Abstract
Background/aims
Intestinal ischaemia (II) is the most critical factor to determine in patients with adhesive small bowel obstruction (ASBO) because intestinal ischaemia could be reversible. The aim of this study was to create a clinicoradiological score to predict II in patients with ASBO.
Methods
We conducted a retrospective study including 124 patients with ASBO. Logistic regression analysis was used to identify predictive factors of II. We assigned points for the score according to the regression coefficient. The area under the curve (AUC) was determined using receiver operating characteristic curves.
Results
Six independent predictive factors of II were identified: age, pain duration, body temperature, WBC, reduced wall enhancement and segmental mesenteric fluid at CT scan. According to the regression, coefficient points were assigned to each of the variables associated with II. The estimated rates of II were calculated for the total scores ranging from 0 to 24. The AUC of this clinicoradiological score was 0.92. A cut-off score of 6 was used for the low-probability group (the risk of II was 1.13%). A score ranging from 7 to 15 defined intermediate-probability group (the risk of II was 44%). A score ≥16 defined high-probability group (100% of patients in this group had II).
Conclusions
We performed a score to predict the risk of intestinal II with a good accuracy (the AUC of our score exceeded 0.90). This score is reliable and reproducible, so it can help surgeon to prioritize patients with II for surgery because ischaemia could be reversible, avoiding thus intestinal necrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adhesions are frequent complications of abdominal surgery and are the most frequent aetiology of small bowel obstruction (SBO) (60–70%) [1]. There is a dilemma in the management of patients with adhesive small bowel obstruction (ASBO): delay in surgery may result in intestinal ischaemia (II) and necrosis, but unnecessary laparotomy may result in new adhesion formation [2, 3].
The accurate and early recognition of the presence II in patients with ASBO is important, to plan an early exploratory laparotomy as ischaemia could be reversible [4], but delayed diagnosis and intervention can result in higher incidence of bowel resections and postoperative morbidity and mortality. The decision of emergent surgery in patients with ASBO is not well standardized and is based on surgeon’s judgement [5]. The aim of this study was to devise clinicoradiological score for predicting the risk of II in patients with ASBO.
Methods
Patients
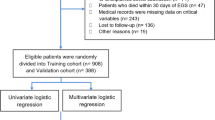
Two hundred and eight consecutive cases of small bowel obstruction (SBO) were collected from January 2008 to December 2017 by using the database from the Department of Surgery at Mohamed Tahar Maamouri Hospital. This study was carried out in compliance with the Declaration of Helsinki and the current ethical guidelines and was approved by the institutional research and ethics board of our hospital. The medical records, including data for symptoms and signs, laboratory tests and imaging examinations, were reviewed.
Inclusion criteria included patients older than 16 years old, with medical history of previous laparotomy, with ASBO confirmed by morphological examination and/or by surgery.
Exclusion criteria included patients younger than 16 years old (20 patients), patients with Crohn’s disease (20 patients), ulcerative colitis (2 patients), intestinal obstruction secondary to colon cancer (10 patients), small bowel carcinoma (6 patients), gastrointestinal stromal tumours (2 patients), abdominal tuberculosis (7 patients) and internal hernia (9 patients). Patients with thrombointestinal ischaemia were also excluded. Patients operated 1 month or less before the current episode were excluded because the obstruction may be the result of adhesive and/or inflammatory phenomena (11 patients). There were no restrictions on the type of first surgery and no upper age limit.
Finally, 124 patients met the inclusion criteria and were enrolled in the study.
Data collection
All clinical and biological data were collected during admission and included age, sex, past history of surgery, duration of symptoms before admission, vomiting, body temperature, heart and respiratory rates, peritoneal irritation signs and white blood cell count (WBC). Radiological data included the presence of a transition zone, free peritoneal fluid, bowel wall enhancement after intravenous contrast of dilated intestine, maximum thickness of bowel wall, maximum diameter of the distended intestine, the presence of segmental mesenteric fluid and the presence of faeces sign.
Treatment and subgroup definitions
Based on clinical judgement, patients with suspected simple obstruction did not undergo emergent laparotomy and had medical treatment. Patients with suspected complicated ASBO underwent urgent laparotomy.
There were three clinical outcome categories: patients with ASBO and successful conservative treatment until discharge, patients who underwent operation but had no evidence of II and patients who underwent urgent laparotomy with evidence of II.
Finally, 101 patients with no II (patients successfully managed without surgical intervention, and patients without evidence of II at laparotomy) were compared to 23 patients complicated by II.
Statistical analysis
All statistical analyses were carried out using the IBM SPSS Statistics software program, version 22.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables were presented as the median and range. Categorical variables were presented as numbers and percentages. Univariate analysis was performed with the Student t test for continuous variables and with the Chi-square test for categorical variables. Logistic regression analysis was used to identify independent predictive factors of II by calculation of odds ratios and its 95% CI. A p ≤ 0.05 was considered statistically significant. Significant continuous variables were transformed into categorical variables using receiver operating characteristic (ROC) curves. The optimal cut-off point with the highest sum of sensitivity and specificity was chosen for each variable.
Score derivation
A score was calculated for each patient according to the regression coefficient of variables identified in multivariate analysis. A ROC curve was drawn to assess the ability of the score to predict II. The resulting statistical information was presented using forest plots. The optimal cut-off points of the factors were evaluated using ROC curves.
Finally, patients were divided into three groups: (1) a low-probability group with a low risk of II (<5%); (2) a high-probability group with high risk of II(>90%); (3) an intermediate-probability group.
Results
Characteristics of study population
Between January 2008 and December 2017, 124 patients with ASBO were admitted to our department. Of these 124 patients, there were 80 males (64.5%) and 44 females (35.5%) with ages ranging from 20 to 87 years old, with a median age of 52 years (45–75 years).
59.6% of patients were operated by midline laparotomy, 24% by McBurney’s incision, 8% by Pfannenstiel incision, 5.2% by right subcostal incision, 1.6% by left subcostal incision and 1.6% by laparoscopy. The average duration from the first surgical intervention to the ASBO episode was 7.5 years (6 months–15 years). The average pain duration before admission was 52 h (2–96 h).
Thirty-five patients (28.22%) were successfully managed conservatively. Eighty-nine patients (72.78%) required surgery.
Among operated patients, sixty-six patients had no evidence of II and underwent lysis of adhesions.
Twenty-three patients were complicated by II. Among these patients, 15 patients (with irreversible II) had bowel resection, while eight patients were successfully managed conservatively without intestinal resection because the II was reversible after lysis of adhesions and irrigation of the ischaemic loop with warm saline solution, the ischaemic bowel improved in colour and peristalsis.
In summary, 101 patients with no II (patients successfully managed without surgical intervention (35 patients), and patients undergoing laparotomy without evidence of II (66 patients)) were compared to 23 patients complicated by II.
Univariate analysis
Univariate analysis identified a number of parameters present at a higher frequency in patients with II (Table 1).
On univariate analysis, II was significantly associated with age, hypertension, duration from the first surgical intervention, pain duration before admission, respiratory rate, body temperature, peritoneal signs and WBC.
Multiple CT scan findings were significantly more common in patients with II, including ascites, thick-walled small bowel, segmental mesenteric fluid and reduced wall enhancement.
Significant continuous variables were transformed into categorical variables using receiver operating characteristic (ROC) curves. The optimal cut-off point with the highest sum of sensitivity and specificity was chosen for each variable (the cut-off point was 67.5 years for age, 72 h for pain duration before admission, 37.8 °C for body temperature, 10,000/mm3 for WBC, one year for the duration from the first surgical intervention, 20/min for the respiratory rate).
Multivariate analysis
Six independent predictive factors significantly associated with II were identified in multivariate analysis (Table 1):
Age ≥ 67.5 years: OR = 9.20, CI95 % [1.06–35.33].
Pain duration before admission > 72 h: OR = 4.00, CI95 % [0.99–18.52].
Body temperature ≥ 37.8 °C: OR = 2.13, CI95 % [1.28–29.31].
WBC (×109/L) > 10: OR = 2.75, CI95 % [1.24–11.65].
Reduced wall enhancement: OR = 5.57, CI95 % [3.78–16.34].
Segmental mesenteric fluid: OR = 2.08, CI95 % [1.35–12.31].
Elaboration of a clinicoradiological score for prediction of II
According to the regression, coefficient points were assigned to each of the 6 variables:
Age ≥ 67.5 years: 9 points.
Pain duration before admission > 72 h: 4 points.
Body temperature ≥ 37.8 °C: 2 points.
WBC (×109/L) > 10: 2 points.
Reduced wall enhancement: 5 points.
Segmental mesenteric fluid: 2 points.
The estimated rates of II were calculated for the total scores ranging from 0 to 24.
ROC curve was generated to assess the predictive ability of this clinicoradiological score for prediction of II (Fig. 1).
The optimal cut-off point with was for the score of 7. Sensitivity at the cut-off point was 95.7%, specificity was 86.1%, and the AUC at the cut-off point was 0.92 (CI95 % [0.85–0.98]).
The positive predictive value (PPV) of this score was 60%, and the negative predictive value (NPV) was 97.7%.
Probability categories (low-, intermediate-, or high-probability) were then divided using cut-offs to create the low or high incidence of II in each category (Fig. 2):
A cut-off score of 6 was used for the low-probability group (only one/88 patients with a score ≤ 6 had II); PPV in this group was 1.13%; and NPV was 53.8%.
A score ranging from 7 to 15 defined intermediate-probability group (11/25 patients in this group had II); PPV in this group was 44%; and NPV was 87.87%.
A score ≥ 16 defined high-probability group (11/11 patients in this group had II); PPV in this group was 100%; and NPV was 89.39%.
Discussion
In the current study, six independent predictive factors of II in patients with ASBO were identified: age, pain duration before admission, body temperature, WBC, reduced wall enhancement and segmental mesenteric fluid at CT scan. A predictive score of II was established based on these independent predictive factors.
Adhesions are frequent complications of abdominal surgery and are the most frequent aetiology of SBO (60–70%) [6, 7]. There is a dilemma in the management of patients with ASBO: delay in surgery may result in intestinal ischaemia (II) and necrosis, but unnecessary laparotomy may result in new adhesions formation, but II can be difficult to determine clinically [1]. In fact, physical examination for detection of strangulation had low sensitivity (48%) [8, 9].
To the best of our knowledge, only two previous studies tried to establish clinicoradiological scores to predict the risk of strangulated small bowel obstructions [5, 10].
There were two major methodological differences between these studies and our study.
First, concerning the inclusion criteria, the previous studies focused on all patients with small bowel obstruction regardless the aetiology, whereas we included only patients with ASBO, in the current study.
Second, the primary endpoint was the need of bowel resection for the study of Schwenter et al. [5], the presence of bowel strangulation for the study of Huang et al. [10] and the presence of II for our study.
We think that II is the most critical factor to determine in patients with ASBO. II requires prompt recognition and early intervention to avoid resections, because II could be reversible.
As, reported by Di Saverio et al. [4], the assessment of reversibility/irreversibility of the ischaemia could be determined when five criteria are taken into consideration:
Reversibility of the discoloration of the bowel wall;
The presence or not of pallid areas in the bowel wall;
The presence or not of peristalsis;
The presence or not of pulsation in mesenteric vessels;
The presence or not of microcirculation on the bowel wall.
If the ischaemic bowel improved in colour and peristalsis, after lysis of adhesions and irrigation of the ischaemic loop with warm saline solution, ischaemia could be considered reversible and conservation of the bowel could be attempted (eight cases in our study). As reported by Duron et al. [11], a more accurate assessment of intestine viability might be suggested through the use of a fluorescein test and a Doppler examination.
Our study showed that age, pain duration before admission, body temperature, WBC, reduced wall enhancement and segmental mesenteric fluid at CT scan significantly associated with occurrence of II in ASBO.
The six predictive factors were determined by logistic regression analysis, which was used to identify independent predictive factors of II by calculation of odds ratios and its 95% CI. In the current study, age had the strongest independent association with II. This finding was consistent with previous investigations [12,13,14,15].
Like our study, several previous publications found a significant correlation between II (and/or strangulation and/or bowel resection) on the one hand, and pain duration before admission [5, 15], body temperature [10, 16], and WBC [1, 5, 16,17,18] on the other hand.
Recently, some studies highlighted the value of other inflammatory indicators for the prediction of II in ASBO, such as C-reactive protein (CRP) [9], and procalcitonin [19].
The value of plain X-rays was recently discussed and judged limited [9] as plain X-ray does not detect the early signs of peritonitis or strangulation [9].
Recently, CT scan has made remarkable progress and has become a valuable imaging modality for determining the risk of strangulation in patients with SBO [20,21,22]. Zalcman et al. [23] estimated a sensitivity for contrast CT scanning of 96% and an NPV of 99% for II in the presence of SBO. As reported in our study, Sheedy et al. [24] found that reduced wall bowel enhancement was the most specific sign for II. Hayakawa et al. [25] added two other signs suggesting ischaemia: localized mesenteric fluid accumulation and localized pneumatosis.
In this reports, we tried to generate a simple clinicoradiological score to predict II in patients with ASBO. The AUC of this clinicoradiological score was 0.92 (the AUC exceeded 0.90, indicating the good accuracy of the score). Our score is most useful in the high- and low-probability groups. The intermediate-probability group requires more careful interpretation.
Limitations
This study has some limitations: first, this is a retrospective study, such as incomplete or missing data acquired from chart reviews. For instance, some inflammatory biomarkers were not frequently used in our department such as CRP, procalcitonin, erythrocyte sedimentation and neutrophil-to-lymphocyte ratio. Second, it is a mono-centric work, so it requires further validation. Further large volume multicentric studies are needed.
Conclusions
Six independent predictive factors of II in patients with ASBO were identified: age, pain duration before admission, body temperature, WBC, reduced wall enhancement and segmental mesenteric fluid at CT scan. Our prediction model can help in evaluating the risk of II in patients with ASBO, and thus, this would help the busy surgeon in prioritizing patients with II for an emergent laparotomy to avoid intestinal necrosis and to decrease the need of intestinal resections. Further large volume multicentric studies are needed.
References
Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L et al (2013) Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 8:42
Lorentzen L, Øines MN, Oma E, Jensen KK, Jorgensen LN (2017) Recurrence after operative treatment of adhesive small-bowel obstruction. J Gastrointest Surg 13:329–334
Bilderback PA, Massman JD 3rd, Smith RK, La Selva D, Helton WS (2015) Small bowel obstruction is a surgical disease: patients with adhesive small bowel obstruction requiring operation have more cost-effective care when admitted to a surgical service. J Am Coll Surg 221:7–13
Di Saverio S, Gori A, Chisari E, Wheeler J, Lim R (2019) Laparoscopic management of adhesive small bowel obstruction with strangulation: when to resect and how to distinguish reversible from nonreversible bowel ischaemia—a video vignette. Colorectal Dis 21(6):727–729
Schwenter F, Poletti PA, Platon Perneger T, Morel P, Gervaz P (2010) Clinicoradiological score for predicting the risk of strangulated small bowel obstruction. Br J Surg 37(7):1119–1125
Parker MC, Ellis H, Moran BJ, Thompson JN, Wilson MS, Menzies D et al (2001) Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis Colon Rectum 44(6):829–830
Ellis H (1998) The magnitude of adhesion related problems. Ann Chir Gynaecol 87(1):9–11
Choudhry AJ, Haddad NN, Rivera M, Morris DS, Zietlow SP, Schiller HJ et al (2016) Medical malpractice in the management of small bowel obstruction: A 33-year review of case law. Surgery 160(4):1017–1027
Ten Broek RPG, Krielen P, Di Saverio S, Coccolini F, Biffl WL, Ansaloni L et al (2018) Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2017 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 13:24
Huang X, Fang G, Lin J, Xu K, Shi H, Zhuang L (2018) A prediction model for recognizing strangulated small bowel obstruction. Gastroenterol Res Pract 2018:7164648
Duron JJ, du Montcel ST, Berger A, Muscari F, Hennet H, Veyrieres M et al (2008) Prevalence and risk factors of mortality and morbidity after operation for adhesive postoperative small bowel obstruction. Am J Surg 195(6):726–734
Duron JJ (2001) Acute intestinal occlusion. Rev Pract 51(15):1670–1674
Fevang BT, Jensen D, Fevang J, Søndenaa K, Ovrebø K, Røkke O et al (2000) Upper gastrointestinal contrast study in the management of small bowel obstruction–a prospective randomised study. Eur J Surg 166(1):39–43
Bizer L, Liebling RW, Delany HM, Gliedman ML (1981) Small bowel obstruction: the role of nonoperative treatment in simple intestinal obstruction and predictive criteria for strangulation obstruction. Surgery 89(4):407–413
Lo AM, Evans WE, Carey LC (1966) Review of small bowel obstruction at Milwaukee County General Hospital. Am J Surg 111(6):884–887
Nandyala VN, Chintakindi SB, Kundarapu G (2016) A study of preoperative predictive factors of strangulation in acute small intestinal obstruction. Int Surg J 3(3):1386–1391
Lefall LD, Syphax B (1970) Clinical aids in strangulation intestinal obstruction. Am J Surg 120:756–759
Strik C, Stommel MW, Schipper LJ, van Goor H, Ten Broek RP (2016) Long term impact of adhesions on bowel obstruction. Surgery 159(5):1351–1359
Cosse C, Regimbeau JM, Fuks D, Mauvais F, Scotte M (2013) Serum procalcitonin for predicting the failure of conservative management and the need for bowel resection in patients with small bowel obstruction. J Am Coll Surg 216(5):997–1004
Mu JF, Wang Q, Wang SD, Wang C, Song JX, Jiang J et al (2018) Clinical factors associated with intestinal strangulating obstruction and recurrence in adhesive small bowel obstruction: A retrospective study of 288 cases. Medicine (Baltimore) 97(34):e12011
Matsushima K, Inaba K, Dollbaum R, Cheng V, Khan M, Herr K et al (2016) High-density free fluid on computed tomography: a predictor of surgical intervention in patients with adhesive small bowel obstruction. Gastrointestinal Surg 20:1861–1866
Hwang JY, Lee JK, Lee JE, Baek SY (2009) Value of multidetector CT in decision making regarding surgery in patients with small-bowel obstruction due to adhesion. Eur Radiol 19:2424–2431
Zalcman M, Sy M, Donckier V, Closset J, Gansbeke DV (2000) Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am J Roentgenol 175:1601–1607
Sheedy SP, Earnest F 4th, Fletcher JG, Fidler JL, Hoskin TL (2006) CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 241(3):729–736
Hayakawa K, Tanikake M, Yoshida S, Yamamoto A, Yamamoto E, Morimoto T (2013) CT findings of small bowel strangulation: the importance of contrast enhancement. Emerg Radiol 20(1):3–9
Funding
No grant support for the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Ethical approval
The study was approved by the institutional research and ethics board of Mohamed Tahar Maamouri Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouassida, M., Laamiri, G., Zribi, S. et al. Predicting Intestinal Ischaemia in Patients with Adhesive Small Bowel Obstruction: A Simple Score. World J Surg 44, 1444–1449 (2020). https://doi.org/10.1007/s00268-020-05377-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05377-6