Abstract
Background
Internal hernia is a well-known postoperative complication after Roux-en-Y gastric bypass. However, it has not been considered a recognized complication for gastric cancer.
Methods
We reviewed the literature in the past decade to clarify the current status of internal hernia after gastrectomy including its incidence, high-risk factors, and treatment.
Results
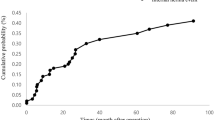
The incidence of internal hernia after gastrectomy was found to be between 0.2 and 5.63%, and the median interval time was less than 2 years. High-risk factors include laparoscopic approach, non-closure of all the mesenteric defects, and Roux-en-Y reconstruction. The rate of bowel resection was significantly higher than that of adhesive small bowel obstruction.
Conclusion
The true incidence of internal hernia after gastrectomy is generally underestimated. Closure of all the mesenteric defects is one of the most effective methods to prevent postoperative internal hernia. Early surgical exploration is necessary when internal hernia is suspected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Internal hernia (IH) is a well-known post-surgical complication after Roux-en-Y gastric bypass (RYGB) [1]. Although closure of defects is laborious and challenging, routine closure of mesenteric defects has been suggested in bariatric surgery [2, 3]. However, IH has not been considered a recognized complication post-gastrectomy for gastric cancer (GC), which generates the same defects during the surgical procedure. Furthermore, the actual prevalence of this complication is mostly undervalued because the duration of follow-up is limited, and few studies investigated IH as a primary endpoint.
Total gastrectomy (TG) or distal gastrectomy (DG) with Roux-en-Y reconstruction creates at least two notable hernia spaces. One is between the Roux limb and the transverse colon, the so-called Petersen’s space. The other is the mesenteric defect around the jejunojejunostomy, if retrocolic placement of the Roux limb is performed, and also the transverse mesocolon defect. These potential hernia spaces are also present during proximal gastrectomy (PG) with double-tract reconstruction (DTR) or jejunal interposition (JI). Each defect becomes a potential orifice for IH. This study reviewed the IH after gastrectomy due to GC using PubMed, Elsevier, and Baidu Scholar. We evaluated the literature in the past decade to elucidate the actual status of IH after gastrectomy, discussing its prevalence, treatments, and outcomes.
Incidence of IH
In the literature, the occurrence of IH after gastrectomy was found to be between 0.20 and 5.63%, and the median interval time was less than 2 years (Table 1) [4,5,6,7,8,9,10,11,12,13,14]. These differences may be due to different proportions of laparoscopy, reconstruction method, and closure of defects. Miyagaki et al. [7] reported 18 (0.20%) cases of IH among 8983 patients who underwent gastrectomy from 24 participating centers, indicating that IH was a negligible postoperative complication. However, this study included those with little possibility of IH such as Billroth I anastomosis and PG with esophagogastrostomy. In this report, IH was observed only in patients with Roux-en-Y reconstruction, and the authors did not describe the proportion of various reconstruction methods in detail. In addition, another study revealed that the incidence of IH after Roux-en-Y reconstruction reached up to 5.37% [12].
Petersen’s hernia (PH)
Approximately 7.9–27% of IH occur at Petersen’s space in laparoscopic RYGB, with a high rate of bowel resection and mortality [15]. An apparent long biliopancreatic limb results in a greater gap from the root of the small bowel mesentery to the transverse colon and a larger Petersen’s space in RYGB than that in Roux-en-Y reconstruction after gastrectomy. In addition, the lower edge of Petersen’s space is higher in gastrectomy, making the small bowel difficult to pass through. Thus, we intuitively believe that PH after gastrectomy is rare, and it was not routinely closed in the past [7, 11]. However, previous literature showed that the proportion of PH was higher after gastrectomy than that after RYGB, which varied between 20 and 100% (Table 1). Many centers have routinely closed Petersen’s space after reconstruction in recent years [11, 16]. A retrospective study from China showed that the incidence of PH in the group with closed Petersen’s space was considerably lesser than that in the non-closure group (p=0.04) [17].
Factors associated with IH
Laparoscopic approach
Laparoscopic surgery is less likely to cause adhesion than open surgery (Table 2). Han et al. [6] revealed that the occurrence of IH is significantly elevated after laparoscopic gastrectomy than open surgery (0.81% vs. 0.19%, p<0.01). Multivariable analysis demonstrated that laparoscopic surgery is an independent risk factor for postoperative IH (p<0.01). These results were similar to those of other studies, in which the laparoscopic method was found to be a risk factor for IH [7, 8, 12]. Two other studies found no difference between open surgery and laparoscopy [4, 10], possibly due to the limited number of cases. Despite the lack of strong evidence from prospective research, most surgeons gradually accepted the fact that laparoscopic gastrectomy is a risk factor for IH than open surgery. Considering the widespread usage of laparoscopy for GC, the prevention and treatment of IH should cause more concern.
Closure of mesenteric defects
The high risk of postoperative IH is closely related to defects after digestive tract reconstruction. Some studies have revealed a considerable reduction in IH incidence or no incidence after hernia site defect closure after gastrectomy (Table 3). The study with closure of jejunojejunostomy mesenteric defect or Petersen’s space alone also supports this fact. The results showed that IH at the jejunojejunostomy mesenteric defect was observed in 3.4% (5/149) patients without closure of this defect, while no IH was found in 206 patients with closure (p<0.01); PH was observed in 2.8% (7/250) patients without closure of this defect, and no PH was found in the 105 patients with closure [13]. On the contrary, Kelly et al. [12] concluded that the occurrence of IH is significantly lowered post-gastrectomy, and regular closure of mesenteric defects may not considerably affect the occurrence of IH. Currently, no consensus exists on the management of mesenteric defects after gastrectomy. We insist that closing all defects contributes to reducing IH after gastrectomy.
Route of Roux limb
As for the position of the Roux limb, the retrocolic route has three potential sites for IH, namely, the transverse mesocolon defect, Petersen’s space, and the jejunojejunostomy mesenteric defect. The antecolic route lowers the potential sites from three to two, including Petersen’s space and the jejunojejunostomy mesenteric defect. The antecolic route might be better as it eliminates one of the most common sites for herniation. In fact, most reports showed no considerable variation in the occurrence of IH between the retrocolic and antecolic routes after gastrectomy (Table 4). Only one study reported that the incidence of IH (10%, 1/10) in the retrocolic route was significantly higher than that in the antecolic route (2.63%, 11/418; p=0.013) [10]. However, Kimura et al. [13] revealed that IH in the retrocolic route occurred earlier than that in the antecolic route. Furthermore, the retrocolic route is a risk factor for intestinal obstruction compared with the antecolic route, and massive bowel resection was mostly conducted in patients in the retrocolic group.
Reconstruction methods
IH was observed mostly in patients with Roux-en-Y reconstruction after TG or DG. This finding was attributed to the fact that Roux-en-Y reconstruction has at least two defects (jejunojejunostomy mesenteric defect and Petersen’s space) for IH; however, Billroth II reconstruction has Petersen’s space only, and no mesenteric defect is created in Billroth I reconstruction. Han et al. [6] revealed that the occurrence of IH with Roux-en-Y reconstruction was higher than that of Billroth II and other reconstruction methods (7.1% vs. 1.4%, p<0.01). Kang et al. [5] also reported that Roux-en-Y reconstructions after DG lead to a considerably elevated incidence of IH relative to Billroth II reconstruction (5.93 vs. 1.14%, p<0.01), and no IH was observed in 2361 patients with Billroth I reconstruction. Another operation involving different reconstruction methods is PG. Considering reflux esophagitis and anastomotic stenosis post-esophagogastrostomy, DTR and JI have been considered to be the most significant alternative reconstruction methods for PG [18, 19]. Takayama et al. [4] reported four cases of PH among 71 patients after PG with JI reconstruction. Another study reported six cases of IH in 264 patients with DTR, and no IH occurred in 39 patients with EG during the same period [5].
Diagnosis of IH
The majority of patients show nonspecific symptoms including nausea, abdominal pain, vomiting, and abdominal pain that occasionally radiates to the back. Tachycardia has been reported as a key clinical predictor in postoperative IH after bariatric surgery [20]. Yoshikawa et al. [10] reported the same results, i.e., 83% of patients presented with IH after gastrectomy with tachycardia (>90 bpm). The white blood cell count and CRP level are usually normal. CT is the preferred choice for patients with clinical suspicion of IH. Miyagaki et al. [7] reported that 14 cases (78%) of 16 patients with IH after gastrectomy were diagnosed with preoperative CT. Yoshikawa et al. [10] reviewed 12 patients with IH after gastrectomy, and small intestinal dilation and whirl sign were present in 100% and 83%, respectively. Instead of focusing on a specific examination, the symptoms of patients and different tests should be combined to more expeditiously determine the proper treatment.
Treatment of IH
IH may cause massive small bowel necrosis and serious consequences, such as short bowel or even death, particularly when early detection and surgical treatment have been delayed. Kang et al. [5] reported 111 cases of IH after gastrectomy identified by CT or surgical exploration, and 52 (46.8%) patients underwent non-surgical treatment. Considering the specificity of CT for IH, the indication could not be established for non-surgical treatment of IH. Given the differences in the basic characteristics of patients, the mortality rates may vary. Thus, we chose the rate of bowel resection as a relatively objective sign to reflect the consequences of IH. The rate of bowel resection caused by IH after gastrectomy is shown in Table 1, which was significantly higher than the rate of adhesive small bowel obstruction [21]. Another study compared the surgical outcome on the basis of time interval from symptom onset to surgery for IH [6]. The result showed that bowel resection was more frequently observed in the late intervention group, and poor surgical results were revealed in the late intervention group. This result suggested that early surgical treatment is needed when IH is suspected after gastrectomy. Because of the dilated intestine, emergency surgical treatment is mostly performed in an open fashion, and laparoscopic exploration can also be considered with no obvious expansion of the bowel.
Surgical treatment involves hernia reduction and closure of each defect or bowel resection. At times, massive bowel resection is inevitable because of extensive bowel necrosis, resulting in short bowel syndrome and even death. Jang et al. [22] reported a patient with the whole small bowel and Roux-en-Y limb necrosis caused by PH after subtotal gastrectomy with Roux-en-Y reconstruction. They connected the remnant stomach and the jejunum by forming a new Roux-en-Y anastomosis using a segment of the transverse colon as a new Roux limb by two-phase operation. In IH patients without bowel necrosis, adhesion often makes it difficult to distinguish the direction of the hernia. It is feasible to look for the hernia into the intestinal canal through the ileocecal area and pulling it toward the ileocecal area. Another aspect is to close all defects. Currently, no consensus exists on the management of mesenteric defects created after gastrectomy. A study recommended that the space between stitches should be established at ≤1 cm during closure of mesenteric defects [23]. Paroz et al. [24] suggested the closure of each mesenteric defect via running nonabsorbable suture after laparoscopic RYGB. Intuitively, nonabsorbable suture should be a better choice in this procedure. However, Ojima et al. [8] routinely closed all mesenteric defects using a 3-0 absorbable suture and reported that no patients developed IH since 2013. Aghajani E et al. [25] reported the technique of closing the defects with a hernia stapling device for obesity surgery, which can easily be used in gastrectomy for GC as well.
Conclusions
The true incidence of IH after gastrectomy is generally underestimated because the duration of follow-up is limited and few studies investigated it as a primary endpoint. We admitted that selection bias is unavoidable in retrospective studies, but a randomized controlled trial is always difficult in such a relatively rare postoperative complication. Although closure of all mesenteric defects is technically difficult, time-consuming, and laborious, it is the most effective method to prevent postoperative IH. Furthermore, surgeons should emphasize the early recognition of IH. Instead of focusing on a specific examination, the symptoms of patients and different tests should be combined to more expeditiously determine the proper treatment. Early surgical exploration is necessary when IH is suspected.
Availability of data and material
Not applicable.
References
Geubbels N, Lijftogt N, Fiocco M, van Leersum NJ, Wouters MW, de Brauw LM (2015) Meta-analysis of internal herniation after gastric bypass surgery. Br J Surg 102(5):451–460
Bauman RW, Pirrello JR (2009) Internal hernia at Petersen’s space after laparoscopic Roux-en-Y gastric bypass: 6.2% incidence without closure–a single surgeon series of 1047 cases. Surg Obes Relat Dis 5(5):565–570
Steele KE, Prokopowicz GP, Magnuson T, Lidor A, Schweitzer M (2008) Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc 22(9):2056–2061
Takayama Y, Kaneoka Y, Maeda A, Fukami Y, Takahashi T, Onoe S, Uji M (2018) Internal hernia after proximal gastrectomy with jejunal interposition. Updates Surg 70(1):85–90
Kang KM, Cho YS, Min SH, Lee Y, Park KB, Park YS, Ahn SH, Park DJ, Kim HH (2019) Internal hernia after gastrectomy for gastric cancer in minimally invasive surgery era. Gastric Cancer 22(5):1009–1015
Han WH, Eom BW, Yoon HM, Kim YW, Ryu KW (2019) Clinical characteristics and surgical outcomes of internal hernia after gastrectomy in gastric cancer patients: retrospective case control study. Surg Endosc 33(9):2873–2879
Miyagaki H, Takiguchi S, Kurokawa Y, Hirao M, Tamura S, Nishida T, Kimura Y, Fujiwara Y, Mori M, Doki Y (2012) Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J Surg 36(4):851–857
Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Kato T, Tsuji T, Yamaue H (2017) Internal hernia after laparoscopic total gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 27(6):470–473
Gong CS, Ko CS, Kim BS, Kim HS (2019) Diaphragmatic hernia after totally laparoscopic total gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 29(3):194–199
Yoshikawa K, Shimada M, Kurita N, Sato H, Iwata T, Higashijima J, Chikakiyo M, Nishi M, Kashihara H, Takasu C, Matsumoto N, Eto S (2014) Characteristics of internal hernia after gastrectomy with Roux-en-Y reconstruction for gastric cancer. Surg Endosc 28(6):1774–1778
Kojima K, Inokuchi M, Kato K, Motoyama K, Sugihara K (2014) Petersen’s hernia after laparoscopic distal gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer 17(1):146–151
Kelly KJ, Allen PJ, Brennan MF, Gollub MJ, Coit DG, Strong VE (2013) Internal hernia after gastrectomy for cancer with Roux-Y reconstruction. Surgery 154(2):305–311
Kimura H, Ishikawa M, Nabae T, Matsunaga T, Murakami S, Kawamoto M, Kamimura T, Uchiyama A (2017) Internal hernia after laparoscopic gastrectomy with Roux-en-Y reconstruction for gastric cancer. Asian J Surg 40(3):203–209
Hanada K, Hata H, Kikuchi S (2015) Internal hernia after gastrectomy with Roux-en-Y reconstruction. Jpn J Gastroenterol Surg 48(11):890–896
Capella RF, Iannace VA, Capella JF (2006) Bowel obstruction after open and laparoscopic gastric bypass surgery for morbid obesity. J Am Coll Surg 203(3):328–335
Hosoya Y, Lefor A, Ui T, Haruta H, Kurashina K, Saito S, Zuiki T, Sata N, Yasuda Y (2011) Internal hernia after laparoscopic gastric resection with antecolic Roux-en-Y reconstruction for gastric cancer. Surg Endosc 25(10):3400–3404
Pan T, Wang H, Liu K, Chen XZ, Zhang WH, Chen XL, Yang K, Zhang B, Zhou ZG, Hu JK (2021) Closure of Petersen’s defect in gastrectomy for gastric cancer: an interrupted time series analysis from a high-volume institution in China. Langenbecks Arch Surg 406(2):427–436
Aburatani T, Kojima K, Otsuki S, Murase H, Okuno K, Gokita K, Tomii C, Tanioka T, Inokuchi M (2017) Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc 31(11):4848–4856
Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, Matsumura S, Kato T, Kitadani J, Iwahashi M, Yamaue H (2014) Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery 156(1):57–63
Ahmed AR, Miskovic D, Vijayaseelan T, O’Malley W, Hanna GB (2012) Root cause analysis of internal hernia and Roux limb compression after laparoscopic Roux-en-Y gastric bypass using observational clinical human reliability assessment. Surg Obes Relat Dis 8(2):158–163
Long B, Robertson J, Koyfman A (2019) Emergency medicine evaluation and management of small bowel obstruction: evidence-based recommendations. J Emerg Med 56(2):166–176
Jang JS, Shin DG (2013) A Peterson’s hernia and subsequent small bowel volvulus: surgical reconstruction utilizing transverse colon as a new Roux-en-Y limb - 1 case. J Korean Surg Soc 85(6):309–313
Müller MK, Räder S, Wildi S, Hauser R, Clavien PA, Weber M (2008) Long-term follow-up of proximal versus distal laparoscopic gastric bypass for morbid obesity. Br J Surg 95(11):1375–1379
Paroz A, Calmes JM, Giusti V, Suter M (2006) Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a continuous challenge in bariatric surgery. Obes Surg 16(11):1482–1487
Aghajani E, Nergaard BJ, Leifson BG, Hedenbro J, Gislason H (2017) The mesenteric defects in laparoscopic Roux-en-Y gastric bypass: 5 years follow-up of non-closure versus closure using the stapler technique. Surg Endosc 31(9):3743–3748
Acknowledgements
We would like to thank colleagues at the Department of Gastrointestinal Surgery in our hospital.
Funding
This study was funded by the gastrointestinal oncology international team cooperation project [NO. SZYJTD201804].
Author information
Authors and Affiliations
Contributions
Sun Ke-kang performed the research and wrote the paper; Wu Yong-you designed the research and supervised the report. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The Second Affiliated Hospital of Soochow University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Kk., Wu, Yy. Current status of internal hernia after gastrectomy for gastric cancer. Langenbecks Arch Surg 407, 99–104 (2022). https://doi.org/10.1007/s00423-021-02371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02371-x