative albumin levels and ASBO, indicating that preoperative nutritional risk or malnutrition heightened the risk of ASBO after EGS (OR = 0.664). Hypoalbuminemia contributes to the leakage of fluid from the intestinal plasma membrane into the peritoneal cavity and the formation of ascites. An increasing body of evidence indicates that hypoalbuminemia triggers hyperfibrinogenemia and promotes the organization of the fibronectin matrix and fibrillar adhesions. In addition, preoperative albumin levels reflect the body's response to inflammation and continuous exposure to various inflammatory factors, and in the presence of hypoalbuminemia, the function of the immune system is significantly reduced [14, 15]. Spontaneous peritonitis is a frequent complication associated with ascites, and the repeated occurrences of peritonitis additionally facilitate the development of adhesions [16]. Ryash et al. [17] conducted a prospective study and confirmed hypoalbuminemia as an independent risk factor by analyzing 92 perioperative variables. This finding is consistent with that of the present study. Consequently, it is crucial to identify patients preoperatively who have hypoalbuminemia or are at nutritional risk to ensure timely and effective initiation of nutritional support.
Additionally, the findings of the current study clearly showed that the probability of ASBO after EGS was significantly higher in males than females. However, the reason for this association is not yet known. At present, literature concerning this aspect has reported inconsistent results. Riber et al. [18] examined the role of sex in emergency open appendectomy for complicated appendicitis and concluded that females had an almost fourfold higher overall risk for ASBO requiring surgical intervention. In Contrast, Andresson et al. [19] suggested that females were at a slightly lower risk of developing ASBO (OR = 0.8) in a similar population. In this case, we speculate that this result may be related to the narrow pelvic space and high visceral fat content. Therefore, conclusions regarding the role of sex cannot be withdrawn.
An increasing number of studies have demonstrated the feasibility and advantages of adjuvant radiotherapy. Nonetheless, the long-term effects of radiotherapy should be thoroughly evaluated to avoid undesired side effects. Small bowel obstruction is known to be a long-term complication associated with radiotherapy, and its incidence increases when a large area of the small bowel is irradiated, especially at irradiation doses exceeding 50–55 Gy [20]. Over the short term, radiotherapy may cause intestinal edema and decrease bowel motility. In the long run, heavily irradiated small bowels may develop fibrosis and ischemia, which further contribute to the occurrence of ASBO. However, the incidence of ASBO after radiotherapy varies widely from literature to literature, and there is no explicit rationale to explain this variation. In addition, fixed loops of the small bowel in the pelvis were observed in 65% of patients receiving postoperative irradiation relative to 18% of patients without surgery [21]. Peeters et al. [22] found no significant difference in the incidence of ASBO between the surgical group and the group that underwent surgery followed by a short-course of radiotherapy. Taken together, these results emphasize the superiority of preoperative RT in reducing the long-term risk of ASBO.
We found that multiple abdominal surgeries played a role in the onset of postoperative ASBO, which has been confirmed in previous studies [5, 8, 23, 24]. However, our findings did not identify it as an independent risk factor for ASBO after regression analysis and controlling for confounding factors.
The approach of surgery was a well-established risk factor for ASBO. Laparotomy led to an inflammatory response, whereas laparoscopic surgery reduced abdominal injury and direct contact with the intestines, resulting in a reduced inflammatory response and adhesion. This explains why laparotomy is more prone to ASBO. This is consistent with previous studies conducted by Hossein et al. [25], Nakajima et al. [26], and Eto et al. [27]. However, we could not confirm that laparoscopic surgery could replace laparotomy in all the cases. Patients with difficult conditions, hemodynamic instability, or a history of multiple abdominal surgeries are generally considered to be unsuitable for laparoscopic surgery. In addition, the long duration of the procedure may be problematic, as it is associated with postoperative complications. This underscores the importance of careful patient selection when selecting an appropriate emergency surgical approach.
Physicians are increasingly acknowledging the significant correlation between EGS and a disproportionately high risk of ASBO. Additionally, there has been a gradual increase in the annual number of procedures performed to treat various etiologies. However, little effort has been dedicated to enhancing the capability of general surgeons to effectively communicate preoperative individual risks to patients. A nomogram is a graphical model that utilizes mathematical formulas to estimate the probability of an outcome and enhance predictive accuracy for individuals. In our study, we developed a nomogram based on perioperative information that is routinely collected, ensuring its broad applicability and generalizability. Surgeons can use this easy-to-use nomogram to personalize the prediction of ASBO probability after EGS, aligning with the current claims in precision medicine.
We recognized from the lessons learned from perioperative care that there is substantial room for improvement in the treatment of our emergency surgical cases, and we would like to share these insights with readers. Multimodal analgesic methods, including local infiltration anesthesia, epidural self-control analgesia, and a transversus abdominis plane block, may provide better pain control without increasing opioid consumption. Adequate analgesia can contribute to the reduction of postoperative ileus by allowing patients to mobilize earlier. Owing to their inherent side effects, opioids that predominantly agonize the κ receptor are used whenever possible. Moreover, patients without hyperglycemia were encouraged to chew gum from the day after surgery. Chewing gum is a form of pseudo-feeding that stimulates bowel motility and secretion through neurohumoral reflexes, thus enhancing the early recovery of gastrointestinal function after surgery. Excessive fluid administration can lead to intestinal edema. Restricting fluid intake while carefully monitoring vital signs can potentially expedite the recovery of bowel function. Early enteral nutrition stimulates the gut-brain axis, which leads to secretion of gastrointestinal hormones and increased intestinal motility, consequently reducing as a result of ASBO. Furthermore, shortening the duration of gastrointestinal decompression and abdominal drainage seems to be effective in decreasing ASBO in patients with these conditions.
However, there were limitations in our study. First, the retrospective study design resulted in unavoidable selection bias. Second, although our analysis included the use of a bootstrap sampling procedure to estimate the predictive accuracy of new data, this process would benefit from further evaluation of external validity, such as the expected use of the nomogram. Third, it would be pertinent to include information on the types of procedures most commonly performed in their collective, a variable that was not evaluated due to insufficient data, limiting the ability to observe its correlation with ASBO. Finally, the surgeries were performed by several surgeons, which could lead to an inevitable potential bias.
Conclusion
By implementing this practical tool, healthcare professionals will be able to identify high-risk individuals and provide targeted interventions to reduce the occurrence of postoperative adhesions and ASBO. Ultimately, the incorporation of a decision support system based on this nomogram will strengthen clinical practice and improve patient management in emergency gastrointestinal surgery. Acquiring more evidence from multicenter is warranted to validate this model before clinical application in the future.
Data availability
Due to the privacy of patients, the raw data cannot be available for public access but can be obtained from Xiu Wenchao (henry_don@163.com) upon reasonable request.
References
Boden I, Sullivan K, Hackett C et al (2022) Intensive physical therapy after emergency laparotomy: pilot phase of the incidence of complications following emergency abdominal surgery get exercising randomized controlled trial. J Trauma Acute Care Surg 92(6):1020–1030. https://doi.org/10.1097/ta.0000000000003542
Sharoky C, Bailey E, Sellers M et al (2017) Outcomes of hospitalized patients undergoing emergency general surgery remote from admission. Surgery 162(3):612–619. https://doi.org/10.1016/j.surg.2017.05.008
Tengberg L, Cihoric M, Foss N et al (2017) Complications after emergency laparotomy beyond the immediate postoperative period - a retrospective, observational cohort study of 1139 patients. Anaesthesia 72(3):309–316. https://doi.org/10.1111/anae.13721
Weibel M, Majno G (1973) Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg 126(3):345–353. https://doi.org/10.1016/s0002-9610(73)80123-0
Krielen P, Stommel M, Pargmae P et al (2020) Adhesion-related readmissions after open and laparoscopic surgery: a retrospective cohort study (SCAR update). Lancet (London, England) 395(10217):33–41. https://doi.org/10.1016/s0140-6736(19)32636-4
Ellis H, Moran B, Thompson J et al (1999) Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet (London, England) 353(9163):1476–1480. https://doi.org/10.1016/s0140-6736(98)09337-4
Mullan C, Siewert B, Eisenberg R (2012) Small bowel obstruction. AJR Am J Roentgenol 198(2):W105-117. https://doi.org/10.2214/ajr.10.4998
Barmparas G, Branco B, Schnüriger B et al (2010) The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg 14(10):1619–1628. https://doi.org/10.1007/s11605-010-1189-8
Kang W, Park Y, Jo Y et al (2018) Early postoperative small bowel obstruction after laparotomy for trauma: incidence and risk factors. Annals of surgical treatment and research 94(2):94–101. https://doi.org/10.4174/astr.2018.94.2.94
Pan T, Galiullin D, Chen X et al (2021) Incidence of adhesive small bowel obstruction after gastrectomy for gastric cancer and its risk factors: a long-term retrospective cohort study from a high-volume institution in China. Updat Surg 73(2):615–626. https://doi.org/10.1007/s13304-021-00983-y
Zheng H, Liu Y, Chen Z et al (2022) Novel nomogram for predicting risk of early postoperative small bowel obstruction after right colectomy for cancer. World J Surg Oncol 20(1):19. https://doi.org/10.1186/s12957-022-02489-2
Collins GS, Reitsma JB, Altman DG et al (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 350:g7594. https://doi.org/10.1136/bmj.g7594
Mudumbai SC, Pershing S, Bowe T et al (2019) Development and validation of a predictive model for American Society of Anesthesiologists Physical Status. BMC Health Serv Res 19(1):859. https://doi.org/10.1186/s12913-019-4640-x
Jeppesen MH, Tolstrup MB, Kehlet Watt S et al (2016) Risk factors affecting morbidity and mortality following emergency laparotomy for small bowel obstruction: a retrospective cohort study. Int J Surg 28:63–68. https://doi.org/10.1016/j.ijsu.2016.02.059
Kronberg U, Kiran RP, Soliman MS et al (2011) A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg 253(1):78–81. https://doi.org/10.1097/SLA.0b013e3181fcb83e
Raynor MC, Pruthi RS (2013) Postoperative Ileus: time for an evidence-based consensus. Eur Urol 64(4):598–599. https://doi.org/10.1016/j.eururo.2012.12.025
Vather R, Josephson R, Jaung R et al (2015) Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 157(4):764–773. https://doi.org/10.1016/j.surg.2014.12.005
Riber C, Søe K, Jørgensen T et al (1997) Intestinal obstruction after appendectomy. Scand J Gastroenterol 32(11):1125–1128. https://doi.org/10.3109/00365529709002991
Andersson RE (2001) Small bowel obstruction after appendicectomy. Br J Surg 88(10):1387–1391. https://doi.org/10.1046/j.0007-1323.2001.01869.x
Coia LR, Myerson RJ, Tepper JE (1995) Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys 31(5):1213–1236. https://doi.org/10.1016/0360-3016(94)00419-l
Green N (1983) The avoidance of small intestine injury in gynecologic cancer. Int J Radiat Oncol Biol Phys 9(9):1385–1390. https://doi.org/10.1016/0360-3016(83)90271-7
Peeters KC, van de Velde CJ, Leer JW et al (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol 23(25):6199–6206. https://doi.org/10.1200/jco.2005.14.779
Taylor GW, Jayne DG, Brown SR et al (2010) Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. Br J Surg 97(1):70–78. https://doi.org/10.1002/bjs.6742
Yamada T, Okabayashi K, Hasegawa H et al (2016) Meta-analysis of the risk of small bowel obstruction following open or laparoscopic colorectal surgery. Br J Surg 103(5):493–503. https://doi.org/10.1002/bjs.10105
Masoomi H, Kang CY, Chaudhry O et al (2012) Predictive factors of early bowel obstruction in colon and rectal surgery: data from the Nationwide Inpatient Sample, 2006–2008. J Am Coll Surg 214(5):831–837. https://doi.org/10.1016/j.jamcollsurg.2012.01.044
Nakajima J, Sasaki A, Otsuka K et al (2010) Risk factors for early postoperative small bowel obstruction after colectomy for colorectal cancer. World J Surg 34(5):1086–1090. https://doi.org/10.1007/s00268-010-0462-z
Eto K, Kosuge M, Ohkuma M et al (2018) Defunctioning ileostomy is a key risk factor for small bowel obstruction after colorectal cancer resection. Anticancer Res 38(3):1789–1795. https://doi.org/10.21873/anticanres.12417
Funding
This study was conducted without external funding support.
Author information
Authors and Affiliations
Contributions
Study conception and design: Puyue Gao; Zongping Yu; Yiqi Wang; Wenchao Xiu; Acquisition of data: Puyue Gao; Zongping Yu; Analysis and interpretation of data: Puyue Gao; Wenchao Xiu; Drafting of manuscript: Puyue Gao; Critical revision of manuscript: Wenchao Xiu.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, P., Yu, Z., Wang, Y. et al. Nomogram for predicting adhesive small bowel obstruction following emergency gastrointestinal surgery. Langenbecks Arch Surg 408, 388 (2023). https://doi.org/10.1007/s00423-023-03126-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03126-6