Abstract
Adhesive small bowel obstruction (ASBO) has a significant impact on quality of life and medical costs. However, data about ASBO after gastrectomy remain sparse. From January 2009 to December 2017, 3025 patients who underwent gastrectomy for gastric cancer were retrospectively collected and analyzed. Clinicopathological materials were obtained retrospectively, and univariate and multivariate regression analyses were performed to determine risk factors for ASBO. A nomogram for the prediction of ASBO was generated using the results of multivariable analyses. Bootstraps with 1000 resamples were performed for internal validation. The performance of the model was assessed with its discrimination, calibration, and clinical usefulness. A total of 330 (10.9%) patients experienced at least one ASBO episode with a median follow-up of 57.0 (interquartile range 31.0–85.0) months. Logistic regression analysis showed that independent risk factors for ASBO were previous abdominal surgery (odds ratio, OR = 2.03), open gastrectomy (OR = 3.12), non-Billroth-1 reconstruction (Billroth-2, OR = 2.61; Roux-en-Y, OR = 1.99; esophagogastrostomy, OR = 2.79), D2/D2 + lymphadenectomy (OR = 2.64), combined organ resection (OR = 2.76), and postoperative intraabdominal complication (OR = 2.73). The nomogram showed good discrimination, with a C-index of 0.702 and good calibration. Decision curve analysis demonstrated that the nomogram was clinically useful. Several risk factors associated with ASBO after gastrectomy for gastric cancer were identified. Nomogram generated based on these factors could serve as a reliable tool to predict the probability of ASBO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small bowel obstruction is a common reason for admission worldwide, accounting for approximately 17% emergency admissions to a surgical service [1]. In the United States, total healthcare costs associated with it are estimated to exceed 2 billion dollars annually [2]. Approximately 75% of small bowel obstructions are due to intraabdominal adhesions, usually as a consequence of previous operation [3]. Adhesions are thought to occur in almost 95% of all abdominal operations [4], and are related to tissue trauma, the subsequent inflammatory response, and healing process [5].
Gastric cancer is the second leading cause of cancer death and the fourth most common cancer worldwide [6]. Gastrectomy with curative resection has been considered as the mainstay of treatment strategy [6]. Patients underwent gastrectomy experience gastrointestinal manipulation, lymphadenectomy, and damage to the normal anatomy of peritoneum and mesentery. As such, the likelihood of ASBO is increased in these patients. However, there have been few studies [7,8,9] and no large cohort study about the incidence of ASBO after gastrectomy for gastric cancer. In addition, these studies were limited by heterogeneity of the causes of small bowel obstruction (adhesions, obstructive tumor, incarcerated hernia, etc.).
Therefore, we designed the study to specifically investigate the incidence of ASBO after gastrectomy for gastric cancer and analyze the perioperative risk factors for it in a large cohort with long-term follow-up. Determining the modifiable risk factors for ASBO will guide medical providers to prevent this complication. We also developed a predictive nomogram for the future identification of risk of ASBO.
Method
Study population
A total of 3743 consecutive patients who underwent potentially curative gastric cancer surgery from January 2009 to December 2017 were extracted from the database of Surgical Gastric Cancer Patient Registry in West China Hospital (Authorized by Surgical Treatment Group of Gastric cancer under registration number: WCH-SGCPR-2020-3). The establishment of this database was approved by the Research Ethics Committee of West China Hospital. Since the retrospective nature of the study, informed consent was waived. However, patient records were anonymized and de-identified before analysis.
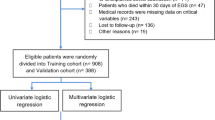
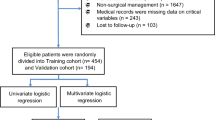
The inclusion criteria were as follows: (1) preoperative upper gastrointestinal endoscopy and biopsy confirmed gastric cancer; (2) preoperative examinations confirmed no distant metastasis. The exclusion criteria were: (1) palliative surgery or exploration; (2) remnant gastric cancer; (3) patients died within 30 days after gastrectomy; (4) patients diagnosed with ASBO or unexplained bowel obstruction within 5 years prior to gastrectomy for gastric cancer; (5) patients with incomplete medical data; (6) patients lost to follow-up. Finally, a total of 3025 patients were enrolled in the study. The diagram is summarized in Fig. 1.
Clinicopathological materials
The demographic and surgical-related parameters of the patients, including gender, age, body mass index (BMI), previous abdominal surgery, surgical approach, surgical procedure, reconstruction method, number of retrieved lymph nodes, extent of lymphadenectomy, combined organ resection, tumor size (cm), macroscopic type, the pathological TNM stage, perioperative blood transfusion, postoperative intensive-care unit (ICU) admission, postoperative surgical complications, postoperative intraabdominal complications, postoperative non-surgical complications, postoperative hospital stay (day), reoperation after gastrectomy, and postoperative chemotherapy, were retrieved for analysis.
The pathological TNM stage was recorded according to the 8th staging manual of American Joint Committee on Cancer [10]. Postoperative complications were defined as any deviation from the normal postoperative course [11]. The severity of postoperative complications was determined according to the Clavien–Dindo classification [11].
Postoperative complications included surgical complications and non-surgical complications. Surgical complications were defined as one of the following events during the postoperative course: wound problem, anastomotic/stump leakage, intraabdominal collections/abscesses, traumatic pancreatitis, lymphatic leakage, intraabdominal bleeding, intraluminal bleeding, ileus, and cholecystitis. Postoperative complications other than surgical complications were defined as non-surgical complications.
Surgical technique
The principles of gastrectomy were based on the Japanese Gastric Cancer Treatment Guidelines [12, 13]. Intraoperative frozen section was routinely performed to secure tumor-free margins. Standard D2 lymphadenectomy was routinely adopted, while D2 + lymphadenectomy was adopted if the nodes beyond the D2 tier were suspicious for metastasis and D1/D1 + lymphadenectomy was selectively adopted for tumors in early stage. Surgical procedure was determined intraoperatively according to the tumor location and the margin of safety. For the reconstruction, esophagogastrostomy was adopted in proximal gastrectomy and Roux-en-Y anastomosis in total gastrectomy. Billroth-1 anastomosis, Billroth-2 anastomosis, and Roux-en-Y anastomosis were adopted in distal gastrectomy according to the surgeons’ preference and experience. Combined organ resection was selectively performed for the purpose of curative resection, or for patients with other comorbidities (such as cholecystectomy for gallbladder stone).
Follow-up
Patients were systematically followed up through consultations or phone calls. All patients were followed up at 6 months in the first 3 years and annually thereafter. The latest follow-up information was updated on Jan 1, 2020. Duration of follow-up was recorded as time from gastrectomy until time of death, or latest follow-up, whichever came first.
Definition of ASBO
ASBO was confirmed by surgery or based on a combination of clinical symptoms (nausea, vomiting, abdominal pain, abdominal distension, and the absence of defecation or flatus in the previous 24 h), physical findings (abdominal tenderness, accentuation of bowel sounds, and tympanitic sounds on percussion), and positive radiologic findings on abdominal X-ray and/or abdominal computed tomography (CT) scans (small bowel distension with air-fluid level). The etiology for the obstruction was determined on the findings at operation and imaging. Patients with small bowel obstruction due to reasons other than adhesions (such as mesenteric defect internal hernia, carcinomatosis, etc.) were not considered to have developed ASBO. Patients were classified into the ASBO ( +) group if they had any episode of ASBO after gastrectomy; otherwise, the patients were classified into the ASBO (−) group (Fig. 1).
Statistical analysis
Statistical analysis was performed using the statistical software package R version 3.6.1 (http://www.r-project.org/) and SPSS 20.0 (SPSS®, Chicago, IL, USA). Continuous variables are reported as the mean ± standard deviation, while categorical variables are reported as number and percentage. A logistic regression analysis was applied to test univariate and multivariate associations between variables to investigate risk factors for ASBO. The risk of ASBO for the respective groups over time was calculated using Kaplan–Meier method, and the differences among them were calculated using log-rank test. A nomogram for the prediction of ASBO was generated using the results of multivariable analyses and the rms package in R. Bootstraps with 1000 resamples were performed for internal validation of the nomogram. The predictive performance of the nomogram was determined by Harrell’s concordance index (C-index). Calibration plot was generated to assess the calibration of the nomogram. A decision curve analysis was generated to evaluate the clinical usefulness of the nomogram. A p value < 0.05 was considered significant.
Results
Baseline characteristics
A total of 3743 consecutive patients who underwent potentially curative gastric cancer surgery from January 2009 to December 2017 were extracted from the database of Surgical Gastric Cancer Patient Registry in West China Hospital. Finally, a total of 3025 patients were included for analysis. These patients had a mean age of 58.2 ± 11.3 years and a male predominance (69.3%). The median follow-up was 57.0 (interquartile range 31.0–85.0) months. The baseline characteristics are indicated in Table 1. The detailed information on previous abdominal surgery and postoperative complication is indicated in Supplementary Table 1 and Supplementary Table 2, respectively.
Incidence of ASBO after gastrectomy for gastric cancer
A total of 330 patients (10.9%) experienced at least one episode of ASBO. The mean time to the first episode of ASBO was 18.2 ± 17.1 months after gastrectomy. Among 330 ASBO patients, 31 patients (9.4%) had recurrent ASBO. 273 patients (82.7%) were managed non-operatively, while 57 patients (17.3%) required operation for their obstruction. These involved laparotomy and lysis of adhesions without bowel resection in 44 patients (13.3%) and with bowel resection in 13 patients (4.0%). The cumulative incidence of ASBO is shown in Fig. 2a. The 3- and 5-year cumulative incidences of ASBO were 10.2% and 12.2%, respectively.
Kaplan–Meier analysis of each risk factor and the log-rank test among respective groups. a Total patients; b previous abdominal surgery (with vs. without); c surgical approach (open vs. laparoscopic); d reconstruction method (Billroth-1 vs. Billroth-2 vs. Roux-en-Y vs. esophagogastrostomy); e extent of lymphadenectomy (D1/D1 + vs. D2/D2 +); f combined organ resection (with vs. without); g postoperative intraabdominal complication (with vs. without)
Risk factors for ASBO after gastrectomy for gastric cancer
Table 2 shows the results of univariate and multivariate logistic regression analyses for the relationship between factors and ASBO. The results showed that previous abdominal surgery [odds ratio (OR) = 2.03, 95% confidence interval (95% CI) 1.55–2.66, p < 0.001], open gastrectomy (OR = 3.12, 95% CI 2.10–5.55, p < 0.001), non-Billroth-1 reconstruction (Billroth-2, OR = 2.61, 95% CI 1.63–4.16, p < 0.001; Roux-en-Y, OR = 1.99, 95% CI 1.22–3.25, p = 0.008; esophagogastrostomy, OR = 2.79, 95% CI 1.60–4.90, p < 0.001), D2/D2 + lymphadenectomy (OR = 2.64, 95% CI 1.77–3.94, p < 0.001), combined organ resection (OR = 2.76, 95% CI 1.88–4.06, p < 0.001), and postoperative intraabdominal complication (OR = 2.73, 95% CI 1.74–4.28, p < 0.001) were significant independent risk factors for ASBO.
The results of Kaplan–Meier analysis for each risk factor are shown in Fig. 2b–g. The 5-year cumulative incidences for previous abdominal surgery history, open gastrectomy, Billroth-1 reconstruction, D2/D2 + lymphadenectomy, combined organ resection, and postoperative intraabdominal complication were 19.3% (vs 10.7%, without previous abdominal surgery), 13.1% (vs 4.7%, laparoscopic gastrectomy), 4.3% (vs 15.1%, Billroth-2 reconstruction; vs 11.8%, Roux-en-Y reconstruction; vs 12.6%, esophagogastrostomy), 13.5% (vs 5.6%, D1/D1 + lymphadenectomy), 27.3% (vs 10.9%, without combined organ resection), and 29.2% (vs 11.4%, without postoperative intraabdominal complication), respectively.
Nomogram and validation
A nomogram for the prediction of ASBO was generated using the results of multivariable analyses (Fig. 3). The predictive model was internally validated using the bootstrap validation method. The nomogram demonstrated good accuracy in estimating the probability of ASBO, with a C-index of 0.702. In addition, model fit was assessed using a bias-corrected calibration plot with 1000-sample bootstrapping for the prediction of ASBO (Fig. 4a). The calibration plot showed adequate fit of the model predicting the risk of ASBO.
a Calibration plot for nomogram prediction of adhesive small bowel obstruction (ASBO) after gastrectomy for gastric cancer. b Decision curve analysis for the nomogram. The y-axis represents net benefit. The x-axis shows the threshold probability. “All” refers to the assumption that all patients develop ASBO and “None” to the assumption that no patient develops ASBO. When the score is within the range 0.22–0.96 (Relevant), using the nomogram to predict ASBO adds more net benefit than the treat-all or treat-none strategies
Clinical usefulness
The decision curve analysis for the nomogram is presented in Fig. 4b. This analysis indicated that, when the threshold probability is within the range 0.22–0.96, using the nomogram to predict ASBO adds more net benefit than the treat-all or treat-none strategies.
Discussion
The marked morbidity and mortality incidences and the healthcare costs highlight the importance of ASBO. The present study was the first large cohort study with a long-term follow-up that specifically investigated the incidence of ASBO and its risk factors after gastrectomy. Numerous and wide-ranging potential risk factors were included and analyzed. Identified risk factors for ASBO were used to generate a nomogram that was able to predict the probability of ASBO.
In the present study, we excluded patients who died within 30 days after gastrectomy and those with remnant gastric cancer. The reason why we excluded patients who died within 30 days after gastrectomy was that ASBO was a relatively long complication after gastrectomy [14]. For remnant gastric cancer, it differs from primary gastric cancer in the following aspects. First, compared with primary gastric cancer, the staging system and guidelines for remnant gastric cancer are not fully established [15]. Second, remnant gastric cancers are mostly limited to the upper third of the stomach [16]. Since lymph nodes were completely removed at the initial operation, all lymphatic flow from the remnant stomach reportedly goes to the greater curvature, regardless of its location. Therefore, total gastrectomy with splenectomy is recommended for remnant gastric cancer [17]. Third, the mean interval after surgeries for gastric cancer is approximately 10 years. Therefore, the majority of patients with remnant gastric cancer are elderly, a population usually has several comorbidities [18]. Considering the above factors, if remnant gastric cancer was included for analysis, many confounding factors would be introduced.
In the present study, postoperative ASBO occurred in 10.9% of gastric cancer patients. The reported incidence of ASBO after gastrectomy for gastric cancer ranged from 6.3% to 11.0% in the previous studies [7,8,9]. Our reported incidence seems consistent with those reported in the previous studies. However, the studies were limited by small sample size, short follow-up, and heterogeneity of the causes of small bowel obstruction. Even the largest study conducted by Inaba et al. included only 395 patients [7]. There was only one study that addressed the risk factors for small bowel obstruction after gastrectomy [9]. The study included only 136 patients for analysis, and the causes of small bowel obstruction included adhesions and incarcerated hernias. Our study was designed to specifically investigate the incidence of ASBO after gastrectomy for gastric cancer in a large cohort with long-term follow-up to overcome these shortcomings.
The study also identified several independent risk factors for ASBO after gastrectomy for gastric cancer. The first factor was surgical approach. Three clinical trials have investigated the impact of surgical approach on the incidence of ASBO following colorectal cancer surgery, but have reported mixed results. The LAFA study [19] showed a protective effect of laparoscopic surgery, while the COLOR [20] and CLASICC [21] trials did not show a statistically significant effect. However, all of the clinical trials were limited by short follow-up and small sample size. These trials had between 2 and 5 years of follow-up and the largest trial involved 786 patients. The highest reported incidence of ASBO in the three trials was 5.2%, a relatively rare incidence. Therefore, a larger number of patients and longer follow-up are needed to achieve sufficient statistical power. In contrast, a recent meta-analysis has demonstrated a protective effect of laparoscopic approach for several abdominal surgeries [22]. Possible reasons for this is that laparoscopic approach involves less tissue trauma and shorter incisions, leads to less bleeding, and reduces the potential for contamination and desiccation of the bowel [23]. An experimental study has also shown that carbon dioxide insufflation may have anti-inflammatory properties, further decreasing the probability of adhesion formation [24].
In the present study, D2/D2 + lymphadenectomy, combined organ resection, and postoperative intraabdominal complication were also identified as independent risk factors for ASBO. Nowadays, D2 lymphadenectomy has been widely accepted as the standard procedure for gastric cancer patients, since it has been proved to improve the overall survival and reduce the risk of tumor recurrence [25]. In our center, D2 + lymphadenectomy was adopted if the nodes beyond the D2 tier were suspicious for metastasis and D1/D1 + lymphadenectomy was selectively adopted for tumors in the early stage. Combined organ resection was selectively performed for the purpose of curative resection, or for patients with other comorbidities. It is generally accepted that the risk of ASBO depends largely on the magnitude of the operation [26]. To our knowledge, this was the first study that demonstrated an association between postoperative complication and ASBO. Postoperative intraabdominal complications are related to peritoneal inflammation, intraabdominal tissue ischemia, and presence of blood, bile, or feces. All of these factors have been demonstrated to be key factors in the formation of adhesions in the previous studies [26, 27].
Interestingly, Billroth-1 reconstruction was found to be a protective factor for ASBO. The possible reasons why Billroth-2 and Roux-en-Y reconstruction lead to more ASBOs than Billroth-1 reconstruction are as follows. First, Billroth-1 reconstruction involves only one anastomosis site without stumps, while Billroth-2 and R-Y involve more anastomosis sites or stumps. Anastomosis site and stump might elicit stronger local inflammatory response, which in turn eventually have resulted in more adhesions. Second, both Billroth-2 reconstruction and Roux-en-Y reconstruction have potential internal hernia defects, while Billroth-1 reconstruction does not [28]; therefore, it is possible that some of the ASBOs may have been caused by mesenteric defect internal hernia, although we excluded bowel obstructions that were caused by mesenteric defect internal hernia as far as possible. The reasons why esophagogastrostomy leads to more ASBOs than Billroth-1 reconstruction are as follows. The splenic flexure of colon may move upward the spleen recess after esophagogastrostomy, which may cause the small bowel to move upward the surgical site, resulting in adhesions between the small bowel and the surgical site.
To our knowledge, this was the first nomogram model to predict the probability of ASBO. Medical providers could make an individualized prediction of the probability of ASBO with this easy-to-use model, which is consistent with the current trend toward personalized medicine [29]. We can identify which patients were at high risk for ASBO based on the nomogram. First, for high-risk patients, the possibility of ASBO should always be considered when typical symptoms occur. The patients are advised to seek medical advice as soon as possible, which may help to avoid some serious complications, such as bowel necrosis. Second, there were several randomized clinical trials on ASBO prevention, such as the use of bioresorbable membrane [8]. This nomogram may provide shreds for future studies. In our personal opinion, risk factors that were incorporated into this nomogram should be balanced before inclusion. Third, we have identified six risk factors for ASBO in the present study. Take surgical approach as an example, laparoscopic gastrectomy was a protective factor for ASBO in the study. Several randomized-controlled trials have demonstrated laparoscopic distal gastrectomy did not result in inferior disease-free survival among patients with both preoperative clinical stages indicating early [30] and locally advanced gastric cancer [31]. Then, upon achieving radical resection and surgical safety, surgeons could adopt laparoscopic gastrectomy for these patients without contraindications to reduce the risk of ASBO.
The most important argument for the use of the model is based on the need to interpret an individual's need for additional care or treatment. However, the calibration and discrimination cannot capture the clinical consequences of particular degrees of miscalibration or levels of discrimination [32]. Therefore, instead of the multicenter prospective validation of the model, which is largely impractical, we applied decision curve analysis to justify the clinical usefulness in the study. The decision curve demonstrated that using the nomogram in the present study to predict the probability of ASBO is more beneficial than the treat-all or the treat-none strategies if the threshold probability of a patient is within 0.22–0.96.
The study also has some limitations. First, the study is a monocentric retrospective cohort study, it is questionable whether these findings can be applied to other hospitals. Second, our model has not been validated externally. However, we used bootstrapping, which has been shown to provide stable estimates with low bias [33], to internally validate our model, which provided good optimism adjusted estimates of its predictive capability. Furthermore, decision curve analysis demonstrated that the nomogram was clinically useful.
Conclusions
In the present study, several risk factors associated with ASBO after gastrectomy for gastric cancer were identified. Nomogram generated based on these factors could serve as a reliable tool to predict the probability of ASBO. These findings may provide health care providers’ warnings about risk factors for ASBO after gastrectomy, and they may also be useful for further research on the prevention and treatment of ASBO.
References
Foster NM, McGory ML, Zingmond DS, Ko CY (2006) Small bowel obstruction: a population-based appraisal. J Am Coll Surg 203(2):170–176. https://doi.org/10.1016/j.jamcollsurg.2006.04.020
Sikirica V, Bapat B, Candrilli SD, Davis KL, Wilson M, Johns A (2011) The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg 11:13. https://doi.org/10.1186/1471-2482-11-13
Mullan CP, Siewert B, Eisenberg RL (2012) Small bowel obstruction. AJR Am J Roentgenol 198(2):W105-117. https://doi.org/10.2214/AJR.10.4998
Harold E, Brendan J, Jeremy N et al (1999) Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. https://doi.org/10.1016/S0140-6736(98)09337-4
Tingstedt B, Isaksson J, Andersson R (2007) Long-term follow-up and cost analysis following surgery for small bowel obstruction caused by intra-abdominal adhesions. Br J Surg 94(6):743–748. https://doi.org/10.1002/bjs.5634
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388(10060):2654–2664. https://doi.org/10.1016/s0140-6736(16)30354-3
Inaba T, Okinaga K, Fukushima R, Iinuma H, Ogihara T, Ogawa F, Iwasaki K, Tanaka M, Yamada H (2004) Prospective randomized study of two laparotomy incisions for gastrectomy: midline incision versus transverse incision. Gastric Cancer 7(3):167–171. https://doi.org/10.1007/s10120-004-0291-6
Hayashi S, Takayama T, Masuda H, Kochi M, Ishii Y, Matsuda M, Yamagata M, Fujii M (2008) Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: a randomized clinical trial. Ann Surg 247(5):766–770. https://doi.org/10.1097/SLA.0b013e3181656d4e
Keishi S, Daisuke K, Morimasa T et al (2008) Factors influencing the development of small intestinal obstruction following gastrectomy for early gastric cancer. Hepatogastroenterology 55:496–499
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99. https://doi.org/10.3322/caac.21388
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Sano T, Aiko T (2011) New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer 14(2):97–100. https://doi.org/10.1007/s10120-011-0040-6
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4
Krielen P, Stommel MWJ, Pargmae P, Bouvy ND, Bakkum EA, Ellis H, Parker MC, Griffiths EA, van Goor H, ten Broek RPG (2020) Adhesion-related readmissions after open and laparoscopic surgery: a retrospective cohort study (SCAR update). Lancet 395(10217):33–41. https://doi.org/10.1016/s0140-6736(19)32636-4
Oh SE, An JY, Choi MG, Lee JH, Sohn TS, Bae JM (2020) Comparisons of remnant primary, residual, and recurrent gastric cancer and applicability of the 8th AJCC TNM classification for remnant gastric cancer staging. Eur J Surg Oncol 46(12):2236–2242. https://doi.org/10.1016/j.ejso.2020.06.032
Honda S, Bando E, Makuuchi R, Tokunaga M, Tanizawa Y, Kawamura T, Sugiura T, Kinugasa Y, Uesaka K, Terashima M (2017) Effects of initial disease status on lymph flow following gastrectomy in cases of carcinoma in the remnant stomach. Gastric Cancer 20(3):457–464. https://doi.org/10.1007/s10120-016-0640-2
Ohashi M, Morita S, Fukagawa T, Kushima R, Katai H (2015) Surgical treatment of non-early gastric remnant carcinoma developing after distal gastrectomy for gastric cancer. J Surg Oncol 111(2):208–212. https://doi.org/10.1002/jso.23774
Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M (2007) Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg 94(1):92–95. https://doi.org/10.1002/bjs.5538
Bartels SA, Vlug MS, Hollmann MW, Dijkgraaf MG, Ubbink DT, Cense HA, van Wagensveld BA, Engel AF, Gerhards MF, Bemelman WA, Collaborative LSG (2014) Small bowel obstruction, incisional hernia and survival after laparoscopic and open colonic resection (LAFA study). Br J Surg 101(9):1153–1159. https://doi.org/10.1002/bjs.9585
Scholin J, Buunen M, Hop W, Bonjer J, Anderberg B, Cuesta M, Delgado S, Ibarzabal A, Ivarsson ML, Janson M, Lacy A, Lange J, Pahlman L, Skullman S, Haglind E (2011) Bowel obstruction after laparoscopic and open colon resection for cancer: results of 5 years of follow-up in a randomized trial. Surg Endosc 25(12):3755–3760. https://doi.org/10.1007/s00464-011-1782-2
Taylor GW, Jayne DG, Brown SR, Thorpe H, Brown JM, Dewberry SC, Parker MC, Guillou PJ (2010) Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. Br J Surg 97(1):70–78. https://doi.org/10.1002/bjs.6742
Yamada T, Okabayashi K, Hasegawa H, Tsuruta M, Yoo JH, Seishima R, Kitagawa Y (2016) Meta-analysis of the risk of small bowel obstruction following open or laparoscopic colorectal surgery. Br J Surg 103(5):493–503. https://doi.org/10.1002/bjs.10105
Gutt CN, Oniu T, Schemmer P, Mehrabi A, Buchler MW (2004) Fewer adhesions induced by laparoscopic surgery? Surg Endosc 18(6):898–906. https://doi.org/10.1007/s00464-003-9233-3
Hanly EJ, Aurora AR, Fuentes JM, Shih SP, Marohn MR, De Maio A, Talamini MA (2005) Abdominal insufflation with CO2 causes peritoneal acidosis independent of systemic pH. J Gastrointest Surg 9(9):1245–1251. https://doi.org/10.1016/j.gassur.2005.09.007 (discussion 1251–1242)
Songun I, Putter H, Kranenbarg EM-K, Sasako M, van de Velde CJH (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11(5):439–449. https://doi.org/10.1016/s1470-2045(10)70070-x
Hellebrekers BW, Kooistra T (2011) Pathogenesis of postoperative adhesion formation. Br J Surg 98(11):1503–1516. https://doi.org/10.1002/bjs.7657
Luijendijk RW, de Lange D, Wauters C (1996) Foreign material in postoperative adhesions. Ann Surg 223:242–248
Miyagaki H, Takiguchi S, Kurokawa Y, Hirao M, Tamura S, Nishida T, Kimura Y, Fujiwara Y, Mori M, Doki Y (2012) Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J Surg 36(4):851–857. https://doi.org/10.1007/s00268-012-1479-2
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16(4):e173–e180. https://doi.org/10.1016/s1470-2045(14)71116-7
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ, Korean Laparoendoscopic Gastrointestinal Surgery Study Group (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 5(4):506–513. https://doi.org/10.1001/jamaoncol.2018.6727
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study Group (2019) Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA 321(20):1983–1992. https://doi.org/10.1001/jama.2019.5359
Pencina MJ, D’ Agostino RB, D’ Agostino RB, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27(2):157–172. https://doi.org/10.1002/sim.2929
Ewout W, Frank E, Gerard J et al (2001) Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54:774–781
Acknowledgements
The authors would like to thank the substantial work of Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China, for the establishment and updating of gastric cancer database.
Funding
Foundation of Science & Technology Department of Sichuan Province (2019YFS0255); The Ten Thousand Talent Program of Sichuan Province (No. 101); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University, No. ZY2017304.
Author information
Authors and Affiliations
Contributions
JKH, TP, XLC, and DG made substantial contributions to conception and design for this study. TP, XLC, and DG, KL, and WHZ acquired and analyzed data and Tao Pan drafted the article. KY, XZC, and JKH provided a large number of cases and gave many important suggestions for this study. They also participated in writing the paper. TP, LYZ, KL, XZC, WHZ, XLC, and KY also participated in revising it critically for important intellectual content; TP, XLC, and DG contributed equally to this work. JKH gave the final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Disclosures Tao Pan, Danil Galiullin, Xiao-long Chen, Wei-han Zhang, Kun Yang, Kai Liu, Lin-yong Zhao, Xin-zu Chen, and Jian-kun Hu have no conflicts of interest or financial ties to disclose.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, T., Galiullin, D., Chen, XL. et al. Incidence of adhesive small bowel obstruction after gastrectomy for gastric cancer and its risk factors: a long-term retrospective cohort study from a high-volume institution in China. Updates Surg 73, 615–626 (2021). https://doi.org/10.1007/s13304-021-00983-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-00983-y